Introduction
Although HSMI has been formally diagnosed only in Atlantic salmon, other salmonids and some marine fish are also susceptible to infection by PRV (
Wiik-Nielsen et al. 2012;
Morton et al 2017;
Purcell et al. 2018). In the last few years, there has been a surge of PRV-related diseases reported in Pacific salmon in Norway, Chile, Japan, and Canada (
Olsen et al. 2015;
Godoy et al. 2016;
Takano et al. 2016;
Miller et al. 2017). In addition, outbreaks of a disease designated as “HSMI-like” were reported in farmed rainbow trout (
Oncorhynchus mykiss (Walbaum, 1792)) across five hatcheries and two marine farms in Norway, and a PRV strain (variously termed PRV
om or PRV-II, most recently classified as PRV-3) was sequenced from affected fish (
Olsen et al. 2015;
Gjevre et al. 2016;
Hauge et al. 2017). Challenge studies using tissue homogenates from affected rainbow trout resulted in the development of the pathological lesions, but, as with HSMI, not the clinical signs (
Hauge et al. 2017). However, although inflammatory lesions in the heart and skeletal muscle were present, heart inflammation was more concentrated in the spongy layer. In field outbreaks, some clinical signs were similar to HSMI, e.g., anorexia, lethargy, and ascites. Other features of the disease were not common in fish with HSMI, notably anemia, jaundice (yellowish liver), hemosiderosis in the spleen, and hemorrhaging that are all consistent with lysis of red blood cells (RBCs). The same strain of PRV, and possibly PRV-1, has also been implicated in outbreaks in farmed Chilean coho salmon (
Oncorhynchus kisutch (Walbaum, 1792)), in which major hepatic necrosis and erythrophagocytosis in the kidney and spleen were prominent pathological features commonly reported (
Godoy et al. 2016).
Farmed Japanese coho salmon have also been afflicted by a disease caused by PRV-2, a strain that is distinct, based on nucleotide sequence, from PRV-3 (i.e., the Norwegian PRV-II). The disease associated with PRV-2 was named erythrocytic inclusion body syndrome (EIBS) (
Michak et al. 1992), where the inclusions are due to viral factories in the RBCs, but is highly similar in clinical and pathological signs to HSMI-like disease (
Takano et al. 2016). Severe anemia and jaundice were hypothesized to be caused by excess bilirubin in the liver and a mechanism involving viral mediated lysis of RBCs was suspected (
Sakai et al. 1994).
In British Columbia, Canada, the dominant farmed species is Atlantic salmon, whereas chinook ((
Oncorhynchus tshawytscha (Walbaum, 1792)) and coho salmon, both endemic species, currently make up approximately 3% of farmed biomass. A disease characterized by jaundice and anemia, also called jaundice syndrome, has caused low level mortality of farmed chinook salmon for several years (
Garver et al. 2016), and as observed in Japan, Chile, and Norway, the disease is associated with PRV (
Miller et al. 2017). In BC, however, only a single strain of PRV, PRV-1, has been observed. This is the same strain that causes HSMI in Atlantic salmon.
As a cause-and-effect relationship has only been demonstrated between Strain PRV-1 and HSMI in Atlantic salmon and for Strain PRV-2 and EIBS in Japanese coho, this study set out to resolve whether Strain PRV-1 is likely to play a causative role in the development of jaundice/anemia in BC chinook salmon. This question is relevant to understanding the risk associated with PRV transmission from farmed to wild salmon in BC, a topic of interest to the public, government regulators, and industry.
Methods
Farm samples
Farmed chinook and Atlantic salmon were provided by the Fisheries and Oceans Canada (DFO) regulatory farm audit program. This program conducts randomized farm audits across the BC salmon aquaculture industry (
Fig. S1) to assess the role of infectious disease in background losses to production populations and to ensure reporting compliance with World Organization of Animal Health (OIE) listed diseases. In each farm audit, one to nine fresh (recently dead) silver fish were provided from collections undertaken from 2011 to 2013, with 210 chinook and 672 Atlantic salmon samples incorporated into our analysis. At the time of collection, clinical and environmental data and gross lesions were recorded, and tissue samples were taken for histopathology, bacterial and viral culture, as well as molecular analysis. Histopathological assessments of audit salmon were provided by Hugh W. Ferguson and veterinary diagnostics were provided by Ian Keith (DFO Aquaculture Management Division, Pacific Region).
The name “jaundice/anemia” assigned to the condition observed in BC chinook salmon derives from the most striking clinical sign (yellowing of the abdomen and under the eye) occurring during the development of the disease, with anemia preceding the appearance of jaundice. Chinook salmon were classified as “jaundice” if the veterinary diagnostic comments indicated “jaundice syndrome” or “jaundice—no agent”, and (or) the gross lesions indicated “yellow fluid” or “yellow bile” or “yellow bile-like fluid” noted in the peritoneal cavity or on the pyloric caeca and (or) liver. In addition, two fish with pathological notes containing either “(inflammatory) lesions in heart or spongy layer only—CMS-like” or “acute renal tubular necrosis, suggestive of viral infection” were classified as “jaundice”, as these are also features of jaundice/anemia. Fish were classified as “anemia” if clinical data included the terms “anemic” or “pale liver” or “pale gills”.
Although the international standard for diagnosing HSMI (
Biering and Garseth 2012) is, at a farm-level, based on the presence of inflammatory lesions in heart and skeletal muscle, and not pancreas (principally to differentiate HSMI from other virally-mediated heart diseases in Norway), the audit sampling program did not include samples of skeletal muscle tissue until early 2013. However, the viral agents responsible for the other two heart diseases, cardiomyopathy syndrome (CMS) and pancreas disease (PD), were not detected in a surveillance of over 10 000 salmon in British Columbia (K.M. Miller, personal observation, 2018). Therefore HSMI can be recognized in BC simply on the basis of virally-mediated myocarditis lesions in the heart. Hence, Atlantic salmon in this study were diagnosed with “HSMI” if the histopathology notes indicated “CMS/HSMI-like”, or “CMS-like myocarditis”, or “(inflammatory) heart lesions look to be viral”, or “severe viral-type myocarditis/pericarditis”, or “(inflammatory) heart lesions affecting compact layer”.
A longitudinal collection of Atlantic salmon from a single farm outbreak of HSMI (
Di Cicco et al. 2017) was also utilized in our analyses, providing a means to finely study the disease development pathway of HSMI in a single population. These collections included sampling of live and moribund/recently dead fish from a single farm in the Discovery Islands over the 11 month course of the disease outbreak. The pathological investigation showing the development of inflammatory lesions in both heart and skeletal muscle tissues within these sampled fish over time was described by
Di Cicco et al. (2017), and we used the classification of fish along the disease development pathway outlined in that study to identify individuals upon which to attempt the localization of PRV through in situ hybridization (ISH) analysis.
Molecular analyses
Samples for molecular analysis were collected through dissections on the farms, where live-sampled fish were killed by industry personnel by stunning (a blow to the head), as is the industry standard for food fish. For the longitudinal sampling, brain, liver, gill, kidney, and heart tissues were sampled and preserved separately in RNA
later (Ambion, Austin, Texas, USA), and held at 4 °C for 24 h before being transferred into a −80 °C freezer until nucleic acid extraction. Audit tissue samples also included spleen and pyloric caeca, with skeletal muscle added to a the latter third of the samples, in 2013; audit tissue samples were kept on ice until reaching the laboratory by the end of the day and then frozen at −80 °C in a single sterile tube. DNA and RNA were extracted from combined Tri Reagent™ (Invitrogen, Carlsbad, California, USA) homogenates of all collected tissues for the audit samples, and solely the heart tissue for the HSMI longitudinal study, using previously described methods (
Miller et al. 2016).
We employed the BioMark™ (Fluidigm, San Francisco, California, USA) HD microfluidics quantitative PCR platform to assess the presence and load of 45 salmon infectious agents, including viruses, bacteria, fungi, and protozoan parasites for Atlantic salmon in the longitudinal study (
Di Cicco et al. 2017) and for farm audit salmon (
Miller et al. 2017). Viruses with TaqMan (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) assays included in this analysis are as follows: PRV, Atlantic salmon paramyxovirus (ASPV), infectious hematopoietic necrosis virus (IHNV), infectious salmon anemia virus (ISAV), Pacific salmon parvovirus (PSPV), piscine myocarditis virus (PMCV), salmonid alphavirus (SAV), salmonid herpesvirus (OMV), encephalopathy and retinopathy virus (VER), erythrocytic necrosis virus (ENV), and viral hemorrhagic septicemia virus (VHSV). Details on the analytical performance of these and other infectious agent assays on the platform were evaluated by
Miller et al. (2016), who demonstrated the sensitivity (limit of detection), repeatability, and specificity of each assay. To limit false positive amplification, all laboratory processing steps were carried out in dedicated workspaces, with most in UV hoods, separating pre- and post-amplification procedures into different laboratories and adding artificial construct positive controls last, as described by
Miller et al. (2016).
A panel of host biomarkers (genes) that when co-expressed identifies fish in a viral disease state (VDD) was developed and validated by
Miller et al. (2017), who also described their application to the salmon audit samples using the Fluidigm BioMark platform. The original panel evaluated by
Miller et al. (2017) included TaqMan assays to 51 biomarkers, 20 of which had good efficiencies and provided viral disease resolution in chinook salmon, and 22 in Atlantic salmon (
Table S1). However,
Miller et al. (2017) showed that as few as 11 biomarkers were required to differentiate fish merely carrying a virus from those experiencing cellular-level expression of a viral disease (and sensitive to the early phase of disease, when histopathological changes are not yet visible), which was the application of interest for the current study. Moreover, the VDD signature was shown to be predictive of viral disease regardless of the tissue assessed and, as such, worked equally well on the multi-tissue RNA samples from the audit program as opposed to single tissue (heart) analysis.
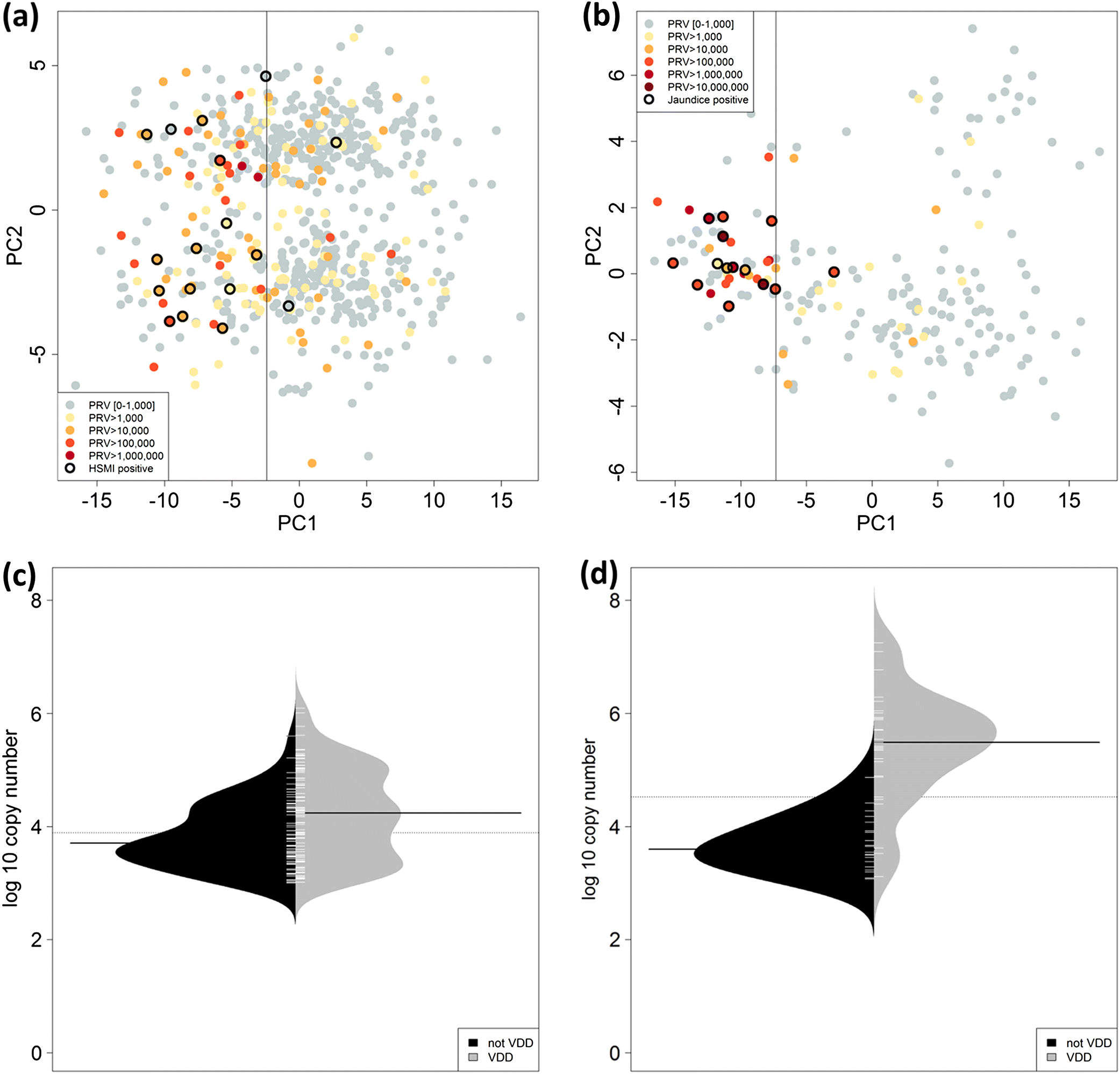
We applied the VDD panel to the audit fish to resolve early disease development associated with PRV in both Atlantic and chinook salmon, and to differentiate fish that were simply carriers of the virus from those that were actually in a viral disease state. The panel was applied to all of the fish in our collections, and hence also included fish for which PRV was not detected and could potentially contain other viral-mediated diseases. Herein, we restrict most of our presentation, and all of our ISH analyses (except for controls), to fish that contained PRV at loads >103 copies per μg nucleic acids (with one exception; most >104 copies per μg), although we calculate prevalence of a viral disease state in sampled farmed salmon.
