Introduction
Given current rapid climate change in the Arctic (
IPCC 2013), it is essential to understand how northern arthropods, which constitute more than 60% of the northern diversity (
CAFF 2013), are distributed and which factors influence spatial patterns of diversity. This is required to develop sustainable long-term conservation strategies. Because these small ectothermic organisms respond to fine variations in microhabitat, but often have large range of distribution (
CAFF 2013;
Hansen et al. 2016), appropriate investigation of diversity patterns and drivers underlying those patterns is required at multiple spatial scales.
Looking to the literature, it is clear that determining patterns at multiple spatial scales can help to unravel some unexpected biological information (e.g., host specificity within butterflies (
Summerville et al. 2003)), reinforce the importance of some habitats for biodiversity conservation (
Paknia and Pfeiffer 2011) or the impact of some disturbances on biodiversity (
Fournier and Loreau 2001), and determine processes driving biodiversity patterns (
Marques and Schoereder 2014). Using a standardized nested sampling design, patterns and processes of tropical and temperate forest arthropods have been successfully described, focusing on local and regional patterns of butterflies and moths (
Summerville et al. 2003;
Beck et al. 2012), flies (
Lévesque-Beaudin and Wheeler 2011), beetles (
Fournier and Loreau 2001;
Gering et al. 2003), ants (
Paknia and Pfeiffer 2011), and spiders (
Cabra-García et al. 2010;
Larrivée and Buddle 2010). Although the effects of spatial scales on arthropods have been extensively studied, there remain relatively few studies that focus on biome transitions.
Ecological patterns and processes in more temperate regions are known to differ from those in tropical regions (
Algar et al. 2011), and it is reasonable to assume patterns and processes in Arctic regions, which have received little attention (but see
Ernst and Buddle 2015;
Hansen et al. 2016;
