The first pterosaur pelvic material from the Dinosaur Park Formation (Campanian) and implications for azhdarchid locomotion
This article has been corrected.
VIEW CORRECTIONAbstract
A partial pterosaur pelvis from the Campanian Dinosaur Park Formation of Canada adds to our knowledge of Late Cretaceous pterosaurs. The pelvis is tentatively referred to Azhdarchidae and represents the first pelvic material from a North American azhdarchid. The morphology of the ilium is bizarre compared with other pterosaurs: it is highly pneumatized, the preacetabular process tapers anteriorly, and muscle scars show that it would have anchored strong adductor musculature for the hindlimb. The acetabulum is deep and faces ventrolaterally, allowing the limb to be positioned underneath the body. These features support previous suggestions that azhdarchids were well adapted to terrestrial locomotion.
Introduction
The pterosaurs of western Canada are relatively poorly known, represented primarily by fragmented skeletons (Currie and Russell 1982; Padian 1984) or isolated elements (Sullivan and Fowler 2011), mostly referable to Azhdarchidae. In Dinosaur Provincial Park, taphonomic biases against small, delicate elements (Brown et al. 2013) mean that already-fragile pterosaur material is rare. Previously described material from the Dinosaur Park Formation (DPF) includes vertebrae (Currie and Russell 1982), several pectoral girdle and wing elements (Godfrey and Currie 2005), and possible hindlimb elements (Godfrey and Currie 2005). However, no cranial or pelvic material has yet been recovered. The paucity of material and its isolated nature means that untangling the taxonomy of the DPF pterosaurs is particularly difficult.
Godfrey and Currie (2005) suggested that, because of the widely disparate sizes of identifiable elements, up to three azhdarchid taxa may have coexisted. The largest of the azhdarchids is represented by a large limb bone (TMP 1980.016.1367) that Currie and Russell (1982) suggested was a femur. If this bone is indeed a femur that individual would have had a wingspan of up to 13 m. Others (Padian and Smith 1992; Averianov 2014) have argued that TMP 1980.016.1367 is in fact a wing element and, therefore, represents a smaller individual, similar in size to Quetzalcoatlus northropi from Texas. It has also been suggested that this element is a large, poorly preserved cervical vertebra (Godfrey and Currie 2005; M Habib, personal communication, 2016). Godfrey and Currie (2005) suggested that the smallest azhdarchid material from the DPF is probably Montanazhdarcho (Padian 1984; Padian et al. 1995), although the azhdarchid identity of that genus is uncertain (Carroll 2015). A partial skeleton described by Currie and Jacobsen (1995) represents an intermediately sized individual (wingspan 6 m), although they point out that this individual was immature. In most cases, a lack of autapomorphic characters in the elements from the DPF prevents their assignment to these other taxa. However, Sullivan and Fowler (2011) suggested that both wing phalanges IV-1 collected from the DPF were referable to Navajodactylus from the Kirtland Formation of New Mexico. Despite the considerable geographic distance, it is conceivable and has been previously suggested (Habib 2010) that, as volant animals, azhdarchids could disperse over such a large area. Pterosaur material from the DPF, therefore, may represent as many as four different taxa.