Host biomarkers in the VDD panel were normalized to three reference genes derived from previous microarray studies (
Table S1), and relative gene expression was assessed using the 2-ΔΔCt method (
Livak and Schmittgen 2001). A pooled sample was used as the relative control. Classification of fish in a viral disease state was conducted through principal component analysis (PCA) of the expression data from 20 assays in chinook and 22 assays in Atlantic salmon using the sample scores across PC1, as described by
Miller et al. (2017). The goal of the VDD classification was to identify PRV-positive fish that may be in an early viral disease state, but not specifically diagnosed through pathology or clinical signs with a PRV-related disease. Hence, the edge of the VDD signature was determined by PC1 sample scores within the range of fish identified in a viral disease state (jaundice/anemia for chinook salmon and HSMI for Atlantic salmon). A species-specific VDD threshold was chosen at the maximum value of Youden’s J statistic for a receiver operating characteristic (ROC) analysis of the PC1 sample scores, where viral disease state (identified/not identified) was used as the binary class for the ROC analysis. The Youden’s J statistic represents the value of (sensitivity + specificity −1) for each point on the ROC curve and takes on values in (−1, 1), with 1 characterizing a perfect test where sensitivity and specificity are both at 100. This approach optimizes the differentiating ability of the classifier when equal weight is given to sensitivity and specificity (
Youden 1950).
Sample selection for ISH
The goal of our ISH study was to track the movement of PRV within salmon tissues during early infection, development, and peak states of disease development in Atlantic and chinook salmon. To study HSMI in Atlantic salmon, we selected 24 samples collected from the longitudinal study that diagnosed HSMI in British Columbia (
Di Cicco et al. 2017) that had already been analyzed through histopathology and scored for heart and skeletal muscle lesions consistent with HSMI (see score system used by
Di Cicco et al. 2017). Following the indications of the previous analysis, we identified three stages of disease development, and conducted ISH analysis on six fish per stage. The stages were classified as:
1.
Fish negative to PRV, and showing no histopathological lesions in any organ: negative control fish.
2.
Fish positive to PRV (>103 copies per μg nucleic acids), and showing no histopathological lesion in any organ (heart score = 0): “initial” stage of infection.
3.
Fish positive to PRV (>103 copies per μg with one exception), showing mild degree lesions in the heart consistent with HSMI, but primarily localized in the epicardium and the compact layer of the myocardium (heart score = 1.5 to 3): “developing” stage of infection.
4.
Fish positive to PRV (>103 copies per μg), showing severe lesions in the whole heart (panmyocarditis) diagnostic of HSMI (heart score ≥4): “HSMI”.
The samples chosen for ISH analysis consisted primarily of live, apparently healthy/asymptomatic fish for the first three groups, and moribund/freshly dead fish for the HSMI group.
Samples potentially representing different stages of development of jaundice/anemia in chinook salmon were chosen on the basis of (1) PRV load (>10
4 copies per μg nucleic acids, with one exception), (2) VDD classification, and (3) whether fish had been diagnosed with jaundice/anemia. From these data, we selected three groups of samples, as follows:
1.
Fish positive for PRV (>103 copies per μg), but VDD negative: “PRV+ only” (three samples).
2.
Fish positive for PRV (>103 copies per μg) and classified as in a VDD state: “PRV+/VDD+” (five samples).
3.
Fish positive for PRV (>103 copies per μg), in a VDD state, and diagnosed with jaundice (most also with anemia): “jaundice/anemia” (nine samples).
All audit chinook samples were moribund/freshly dead. No fish in group 1 and 2 showed signs of jaundice and only one fish in group 2 showed signs of anemia (but no jaundice).
Histopathology
Preparation of the tissues for histopathological evaluation has been described previously by
Di Cicco et al. (2017). Briefly, all tissues fixed in 10% neutral buffered formalin were dehydrated through an ascending gradient of alcohol solutions, embedded in paraffin wax, cut at 3.5 μm thickness, and stained with routine hematoxylin and eosin (H&E) for morphological evaluation by light microscope.
For both Atlantic and chinook salmon, histology slides contained a section of heart (atrium and ventricle), liver, anterior and posterior kidney, spleen, intestine (often both small intestine and colon), skeletal muscle, brain, and gills. All samples were read and scored by two pathologists (HWF and ED), with exception of group 1, 2, and 3 of the HSMI study that were read principally by one pathologist (ED). The scoring system for heart and skeletal muscle lesions were as described by
Di Cicco et al. (2017), and followed
Finstad et al. (2012). For the other organs we utilized the scoring system used for regulatory purposes in the audit program. The severity of lesions was classified as 0 (no lesion), 1 (mild lesion), 2 (moderate lesion), and 3 (severe lesion). Consecutive slides from the same fish were cut for ISH to allow comparison between the presence of histopathological lesions and the localization of PRV.
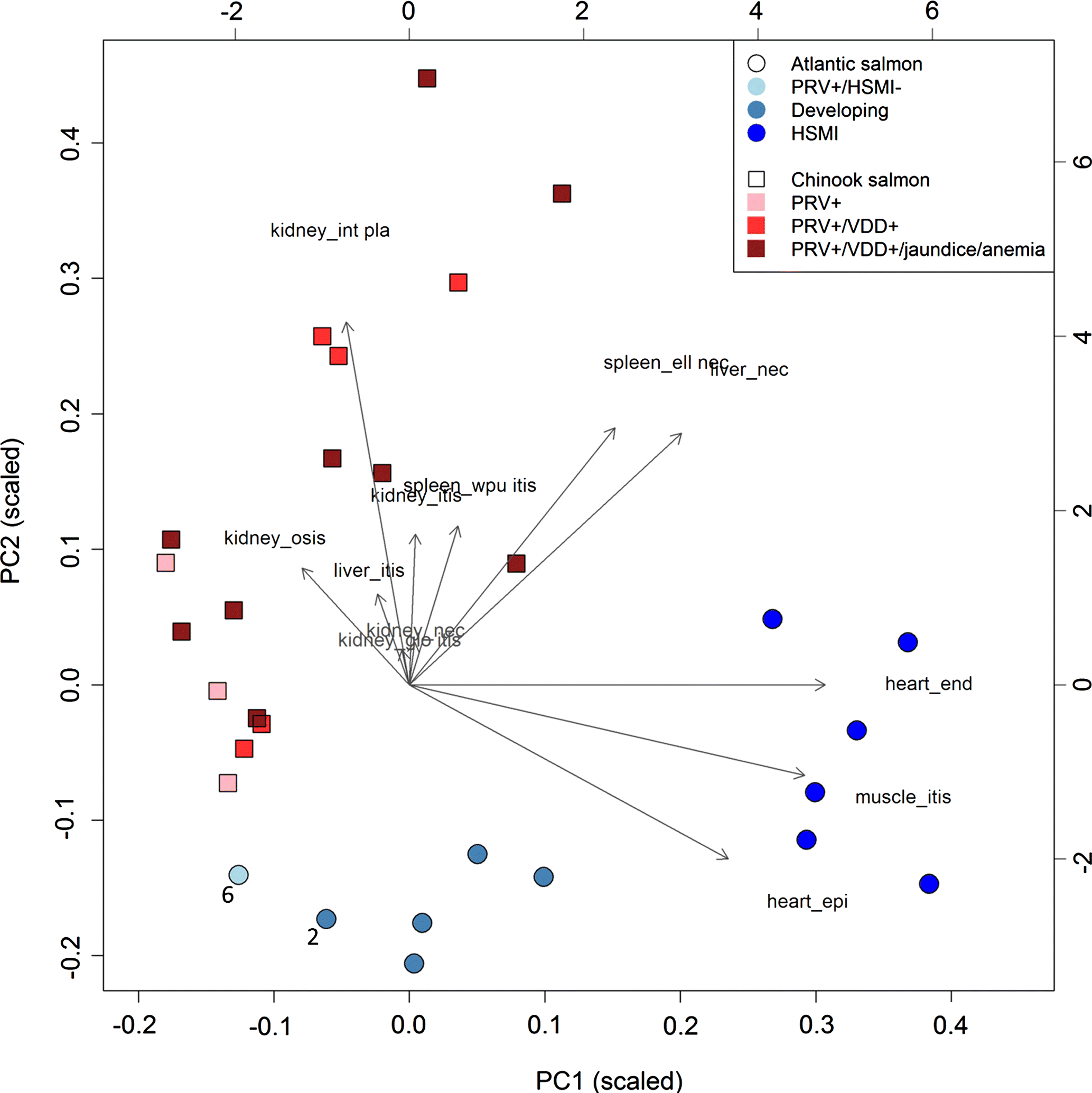
Statistical evaluation of histopathology scores was undertaken to identify lesions showing the strongest interspecies differences. Principal component analysis was applied to the lesion scores to identify the main trajectories in the data and visualize whether Atlantic and chinook salmon clustered separately. A PCA biplot was used for visualization to project the lesions onto the same graph as the samples (
Gabriel 1971;
Ringnér 2008). In addition, lesion scores were treated as binary variables (zero score versus positive score) and contrasted between species for each of the three groups with PRV detections. Barnard’s (exact) test was used to identify lesions that were significantly different between species (
Barnard 1945). The analysis was implemented in R version 3.3.2 based on the ‘Exact’ package (version 1.7).