Cameron and Buddle 2017), will also differ from other major biomes on the planet. Therefore, the objective of this research was to document Arctic biodiversity patterns across multiple geographical scales, from within-site (sample to sample) to regional scales (i.e., across all of northern Canada), using ground-dwelling spiders (Araneae) as a model taxon, and to determine the relative importance of environmental and spatial drivers underlying spatial patterns of diversity in northern Canada.
We addressed the following three research questions:
•
How do ground-dwelling spider species richness, abundance, composition, and structure vary at sample, site, and regional scales, in three major ecoclimatic regions in northern Canada?
•
Are patterns similar among the most dominant families?
•
What is the relative importance of environmental and spatial drivers underlying diversity patterns of spiders in northern Canada?
Results
A total of 28 427 spider specimens representing 14 families was collected (
Table 1); of these, 23 010 adult spiders (28% female and 72% male) were identified to species. Total species richness (γ diversity) was 305 species, with 107 (35%) spider species representing new provincial or territorial records (
Table S2). In total, 68 species were singletons and 27 were doubletons, which represent respectively 22% and 9% of the total diversity. Fewer spider species were collected in the arctic (35 species) compared with the subarctic (173 species) and north boreal (232 species) ecoclimatic regions. Eight species of spiders were present in all three ecoclimatic regions (
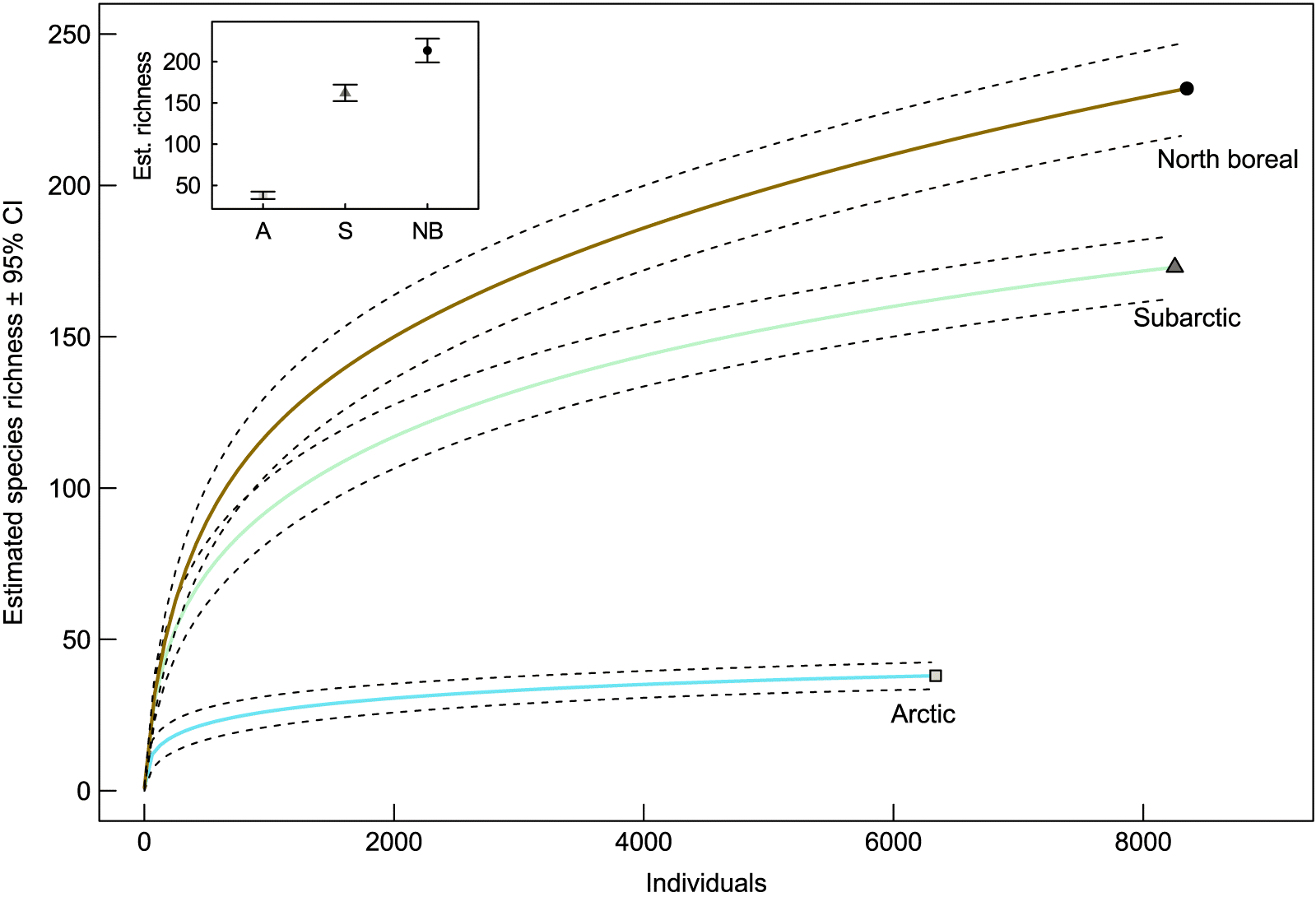
Table S2). Of the species present in the arctic, 65% were shared with another region compared with 73% in the subarctic and 46% in the north boreal ecoclimatic regions. Species rarefaction curves revealed that the arctic and subarctic ecoclimatic regions were better sampled than the north boreal region (
Fig. 1). The completeness of the collection varied between 69% and 100% within site, with lower values in the north boreal region (
Table 1).