ISH
RNA-ISH was performed using BASEscope (RED) (Advanced Cell Diagnostics, Newark, California, USA) according to the manufacturer’s instructions. Briefly, consecutive sections of all Atlantic and chinook salmon samples utilized for the histopathological analysis were dewaxed by incubating for 60 min at 60 °C and endogenous peroxidases were quenched with hydrogen peroxide for 10 min at room temperature. Slides were then boiled for 30 min in RNAscope target retrieval reagents (Advanced Cell Diagnostics, Newark, California, USA) and incubated for 30 min in RNAscope Protease IV reagent prior to hybridization. The slides underwent hybridization with a BASEscope probe against a portion of PRV-1 genome segment L1 (that codes for PRV core shell; Advanced Cell Diagnostics, Newark, California, catalog #705151), to detect PRV in the tissues. A BASEscope probe against the bacterial gene
DapB (#701021) was used as negative control to confirm the absence of background and (or) non-specific cross-reactivity of the assay. Concurrently, PRV/HSMI positive (
n = 2) and negative (
n = 1) heart samples from Atlantic salmon collected during an experimental challenge for HSMI performed in Norway (
Finstad et al. 2014) were used as positive and negative controls for PRV-1 presence and localization. Signal amplification was performed according to the manufacturer’s instructions, followed by counterstaining with Gill’s hematoxylin and visualization by bright field microscopy.
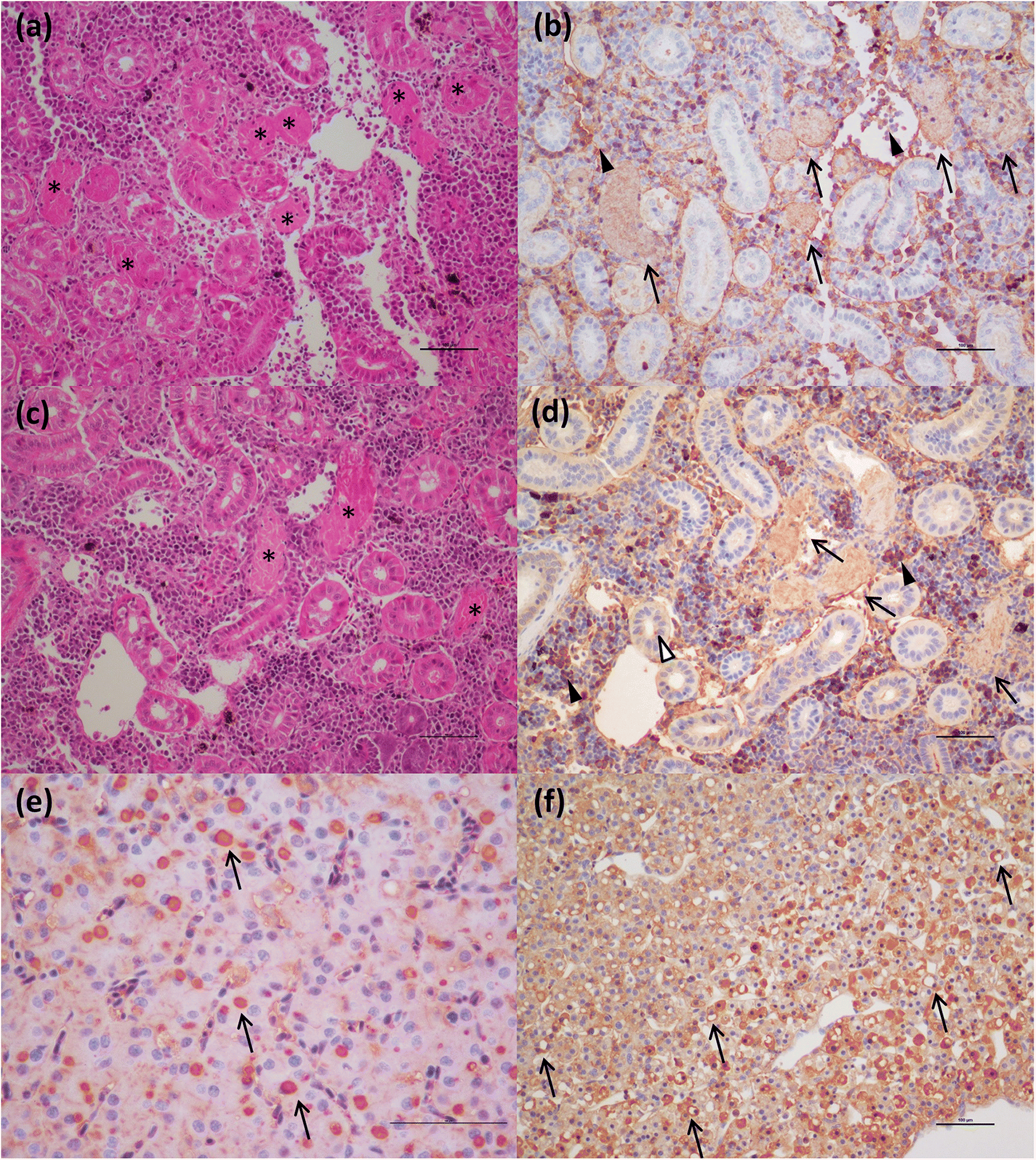
Immunohistochemistry with antibody to hemoglobin
To evaluate the presence of hemoglobin in the damaged tissues of chinook salmon, an antibody raised against hemoglobin (rabbit polyclonal Anti-Hemoglobin subunit beta antibody, ab202399, Abcam Inc., Toronto, Ontario, Canada) was utilized to perform immunohistochemistry on a subset of samples of chinook salmon (affected by various degrees of renal tubular necrosis:
n = 3 fish classified as “PRV+/VDD+” and
n = 3 samples from fish diagnosed with jaundice/anemia) and fish presenting no lesions in the kidney (
n = 2 fish classified as “PRV+ only”). The protocol was previously described by
Di Cicco et al. (2017). Briefly, 3.5 μm thick paraffin wax embedded sections were mounted on Superfrost Plus glass slides (Thermo Fisher Scientific, Portsmouth, New Hampshire, USA). These were heated at 60 °C for 20 min, dewaxed in xylene, and rehydrated through graded alcohols. Antigen retrieval was performed by autoclave treatment (121 °C for 10 min) in citrate buffer (0.1 mol·L
−1, pH 6.0). The sections were successively treated with 3% hydrogen peroxide for 1 h to block endogenous peroxidase activity. Non-specific binding sites were blocked by normal goat serum (Vector Laboratories, Burlingame, California, USA) diluted 1:10 in 1% bovine serum albumin (Vector Laboratories, Burlingame, California, USA) in Tris-buffered saline (TBS) [pH 7.6, 0.05 mol·L
−1 Tris/HCl, 0.15 mol·L
−1 NaCl] for 1 h. The same diluent solution was used for primary antibodies (1:100) and then incubated in a humidity chamber at 4 °C overnight. A Vectastain ABC-peroxidase kit (Vector Laboratories, Burlingame, California, USA) was used for detection of bound antibody according to the manufacturer’s instructions, and ImmPACT NovaRED (Vector Laboratories, Burlingame, California, USA) was utilized as substrate. Finally, the sections were counterstained with Harris’ hematoxylin and mounted (Permount, Fisher Chemical, Fair Lawn, New Jersey, USA). All incubations, except with the primary antibodies, were carried out at room temperature in a humidity chamber. Two PRV negative samples (including all internal organs and not showing histopathological signs of hemolysis and (or) anemia) were used as negative control. Primary and secondary antibody controls were performed by replacing the antibody with the respective diluent alone.
Whole genome sequencing of PRV
RNA viruses exist as groups of closely related genotypes, known as a viral quasispecies. These can exist as several master variants, each with their own cloud of mutants (
Lauring and Andino 2010;
Mordecai et al. 2016).
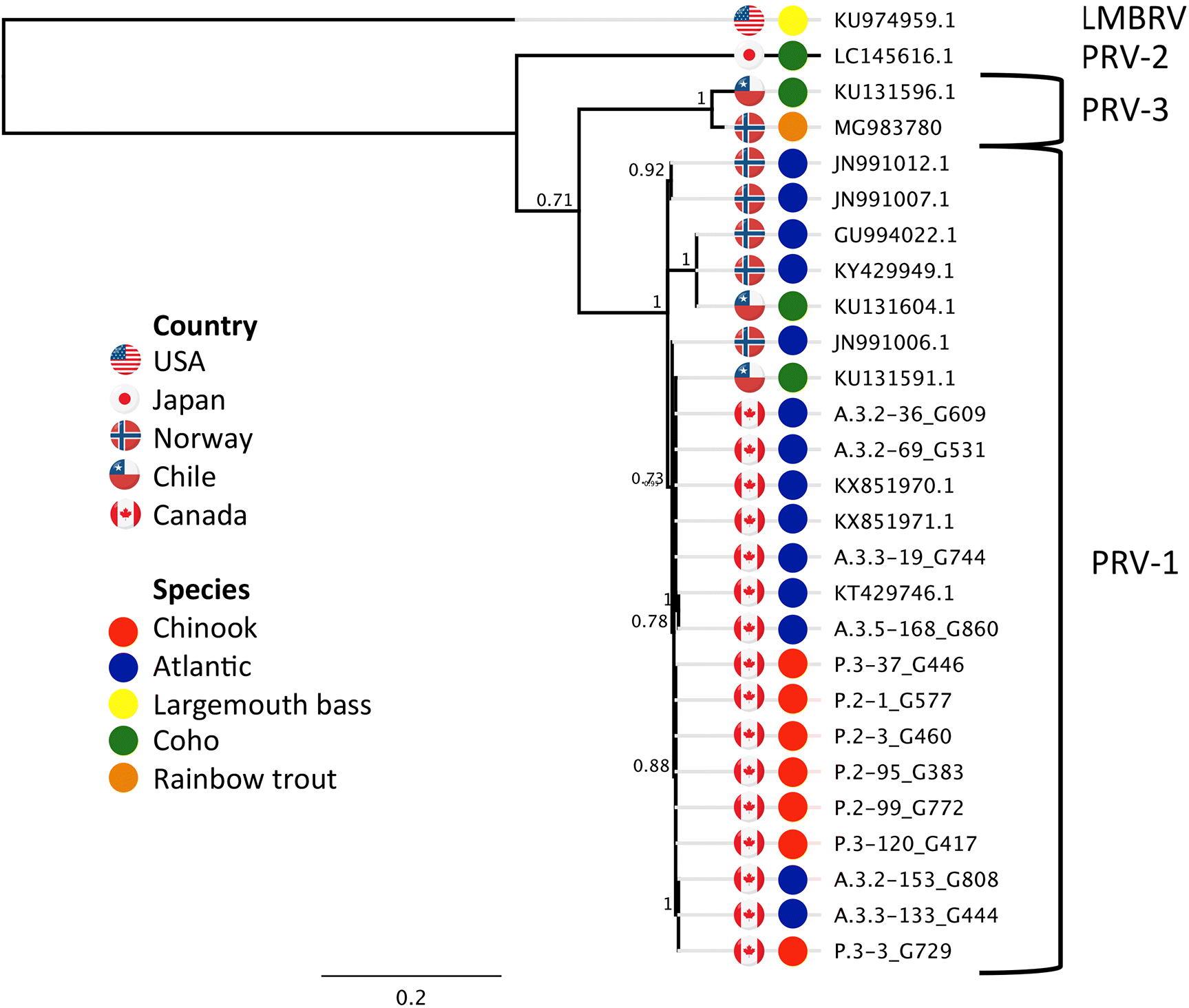
Dhamotharan et al. (2018) recently showed that there are currently three strains of PRV, known as PRV-1, 2, and 3, which they described as variants of PRV. However, to accurately describe the variation found within these three groups, the nomenclature needs to be refined. In this manuscript we refer to PRV-1, 2, and 3 as strains (i.e., master variants). Finally, we refer to each unique consensus sequence (itself made up of individual sequencing reads from different individual virions) as a sequence variant.
Although we know from previous studies that currently only Strain PRV-1 has been observed in British Columbia, we conducted whole viral genome sequencing in individuals across farm audits with fish diagnosed with HSMI (Atlantic salmon; six farms) or jaundice/anemia (chinook salmon; four farms). For chinook salmon, we also sequenced three individuals from farms with nearly all fish collected classified as PRV+/VDD+. This information is critical to determine whether there were any consistent differences in the sequence of the virus associated with each disease, and consequently indicate the potential risk of disease transmission from farmed Atlantic to wild Pacific salmon.
For each farm audit, we sequenced the fish carrying the highest load of PRV, with one exception. We made every attempt to represent disease occurrence broadly distributed over the farming region in British Columbia, as dictated by the 2011 to 2013 audit samples provided to us. For Atlantic salmon, farms were located along the inside passage of Vancouver Island (Discovery Islands (Regulatory area A3.2)) and Broughton Archipelago (A3.3) and the Central Coast (A3.5) (
Fig. S1). These audit collections spanned three seasons, represented three companies, included samples from 2011, 2012, and 2013, and included two collections from the same farm over successive seasons within the same year. For chinook salmon, four farms were on the west coast of BC (P2), and three were located in the Discovery Island/Broughton Archipelago region (P3) (see locations on
Fig. S1). These audit collections spanned three seasons, represented four companies, included samples from 2011, 2012, and 2013, and included sampling from the same site and season collection over two separate years.
Because loads of PRV were very high for chinook salmon (>10
5 copies per μg nucleic acids), we carried out RNA-sequencing with no enrichment, as described by
Di Cicco et al. (2017). However, previous studies performed in our laboratory have suggested that full genome sequences were not guaranteed at loads <10
5 copies per μg nucleic acids, which was the case for most of the Atlantic salmon samples. Hence, we included a SureSelect™ (Agilent, Santa Clara, California, USA) enrichment step for viral content (our Sure Select panel includes oligonucleotides across the full genomes of PRV-1 as well as many other salmonid viruses assessed on our infectious agent monitoring platform).
The Atlantic salmon samples were prepared using the SureSelectXT RNA Direct next-generation sequencing (NGS) target workflow (Agilent, Santa Clara, California, USA). This included preparing the RNAseq library with the SureSelect Strand-Specific RNA library Prep kit (Agilent, Santa Clara, California, USA) according to the manufacturer’s instructions. The adaptor ligated samples were purified with the Agencourt AMPure XP system (Beckman Coulter, Brea, California, USA). High sensitivity (HS) DNA chips were run on the Agilent 2100 Bioanalyzer (Agilent, Santa Clara, California, USA) to determine the final library size and the Qubit dsDNA HS kit (Invitrogen, Carlsbad, California, USA) was used to determine the concentration. Vacuum centrifugation (20 min at 30 °C) was necessary to obtain 200 ng of the adapted libraries in the appropriate volume for hybridization. Hybridization of the adapted cDNA library with the viral SureSelect bait capture library (Agilent, Santa Clara, California, USA) was performed at 65 °C for 24 h according to the manufacturer’s instructions. The cDNA library/capture library hybrids were captured on streptavidin magnetic beads and purified with the Agencourt AMPure XP system (Beckman Coulter, Brea, California, USA). Index tags were added to the post-captured libraries through 15 rounds of amplification and purified using the Agencourt AMPure XP system (Beckman Coulter, Brea, California, USA). HS DNA chips were run on the Agilent 2100 Bioanalyzer (Agilent, Santa Clara, California, USA) to determine the final library size and the concentration was determined using the Qubit dsDNA HS kit (Invitrogen, Carlsbad, California, USA). Sample libraries were normalized to 4 nmol·L−1, and denatured and diluted to obtain a final library of 20 pmol·L−1. The Atlantic salmon enriched RNAseq libraries were processed on one paired end v2 300bp kit on the Illumina MiSeq System (Illumina, San Diego, California, USA), which included a 10% phiX spike-in.
For the chinook salmon RNA-seq, ribosomal removal was performed using the Epicentre ScriptSeq Complete Gold Kit (Epidemiology) (Illumina, San Diego, California, USA) according to the manufacturer’s instructions. A Zymo RNA Clean and Concentrate-5 kit (Zymo Research, Irvine, California, USA) was applied to purify the rRNA depleted total RNA according to the manufacturer’s instructions, and quantified using the Qubit RNA HS kit (Invitrogen, Carlsbad, California, USA). ScriptSeq Index reverse primers (Illumina, San Diego, California, USA) were added to the cDNA during the final amplification step, which involved 14 cycles. The Agencourt AMPure XP system (Beckman Coulter, Brea, California, USA) was applied to purify the 3′-terminal tagged cDNA and final amplified library. HS DNA chips were run on the Agilent 2100 Bioanalyzer (Agilent, Santa Clara, California, USA) to determine the final library size and the concentration was determined using the Qubit dsDNA HS kit (Invitrogen, Carlsbad, California, USA). Sample libraries were normalized to 4 nmol·L−1, and denatured and diluted to obtain a final library of 20 pmol·L−1. The chinook RNA-seq libraries were run over two paired end v3 600bp reagent kits on the Illumina MiSeq System (Illumina, San Diego, California, USA), which included a 2% phiX spike-in.
PRV genome sequence analysis
The quality of the raw reads was checked using FASTQC (
bioinformatics.babraham.ac.uk/projects/fastqc/). Low quality reads or regions of reads and adapter sequences were removed using Trimmomatic (
Bolger et al. 2014). The remaining reads were aligned to the PRV genome segments of the Norwegian isolate Salmo/GP-2010/NOR (
Palacios et al. 2010) using the BWA-mem aligner with default parameters. A consensus sequence of the aligned reads was created within Geneious (V10.1.3) using a threshold of 95%. The consensus sequences were then aligned using Muscle (within Geneious), and trimmed to the shortest resulting contiguous sequence. Trees were constructed using the MrBayes plug in within Geneious.
Discussion
This is the first study to specifically localize PRV across multiple tissues during disease development in salmon. We demonstrate an intimate relationship between viral localization and development of histological lesions that supports an etiological role for the virus in the development of both HSMI in Atlantic salmon and jaundice/anemia in chinook salmon. It is already established that Strain PRV-1 causes HSMI in Atlantic salmon (
Wessel et al. 2017), and that Strain PRV-2 causes EIBS in coho, a jaundice/anemia-related disease in Japanese coho salmon (
Takano et al. 2016). Moreover, strains of PRV have been associated with other diseases. For example, PRV-1 has been associated with jaundice syndrome in farmed chinook (
Miller et al. 2017) and Strain PRV-3 has been associated with HSMI-like disease and anemia in rainbow trout in Norway (
Olsen et al. 2015) and coho salmon in Chile (
Godoy et al. 2016). Therefore, all three recognized strains of PRV have now been affiliated with a jaundice-related disease in three species of Pacific salmon, and the weight of evidence worldwide as well as our findings in BC suggests that PRV-1, the only PRV strain detected in BC salmon, likely causes both diseases, HSMI and jaundice/anemia, in Atlantic and Pacific salmon, respectively.
Full viral genome sequencing of PRV in chinook salmon diagnosed with jaundice/anemia or classified as PRV+/VDD+, sampled from seven farm audits collected over three years, and in Atlantic salmon diagnosed with HSMI, sampled over three years from six farm audits distributed across the BC industry, validated that only closely related sequence variants within PRV-1 occur on BC farms. The sequence variants of PRV-1 from BC share high sequence identity to sequences in Norway (maximum 99.7% identity (JN991006.1)) and Chile (maximum 99.7% identity (KU131591.1 from coho)) for S1, which is within the range of variation observed in BC. Over the entire viral genome maximum sequence identity between BC variants of PRV-1 and the first PRV-1 sequence described by
Palacios et al. (2010) was not as high, and varied among segments from 96.6 (S1) to 99.2 (M1). S1 and M2 are the two segments showing strongest divergence between this Norwegian sequence and BC PRV-1 variants (96.6% and 96.8%, respectively). In
Mammalian orthoreovirus (MRV) these segments code for proteins that confer virulence in mice (
Weiner et al. 1977;
Rubin and Fields 1980), with S1 coding for the cell-attachment protein, which is important for neurovirulence and tissue tropism (
Weiner et al 1977). If these proteins have similar functions in
P. orthoreovirus, it is possible that the minor differences observed between the
Palacios et al. (2010) Norwegian and BC sequence variants of PRV-1 could result in shifts in virulence. This is an area that will be examined in future challenge studies.
Although there were no consistent differences in PRV-1 sequences between Atlantic salmon with HSMI and chinook salmon with jaundice/anemia, sequences from Atlantic salmon clustered separately from chinook salmon for the L1, M1, and M2 segments; however, there were no single nucleotide or amino acid substitutions that were consistently differential between salmon species (data not shown). Hence, the low sequence divergence within PRV-1 is consistent with the virus cross-infecting Atlantic and chinook salmon, and suggests that the virus can transmit diseases between salmon species.
The molecular viral disease development panel, or VDD, was developed in the
Miller et al. (2017) study from meta-analysis of multi-cohort microarray data from viral disease challenge studies for five diseases caused by RNA viruses (infectious hematopoietic necrosis (IHN), infectious pancreatic necrosis (IPN), infectious salmon anemia (ISA), cardiomyopathy syndrome (CMS) and heart and skeletal muscle inflammation (HSMI)), and validated using RNA samples from independent challenge (IHNV across multiple species) and field (HSMI in Atlantic salmon and jaundice/anemia in chinook salmon) samples. In addition to differentiating carrier and disease states for viruses, the VDD panel could also differentiate bacterial and viral disease states. It was shown that a robust set of eleven VDD biomarkers was all that was required; these included SRK2, DEXH, RSAD, UBL1, IFIT5, NFX, HERC6, VHSVIP4, IF44A, CD9, and MX.
The application of the VDD panel was particularly useful in understanding the disease development process, and identifying fish at an early stage of disease progression, before clinical (and sometimes morphological) evidence of disease is recognized. In applying this tool across both Atlantic and Pacific salmon, we demonstrated that almost all fish diagnosed specifically with PRV-associated disease (HSMI or jaundice/anemia) showed a strong host VDD response. Exceptions were two Atlantic salmon with low PRV load that we surmise may have been in a recovery stage whereby lesions persist after the virus is all but cleared, as demonstrated in Norwegian studies (
Finstad et al. 2012). Fish specifically diagnosed with disease account for only 17% of Atlantic salmon and 41% of chinook salmon with PRV loads >10
4 copies per μg nucleic acids classifying as VDD+, suggesting that many more fish may have been in a pre-clinical disease state. However, in both species, there are also fish (33% for Atlantic, 19% for chinook salmon) that contain PRV loads >10
4 copies per μg nucleic acids that do not classify as VDD+. Our research also shows that in these fish, the virus was not observed outside of the RBCs, the primary infective cells for PRV. These data support the hypothesis put forward by
Finstad et al. (2014) and
Wessel et al. (2015) that PRV is highly tolerated by the salmon host (Atlantic salmon) when replicating exclusively in RBCs, and that the host elicits no strong immune reaction or pathological response as long as the virus remains in RBCs (classified in our study as PRV+ only). This explains what has been considered an anomalous finding that fish with high loads of the virus may or may not show any indication of disease, which led some scientists to speculate that PRV is not a primary cause of disease (
Garver et al. 2016), later refuted in the study by
Wessel et al. (2017) demonstrating a cause-and-effect relationship between PRV-1 and HSMI.