Multi-scale patterns of spider diversity
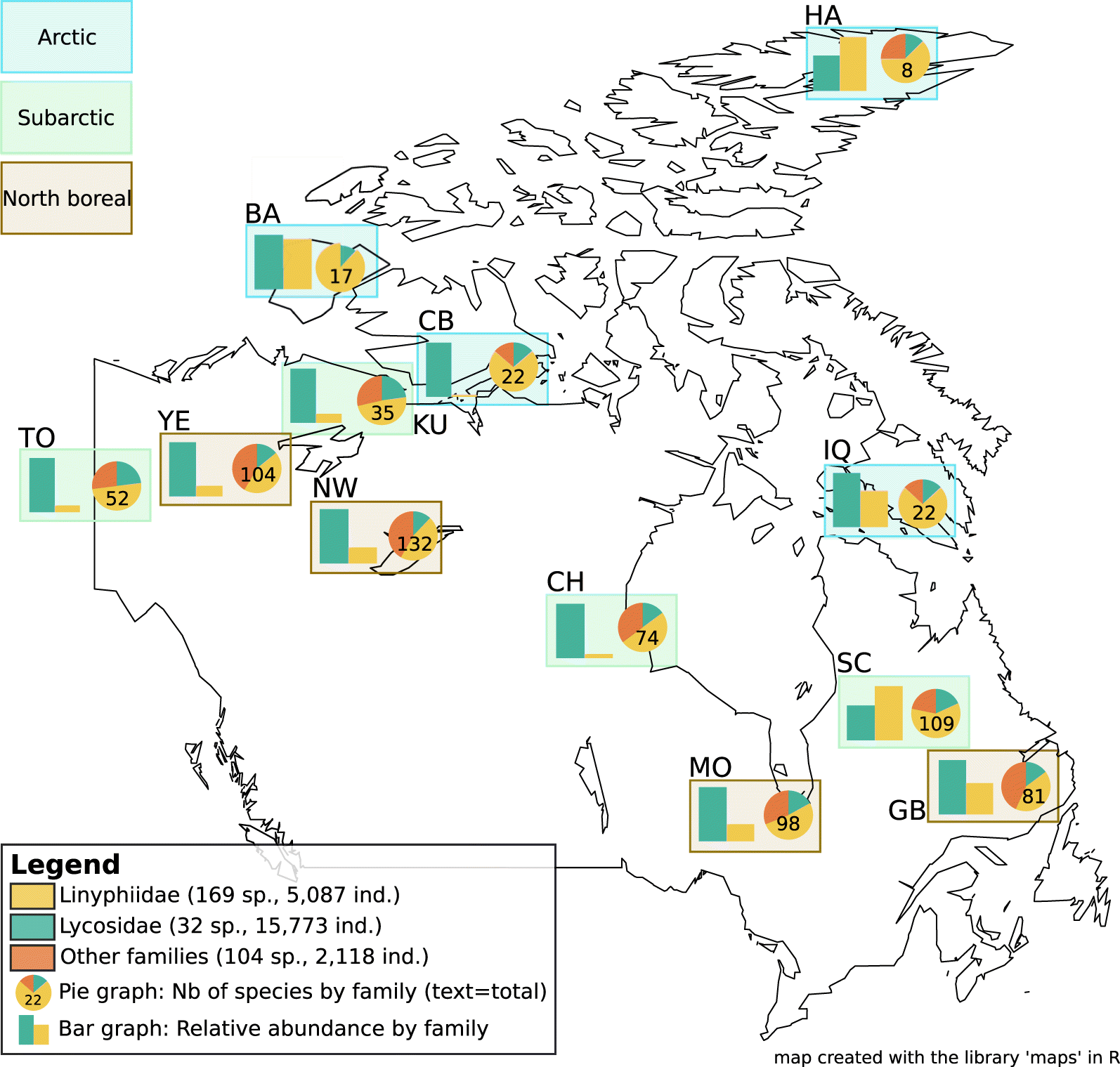
At similar latitudes, species richness was generally higher in western Canada (west of the 96° meridian) than eastern Canada (east of the 96° meridian). For example, the Norman Wells site (NW) in western Canada had a species richness of 132 at 65° latitude, whereas Iqaluit (IQ) at 62° latitude in eastern Canada had a richness of only 22 species (
Fig. 2). With 169 species (55% of the total species richness), the Linyphiidae family was the most diverse family of spiders collected (
Fig. 3). The most abundant family was the Lycosidae, with 15 778 individuals and 32 species, representing 67% of the total abundance (
Fig. 2). The proportion of the total diversity (pie charts) and total abundance (bar charts) represented by Linyphiidae species increased from north boreal sites to arctic sites (
Fig. 2).