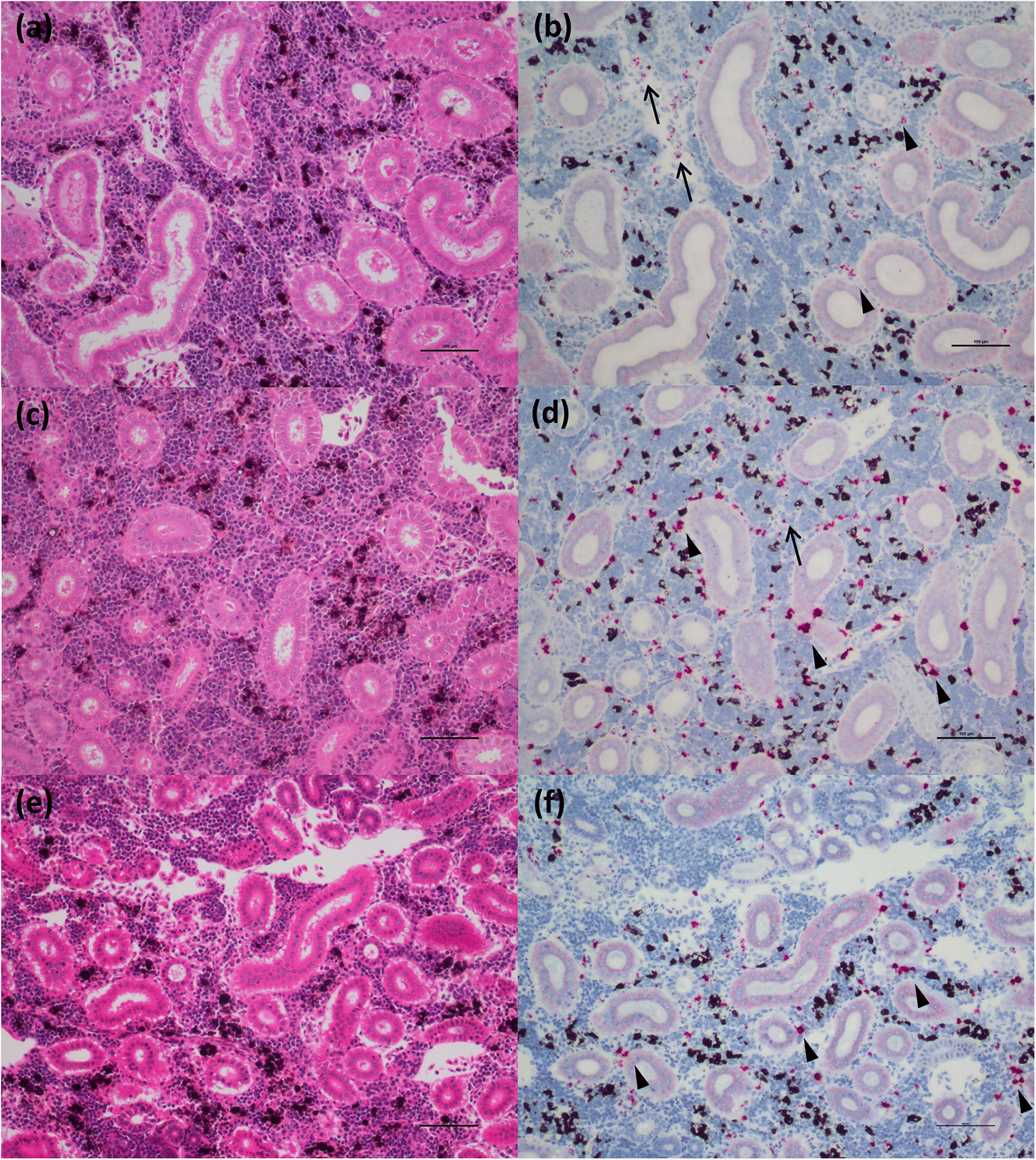
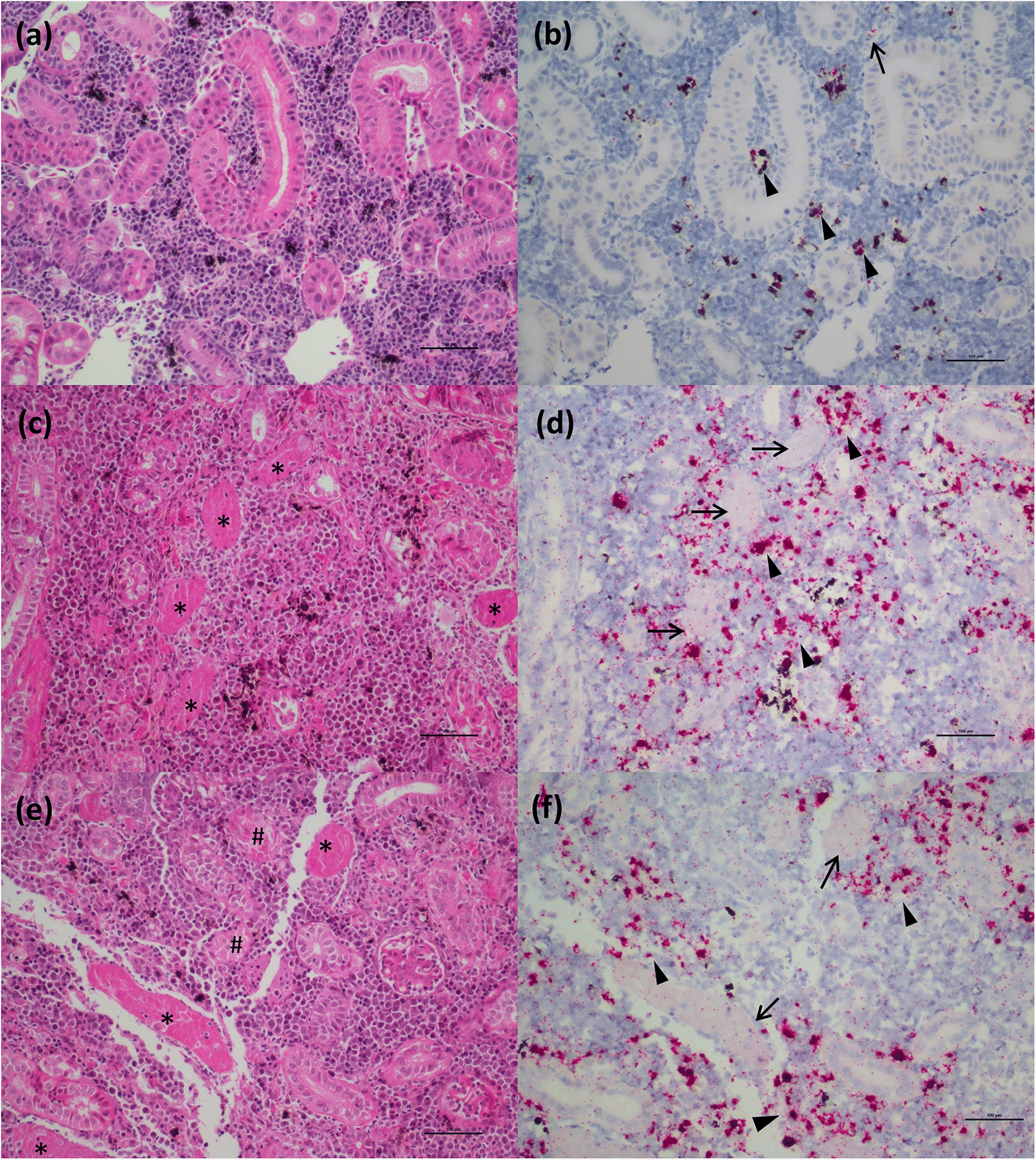
Through application of the molecular VDD tool, and differentiation of fish in a PRV+ carrier state from those at early stages of disease development, we also learn that this anomaly is not as strong in chinook salmon as it is in Atlantic salmon. All chinook salmon in our study with mixed tissue PRV >105 copies per μg nucleic acids classified as VDD+ and showed evidence of RBC rupture, leading to release of virus and high levels of hemoglobin into the tissues. These data are consistent with an upper limit of load tolerance to infection of RBCs in chinook salmon that is not observed in Atlantic salmon, which may result in a higher susceptibility of chinook salmon to disease development. Ideally, an exact tolerance threshold value needs to be determined experimentally, specifically monitoring loads in RBCs, but the use of regulatory audit fish, that were moribund or dead upon sampling, precluded this possibility.
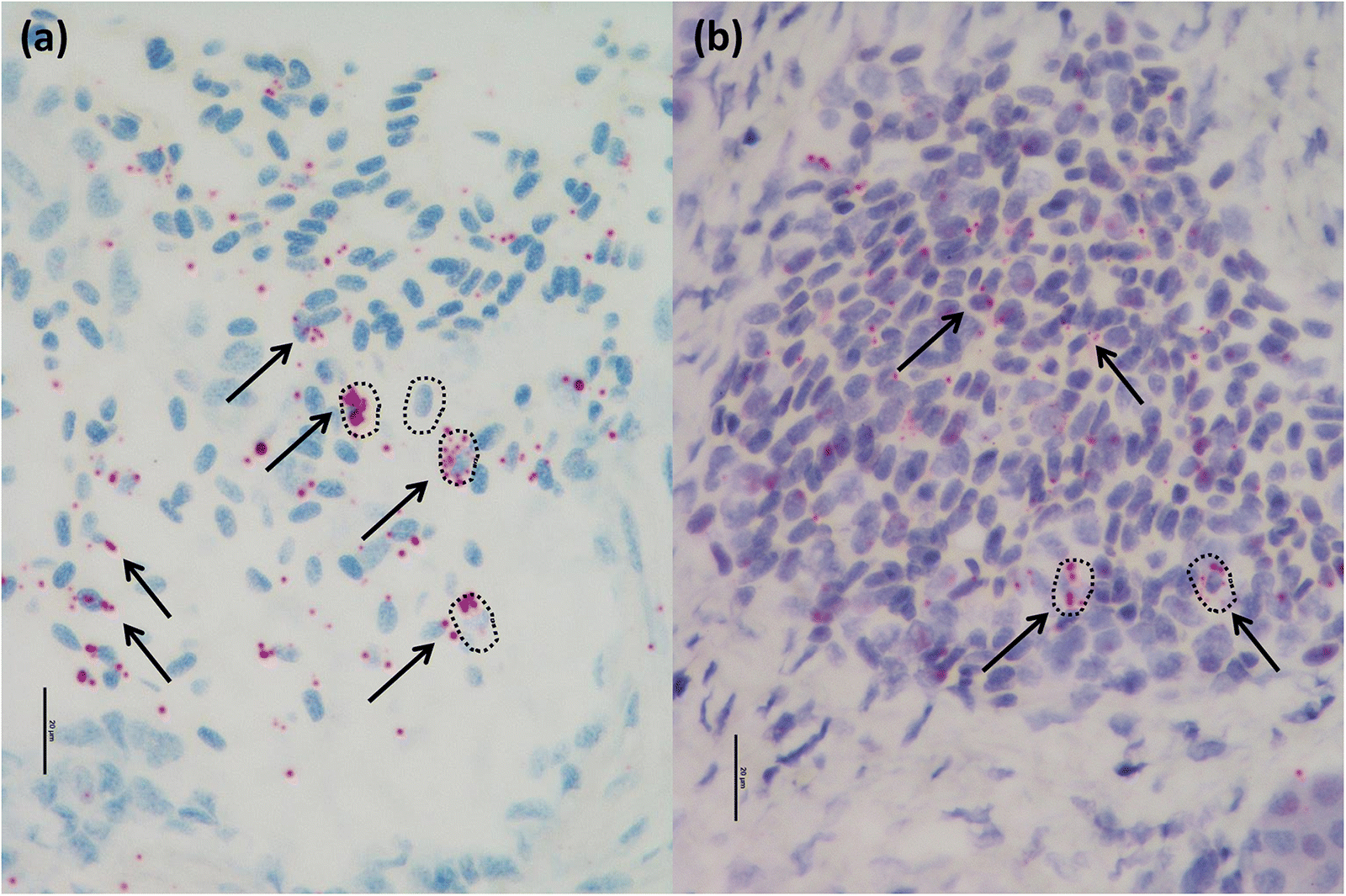
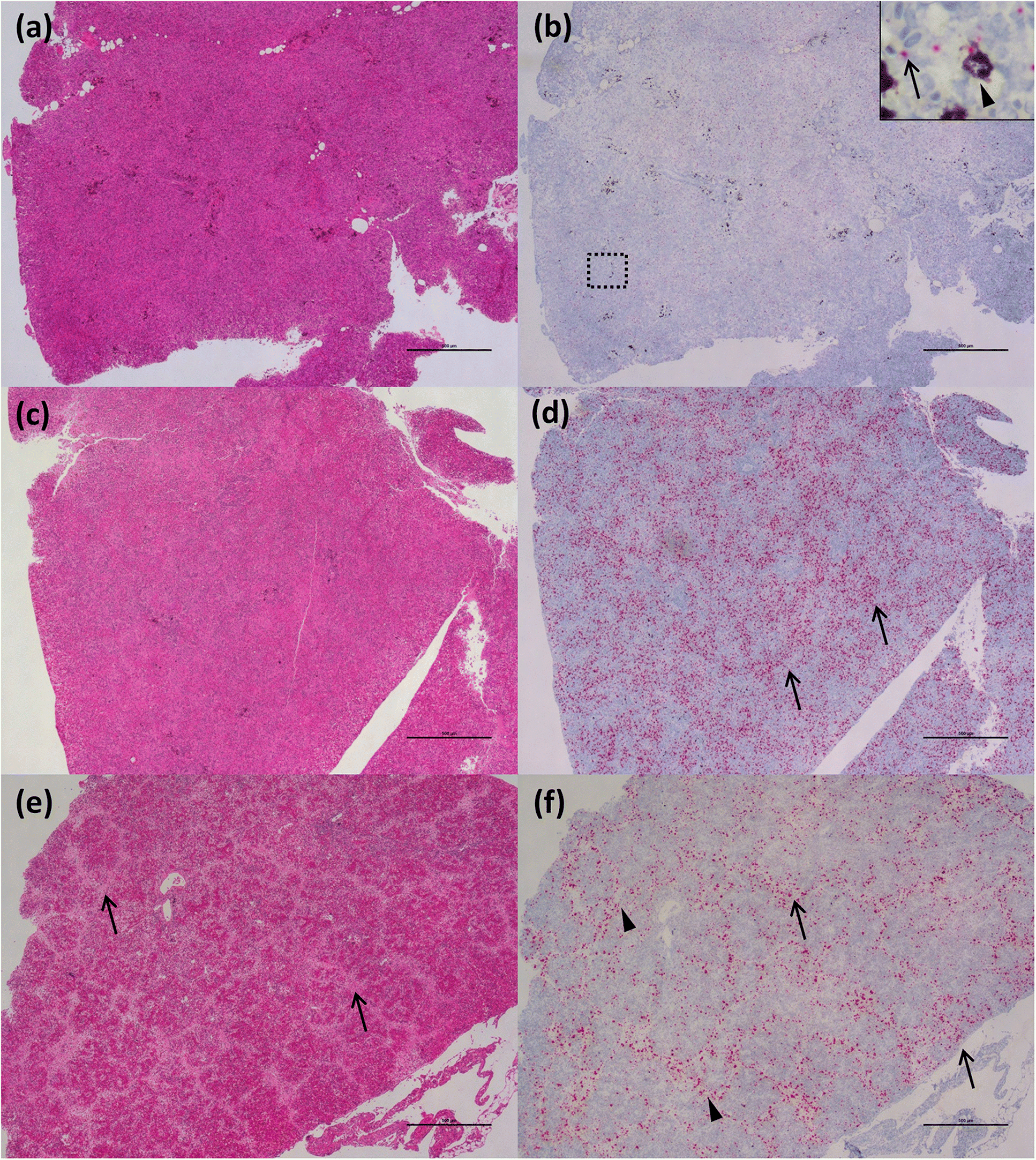
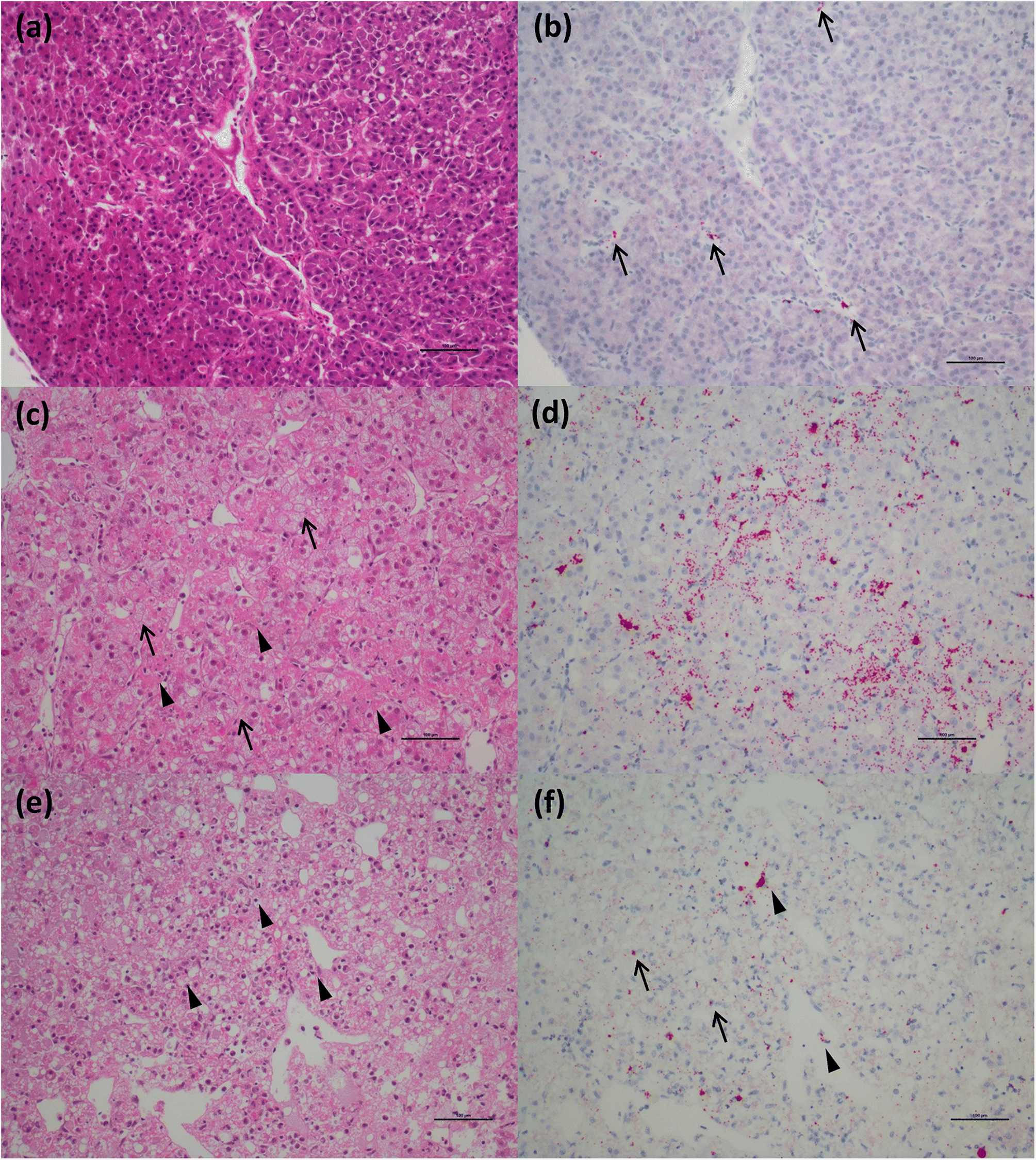
Our ISH data indicate that RBC lysis and release of virus in chinook salmon results in the elicitation of an immediate strong “viral disease” host response (VDD). Additionally, we observed hemosiderin appearance in the spleen of some fish (evidence of metabolized hemoglobin from ruptured RBCs), and massive erythrophagocytosis of damaged/ruptured RBCs (containing PRV) and possibly free virus in spleen and kidney. Immunohistochemistry analysis for hemoglobin also confirmed the presence of high level of hemoglobin in the liver (particularly in damaged/apoptotic hepatocytes) and kidney (in the renal casts resulting from renal tubules necrosis) of fish diagnosed with jaundice/anemia or even just PRV+/VDD+. High levels of hemoglobin, bilirubin, and heme byproduct are known to be toxic for the liver cells (
Sakai et al. 1994) as well as for the epithelial cells of the kidney tubules (
Tracz et al. 2007;
Deuel et al. 2016). These are similar disease characteristics to comparable PRV-related diseases in Pacific salmon in Chile, Japan, and Norway (
Sakai et al. 1994;
Smith et al. 2006;
Olsen et al. 2015;
Godoy et al. 2016;
Takano et al. 2016). Moreover, mild to severe myocarditis primarily involving the spongy layer of the myocardium (as opposed to the whole heart, including mostly the epicardium and compact layer of the myocardium, in HSMI) occurs transiently in chinook salmon and in association with the differential localization of the virus in the spongy layer. This pattern of inflammation has also been observed in PRV-associated diseases in coho salmon and rainbow trout (
Hayakawa et al. 1989;
Olsen et al. 2015;
Godoy et al. 2016).
Interestingly, many of the chinook salmon classified as PRV+/VDD+, but not with jaundice/anemia, pathologically showed advanced states of disease, suggesting that clinical signs (i.e., jaundice and anemia), which were principally used to classify fish in our study, may occur quite late in the disease process, potentially resulting in low diagnostic sensitivity on farms. Moreover, at the peak of the clinical manifestation of the disease, the virus is not always prevalent in the heart, and myocarditis lesions are not always present, suggesting a temporary occurrence of these lesions. By contrast, extensive areas of hepatonecrosis and kidney tubule necrosis are present in regions with high abundance of virus. Whether these degenerative/necrotic lesions are the result of viral infection specifically in the cells that become necrotic, or through the toxic levels of heme being collected, processed and accumulated by these tissues, is not clear, but in either case, these changes are clearly associated with PRV-1 virus-associated hemolysis.