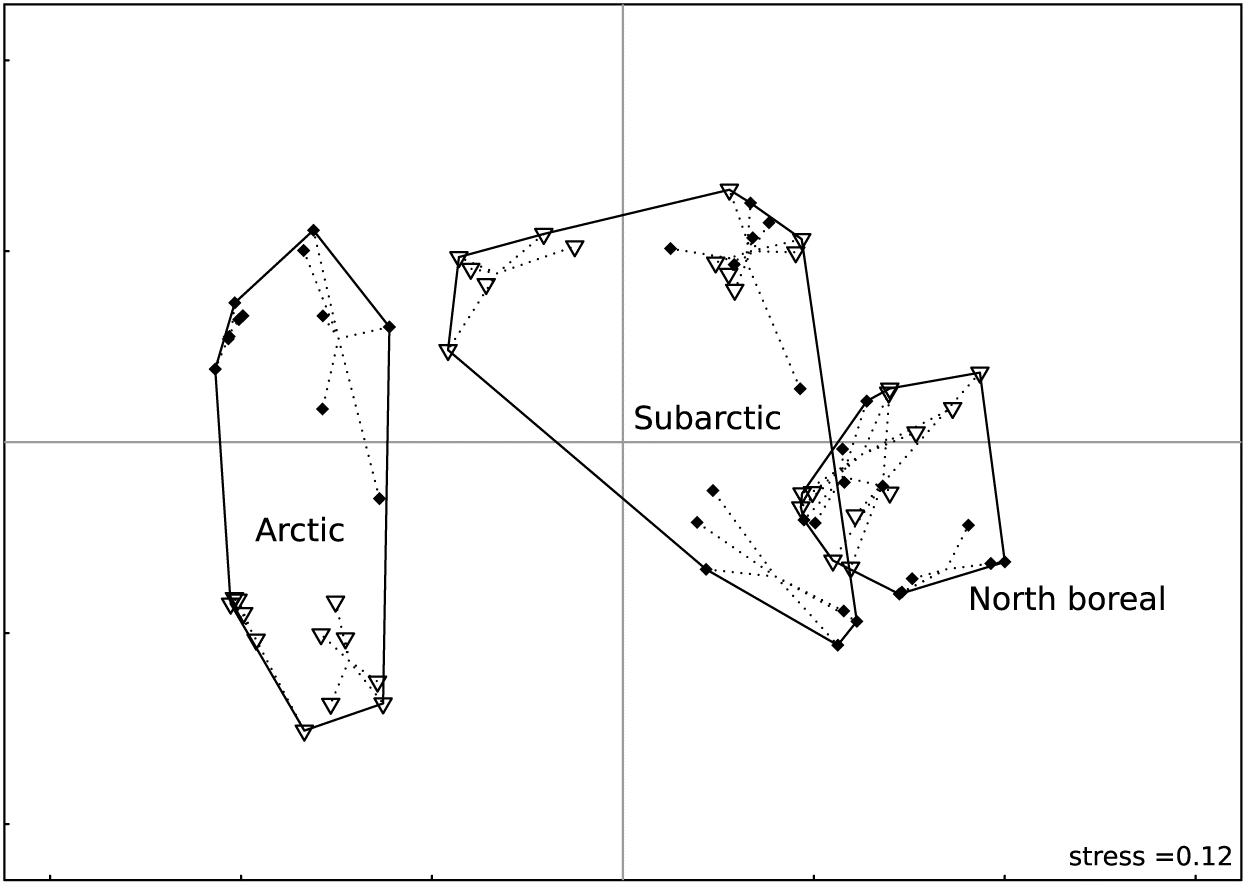
Ecoclimatic regions showed clear differences in species composition (
Fig. 3). Variation in composition between sites was higher in the arctic than in the subarctic or north boreal ecoclimatic regions (
Fig. 3).
Diversity partitioning results showed that spider diversity was structured (i.e., non-random) at the regional, site, and sample scales with lower diversity than expected by chance at lower scales and higher observed diversity at larger spatial scales (
Table 2). The observed values of β4 and β3 were significantly higher than expected with a random distribution (
Table 2). The ecoclimatic region was the most important spatial scale in structuring diversity; β4 contributed to 50% of the total diversity of γ diversity meaning that half of the species are restricted to one ecoclimatic region. The site scale was the second most important spatial scale with β3 contributing to 30% of the total richness. Additive partitioning of diversity within each ecoclimatic region did not show differences between regions. β3 contributed to almost half of the diversity within each region (
Table 2).
Environmental and spatial drivers of diversity patterns
The three explanatory matrices (vegetation, climate, and spatial variables) explained 59.6% of the total variation in spider composition at the regional scale. The vegetation matrix based on local measures explained 38.1% of variation in ground-dwelling spider diversity compared with 35.2% for the climate matrix. Latitude was not an important driver and the two spatial variables explained only 17.1% of the variation in diversity at a large spatial scale. Variance partitioning within each region showed similar results in different proportions. The effects of vegetation explained 61% and 46% of the variance in the arctic and north boreal ecoclimatic regions, respectively.
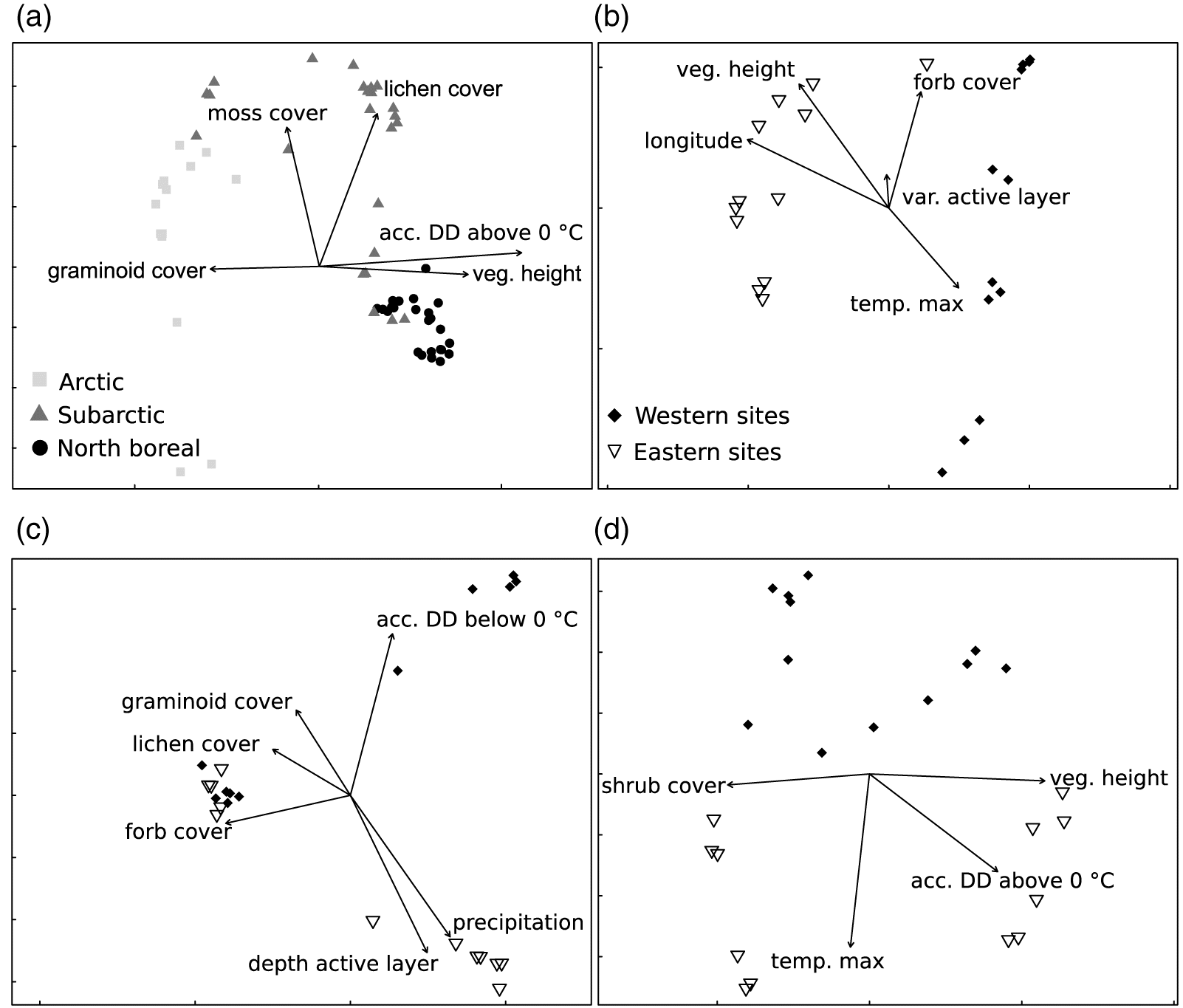
The constrained ordination was performed using all variables, as no variable was discarded by the permutation selection (
Fig. 4a). The first canonical axis explained 16% of the total variance of spider composition, and the first two canonical axes combined explained 26.7% of the variance. The significant variables selected to find parsimonious models were different within each ecoclimatic region (
Figs. 4b,
4c, and
4d). Variables selected in each region were a mix of several local variables and one or two climatic variables, with the exception of the arctic ecoclimatic region where longitude was also significant. For the arctic and the north boreal ecoclimatic regions, maximum temperature of the warmest month seemed to influence differences between the eastern and western sites (
Figs. 4b and