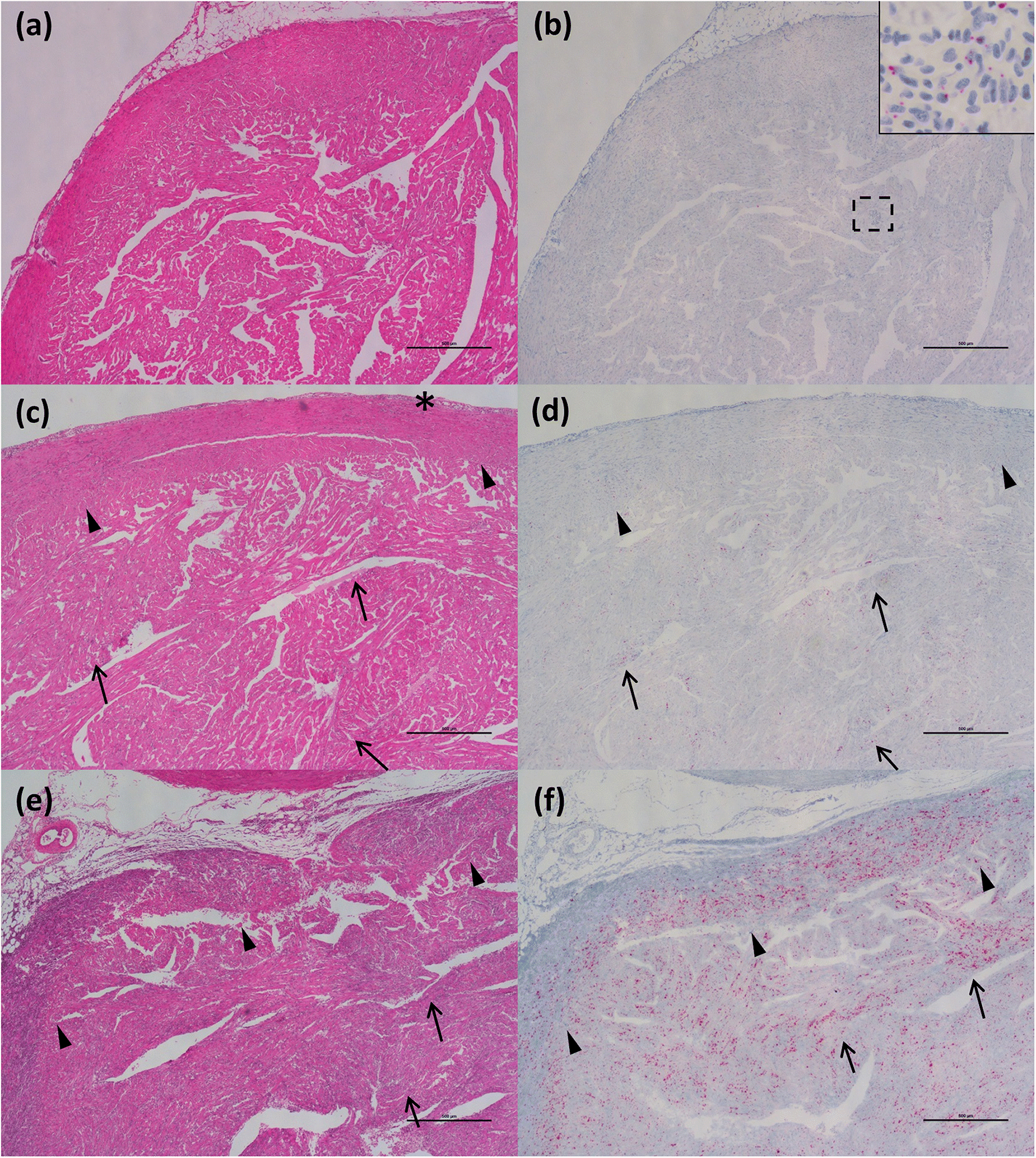
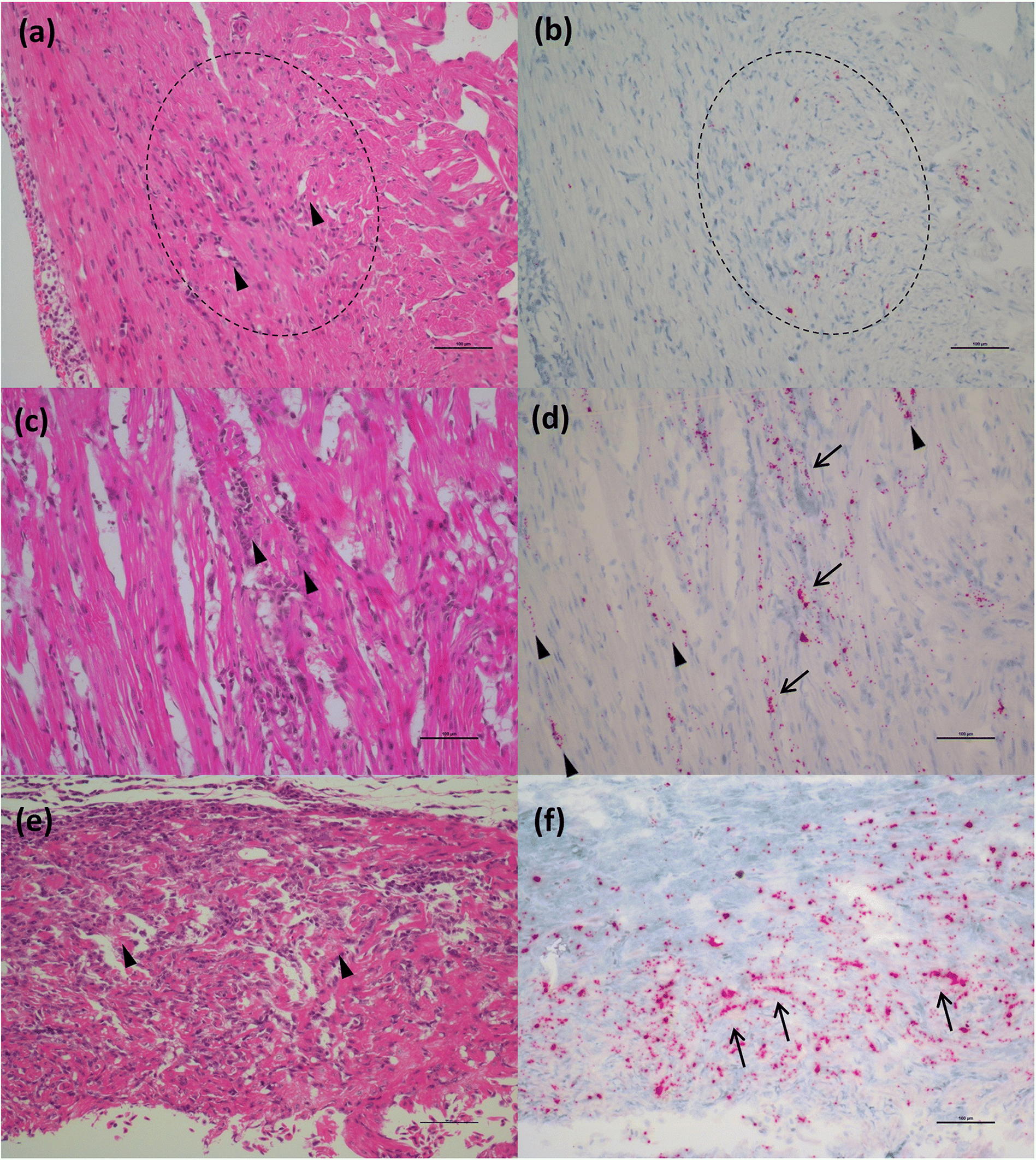
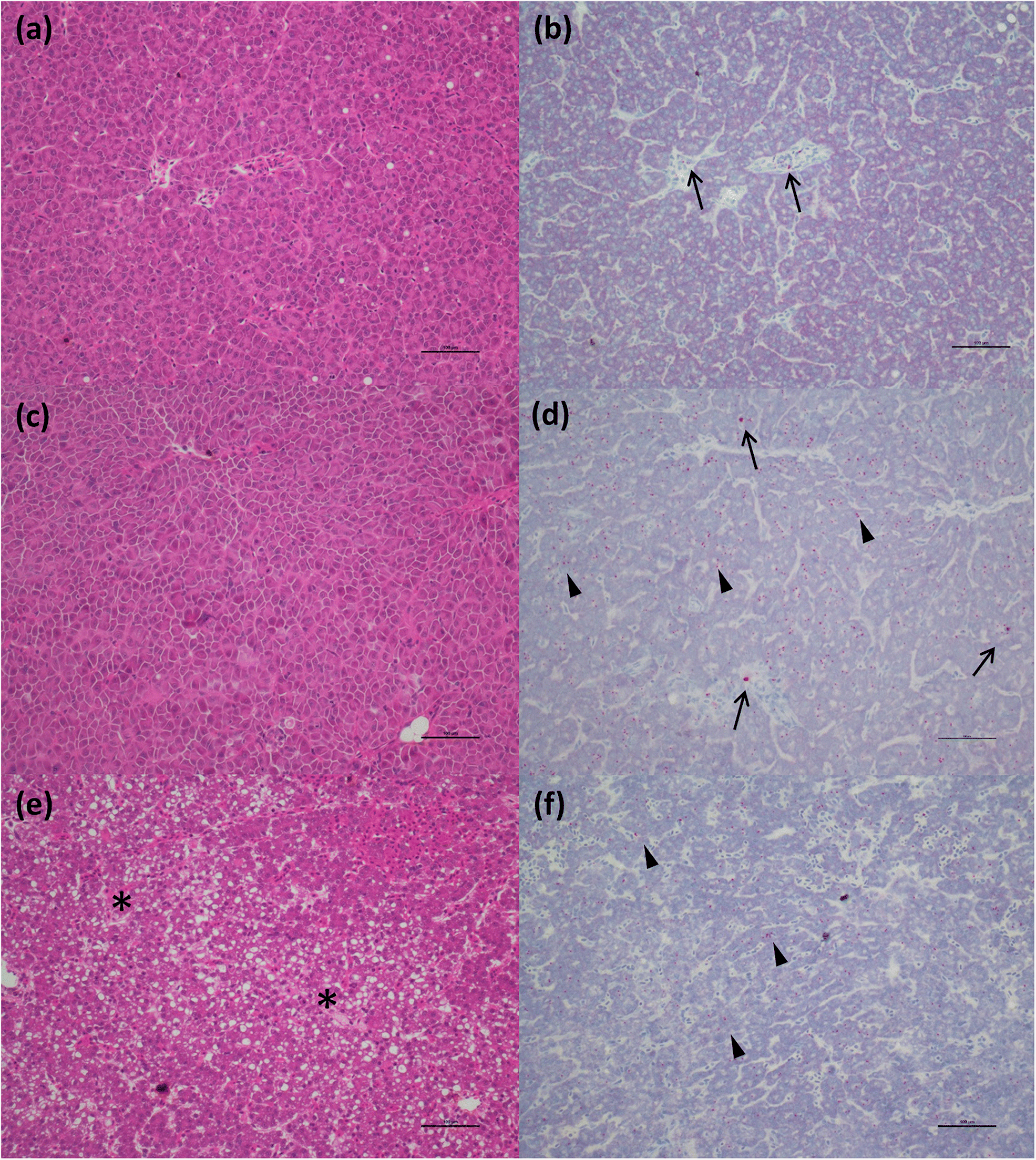
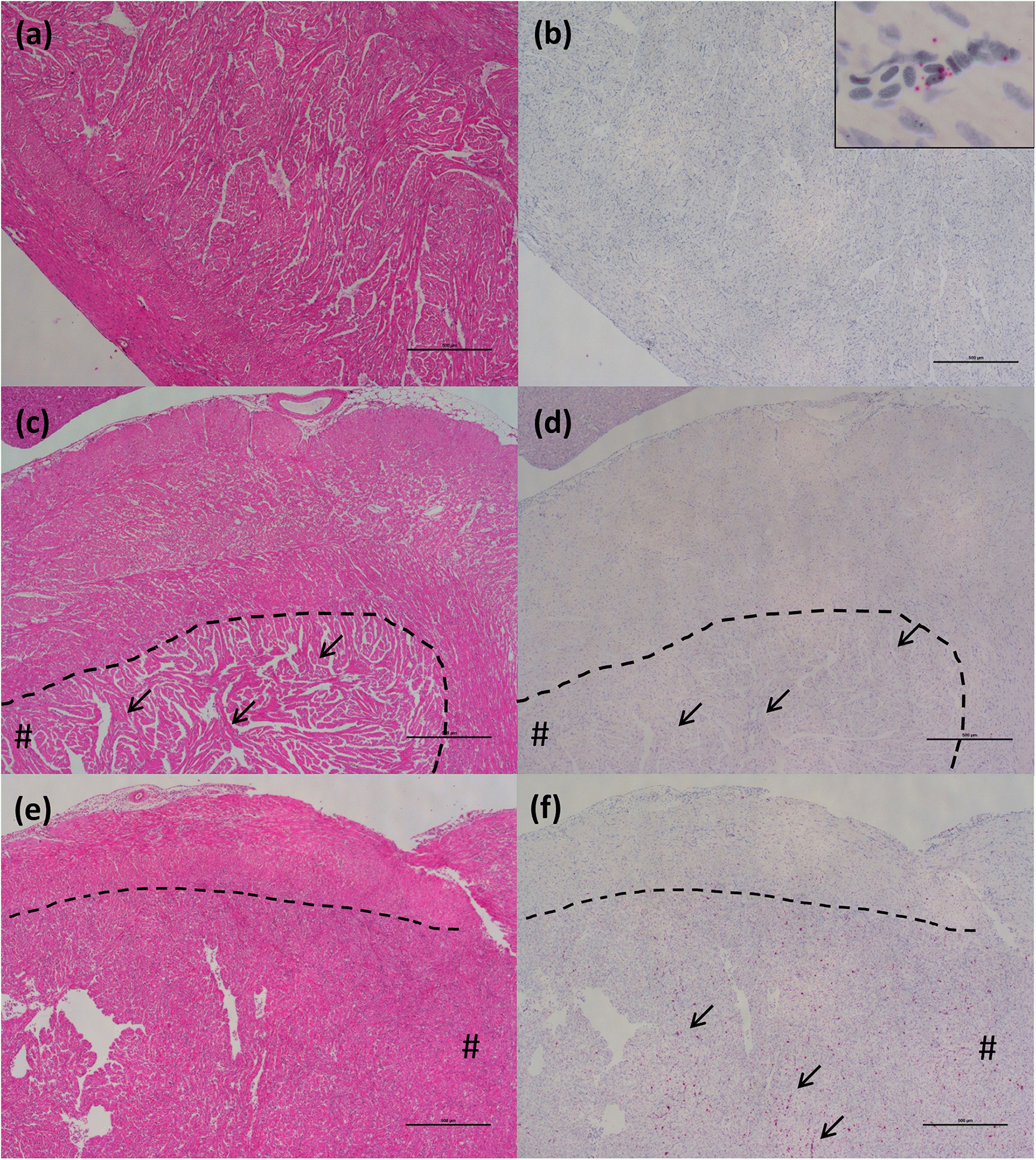
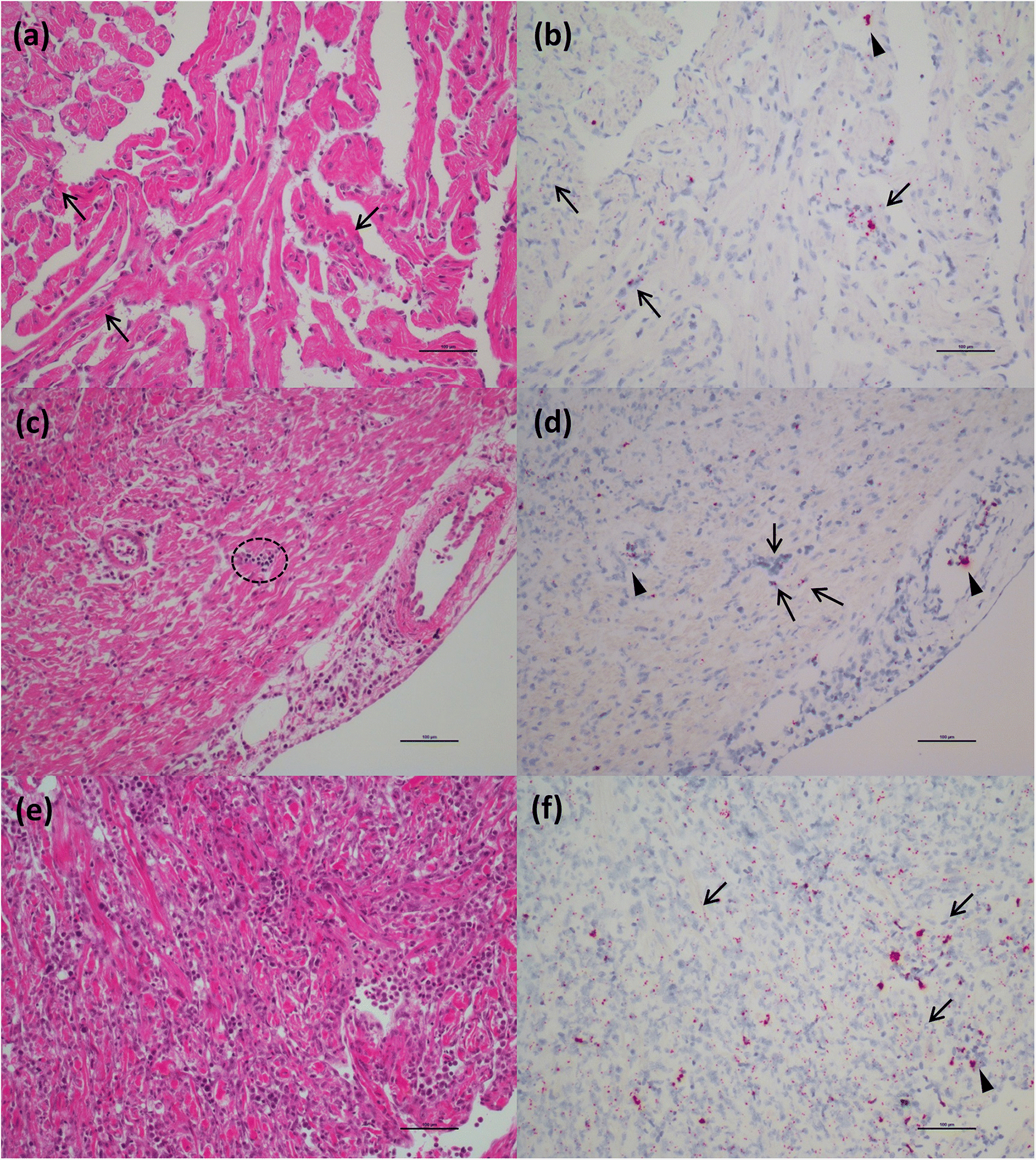
ISH analysis of the HSMI disease development process in Atlantic salmon, known to be caused by PRV-1 infection, showed a strong association of the virus within regions and cells showing tissue damage. In the “developing” stage, the virus begins to move outside of RBCs and infect cardiomyocytes, mostly in the compact layer of the myocardium, but also, to a lesser extent, in the spongy layer, as was also indicated through immunohistochemistry by
Wessel et al. (2017). In this early stage of disease, several cardiomyocytes become infected. The cardiomyocytes damaged by the intracellular activity of the virus induce the inflammatory reaction of the host, identified as foci of inflammation characterized by the presence of lymphocytes and macrophages. When the fish were diagnosed with HSMI, high quantity of the virus, and associated inflammatory lesions, spread throughout the heart, resulting in panmyocarditis by involving both compact and spongy layers of the myocardium, and sometimes even the atrium.
Although our study did not specifically investigate ISH in skeletal muscle lesions (as we had difficulty breaking up muscle tissue sufficiently to allow our RNA probe to enter without causing the tissue to become over-digested or detach from the slide), we have described the temporal patterns of inflammatory lesions in skeletal muscle that track those in the heart (
Di Cicco et al. 2017), and include these data herein. However, in Atlantic salmon, we investigated for the first time the viral localization in tissues other than the heart. Viral distribution in the spleen was similar to that in chinook salmon in the early stages of disease, also showing erythrophagocytosis, but it didn’t reach the massive engulfment of damaged RBCs during the peak of the disease as demonstrated in chinook salmon. Moreover, unlike in chinook salmon, there was no evidence of necrotic lesions in the liver (other than those described as “heart failure” pattern of necrosis) or kidneys in most fish despite a moderate prevalence of the virus in hepatocytes and renal macrophages.
One common finding between HSMI and jaundice/anemia was the localization of PRV particles in the intestine (i.e., enterocytes), particularly during the early stages of disease development, when a high amount of virus is free in the blood. This finding suggests and confirms that the intestine can act not only as an entry point for the virus, but also as a possible route for virus dissemination in the environment, as previously indicated by
Hauge et al. (2017).
Our study was able to illustrate that the key difference in the PRV-associated disease manifestation in Atlantic and Pacific salmon probably lies in the difference between the ability of the species to tolerate high loads of the virus in RBCs. In HSMI, there is no evidence of RBC lysis (and no anemia clinically reported), but rather PRV appears to leak from RBCs upon reaching a high viral load. We hypothesize that at that stage the virus moves into the plasma and infects other cell types, with particular predilection for cardiomyocytes and (to a lesser extent) myocytes of the skeletal muscle. Not being able to support viral replication, these cells undergo structural changes that expose the virus itself or antigenic cellular components that eventually trigger the inflammatory reaction within these tissues. This type of reaction is cell mediated (CD8+ and macrophages) as demonstrated by
Mikalsen et al. (2012), characterizing a typical Type IV hypersensitivity reaction. If inflammation becomes severe it can result in heart failure and death.
By contrast, there is consistent evidence of RBC rupture (e.g., anemia, hemosiderin, enhanced macrophages-erythrophagocytosis, hemorrhaging, excess of free hemoglobin, excess of free bilirubin and jaundice) in PRV-associated diseases in Pacific salmon around the world (
Sakai et al. 1994;
Olsen et al. 2015;
Godoy et al. 2016;
Takano et al. 2016) suggesting that PRV is released en masse during RBC lysis. This would also result in large quantities of hemoglobin being released into the tissues. Hemoglobin is catabolized and removed by hepatocytes, which can be overwhelmed by toxic levels of the breakdown product heme and the yellow compound bilirubin, a byproduct of heme catabolism. Exposure to toxic levels of these byproducts can result in necrosis of the hepatocytes (
Sakai et al. 1994), the degeneration and necrosis of the epithelial cells of kidney tubules (