4d). The first two canonical axes explained 62% of the variance in the arctic (
Fig. 4b), 52% in the subarctic (
Fig. 4c) and 50% in the north boreal ecoclimatic regions (
Fig. 4d).
Discussion
The objective of this research was to quantify the diversity and community structure of Arctic spiders occurring from local to broad spatial scales in northern Canada, and to examine factors determining Arctic species distribution. Our study represents one of the largest standardized biodiversity studies of spiders across large geographical scales, spanning 80 degrees longitude and 30 degrees latitude. This research increased our understanding of the distribution of more than 300 spider species, a dominant arthropod taxon in Arctic ecosystems, including over 100 new provincial and territorial records. Considering the high number of new records, it is clear that baseline data on spider species distribution in Canada are incomplete. Our extensive quantitative dataset will be a benchmark for spider species distribution in northern Canada as it has already facilitated the identification of the conservation status of Canadian spider species (
Canadian Endangered Species Conservation Council 2016).
One third of the spider species found in the Arctic were unique to that ecoclimatic region, but we noted great differences in composition among sites within the arctic ecoclimatic region. The unique geography of the Canadian Arctic archipelago combined with harsh climatic conditions may explain the high variation in composition between sites that were on different islands of the archipelago (
Chernov 1995). These results suggest that at least some Arctic spider species have narrow geographic ranges in Canada, an attribute that might make them more at risk in a context of climate warming (
CAFF 2013;
IPCC 2013). However, the biogeography of the Canadian Arctic archipelago is far from being well understood even though it is the largest archipelago on Earth, with more than 36 000 islands. The study of diversity patterns within and between islands of the archipelago is necessary to complete our understanding of the respective effects of insularity, climate, and geography on biodiversity patterns in the Canadian Arctic. This might be achieved more easily than previously thought, as our results highlight how arthropod sampling in the Arctic, even over a short period of time such as two weeks, could lead to useful information on species diversity.
Despite sampling eastern sites and western sites in different years, the longitudinal pattern observed across Canada was in line with the patterns found for birds, plants, butterflies, and other insect groups in Canada (
Danks 1993;
Chernov 1995;
Callaghan et al. 2004). This peculiar spatial pattern may be best explained by post-glacial migration from different refugia in northern Canada. Western species probably originated from the Beringian refugium or other cryptic western refugia, whereas species in eastern Canada may have originated from southern refugia after the Wisconsin glaciation (e.g.,
Waltari et al. 2004;
Solecki et al. 2016). Moreover, some observed community dissimilarities might result from large-scale obstacles to spider dispersal. For instance, the assemblage composition in Kugluktuk was more similar to that in Churchill, which is nearly 1500 km away, than that in Tombstone Mountains, which is 1000 km away. The presence of natural biogeographic barriers like the sea of Hudson Bay (
Danks 1993) plays an important role in limiting organism dispersion in Canada.
Species richness and evenness at local scales were lower than expected by chance. This suggests that biotic and abiotic factors are more important than stochastic factors in determining the richness of spiders locally. A low local turnover can be explained by the presence of biotic interactions (
Wisz et al. 2013). For example, burrowing spiders in sand dunes showed reduced species density and diversity locally due to competition, and this effect was scale dependent (
Birkhofer et al. 2006). Given that the Lycosidae egg sac parasitism is surprisingly high in some subarctic populations (
Bowden and Buddle 2012), biotic interactions may play a greater role in structuring local diversity in the Arctic than expected. This might be explained by the important biomass of predators compared with herbivores or detritivores in the Arctic (
Ernst and Buddle 2015). Experiments on spider parasitism, cannibalism (see
Asmus 2017 for example), and competition will help to unravel some of the biotic interactions that have largely been forsaken in the recent literature on Arctic food webs based on molecular data (
Roslin et al. 2013;
Wirta et al. 2015).
Spider diversity was structured among ecoclimatic regions, characterized by unique climate, soil type, and vegetation composition (
Strong and Zoltai 1989). Vegetation composition, but not climate variables, best explained variation in the diversity of spiders at a broad scale, as previously observed in other northern systems (e.g.,
Bowden and Buddle 2010a;
Rich et al. 2013) and in other more temperate habitats at regional or local scales (e.g.,
Jimenez Valverde and Lobo 2007;
Jimenez Valverde et al. 2010;
Carvalho et al. 2011). This result may be partly due to the distance between sampling sites and weather station, which was, in one case, greater than 300 km. However, because terrestrial arthropods have strong microhabitat preferences, the strength of correlation between ground-dwelling spider diversity and climate variables may have been clearer if microclimate data were available (
Suggitt et al. 2011). To better understand the drivers of arthropod species distribution, it is, therefore, essential to measure environmental factors, both abiotic and biotic, locally even when examining diversity patterns at large spatial scales.
High beta diversity richness values at broad spatial levels are consistent with other studies of forest beetles (
Gering et al. 2003) and forest spiders (
Larrivée and Buddle 2010). This result suggests that large-scale processes influence spider richness in northern Canada. However, unlike past work on spiders from Europe and Australia (
Finch et al. 2008;
Kumschick et al. 2009;
Whitehouse et al. 2009), we found that latitude was a poor predictor of diversity patterns in Canada. The longitudinal extent of this study and Canada’s geography may partly explain this result. Isotherms bend northward toward the west in Canada, so that habitats in the Yukon Territory have milder climates than eastern habitats at equivalent latitudes (
Danks 1993).
The relative abundance and relative richness of a dominant family of spiders showed strong spatial gradients. The relative number of Linyphiidae species increased toward the Arctic, a trend that has previously been found in Europe (
Koponen 1993). However, our results are the first direct evidence of such a pattern in North America. Linyphiidae are relatively well known taxonomically in the Arctic, with 18% of total linyphiid described species in the world found north of 60° (
Marusik and Koponen 2002). Their overall relative abundance is, however, low compared with the Lycosidae, which represented more than 60% of the total assemblage abundance, on average, with only 10% of total richness. We encourage the use of spiders to further study abundance and diversity patterns in space and time across the Canadian Arctic archipelago. Finally, studies of species food webs in the Arctic may help resolve how biotic interactions affect the range distributions of Arctic species.