Tracz et al. 2007;
Deuel et al. 2016), leading to a yellow appearance of the internal organs (especially the liver) and, in extreme cases, the epithelial cells on the body of the fish. These lesions can also potentially lead to liver and kidney failure, and eventually death. Anemia, reported in all Pacific salmon species affected by strains of PRV (
Olsen et al. 2015;
Godoy et al. 2016;
Takano et al. 2016), also results from a massive reduction in RBCs that could lead to a reduced oxygen uptake capacity and hence a substantially higher short-term mortality rate. Hence, the occurrence of transitory lesions in the heart seems to be characterized by a pathogenic mechanism different from the one affecting liver and kidney. The type of lesions reported for such organs also supports this hypothesis; inflammatory processes that are primarily involved in the heart (just like HSMI, despite a different viral localization/tropism in chinook), and degenerative/necrotic processes in liver and kidney.
Given that
P. orthoreovirus Strain PRV-1 causes HSMI in farmed Atlantic salmon, and likely causes jaundice/anemia in farmed chinook salmon, the absence of consistent sequence variation within PRV-1 in diseased Atlantic and chinook salmon implies a risk of transmission of the virus from farmed salmon to wild Pacific salmon. As Atlantic salmon accounts for 97% of the total biomass of farmed salmon in BC, answering the question of whether the virus causing HSMI in Atlantic salmon causes disease in Pacific salmon is critical when assessing risk. PRV-1 occurred in 65%–75% of farm audit samples of Atlantic and chinook salmon, with approximately 25% of the salmon having high viral loads. In farms where HSMI disease ensues, BC Atlantic salmon show a morbidity rate (i.e., presence of histological lesions) of >80%, similarly to that reported in Norway (
Kongtorp et al. 2004a;
Kongtorp et al. 2006), and retain high loads of the virus and evidence of inflammatory lesions for a prolonged period of time (over 11 months in the study by
Di Cicco et al. 2017). Hence, the environmental footprint and potential for viral transmission to wild salmon from disease-impacted farms is likely high. Although neither disease has yet been identified in wild fish to date, given the high sensitivity of the molecular viral disease development tool to early developing disease states, and of ISH to localize the virus across tissues, we can now apply these approaches to empirically determine whether any evidence exists that PRV-associated diseases may be occurring in migratory salmon in BC.
HSMI is one of the most common infectious diseases of Norwegian farmed salmon (
Garseth et al. 2017). Moreover, disease modeling has identified that fish farming intensity in a region is a major risk factor for HSMI outbreaks (
Kristoffersen et al. 2013;
Morton et al. 2017) suggesting that water-borne transmission may be an important factor in the spread of disease (
Hjeltnes et al. 2016). These data also suggest that farming intensity could enhance the exposure of migrating smolts to PRV (
Garseth et al. 2017). Escaped farmed salmon, which are most often infected with PRV, could also be a transmission vector for freshwater infections in wild fish if they enter rivers (
Madhun et al. 2015;
Hjeltnes et al. 2016,
2017). Importantly, there have been recent reports of HSMI outbreaks occurring in freshwater hatcheries in Norway, which represents a shift in what was considered until recently a disease restricted to sea net pens (
Hjeltnes et al. 2016,
2017). There is also some indication that PRV infection in freshwater may be associated with earlier and more significant HSMI outbreaks in sea net pens and, as a result, some farmers are attempting to produce PRV-free smolts (E. Rimstad, personal communication, 2017;
Hjeltnes et al. 2017).
Prevalence of PRV is low (<3%) in wild migrating BC chinook and sockeye salmon (
Oncorhynchus nerka (Walbaum, 1792)) smolts that have not been exposed to salmon farms (
Siah et al. 2015;
Morton et al. 2017;
Purcell et al. 2018;
Tucker et al. 2018). Higher prevalence is observed in fall, especially in chinook salmon on the west coast of Vancouver Island that remain resident in the same bays and inlets occupied by salmon farms for prolonged periods of time (up to a year) (
Tucker et al. 2011,
2012). An epidemiological study is currently underway to assess the role of salmon farms in the transmission of PRV to wild stocks, based on both patterns of prevalence distributions and full genome sequencing of the virus across farmed and wild populations over multiple years and geographic locations.