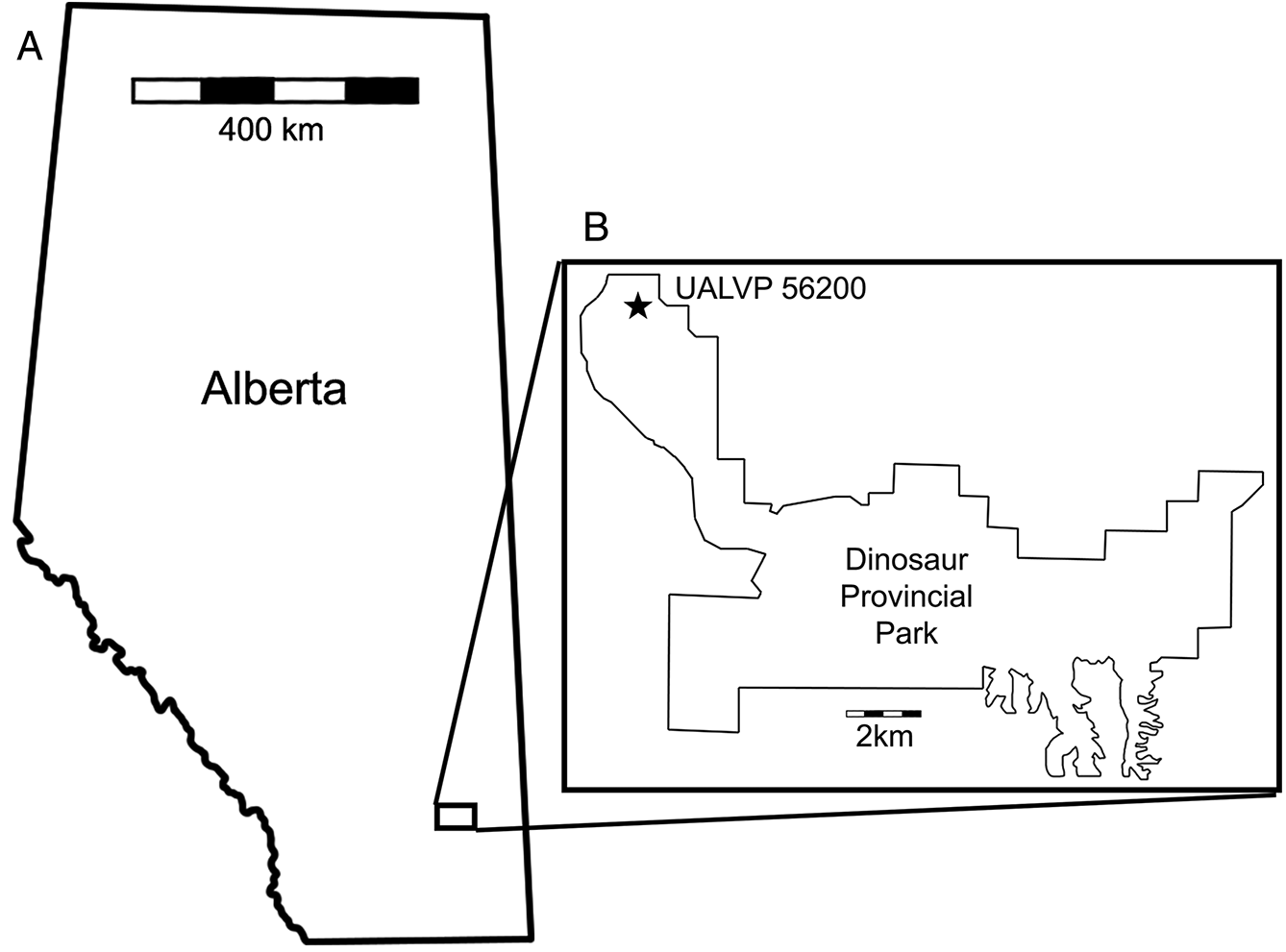
In the summer of 2015, an unusual pterosaur bone (UALVP 56200) was recovered from a multitaxic bone bed at the Steveville locality of Dinosaur Provincial Park (Fig. 1). Comparison with pterosaur pelves strongly suggests that UALVP 56200 is composed mostly of the right preacetabular process of the ilium. The great size (>150 mm) of the element suggests that it may tentatively be referred to Azhdarchidae, although the anatomy of azhdarchid pelves is poorly known. UALVP 56200 provides important anatomical information about the DPF pterosaurs and has implications for the previously proposed terrestrial ecology of large azhdarchids.
Fig. 1.
Material and methods
UALVP 56200—?Azhdarchidae indet. Partial right pelvis including the preacetabular process of the ilium and the acetabular rim of the pubis. The material was collected in 2015 in the DPF from multitaxic bone bed BB151 (GPS: 12U E0458338, N5630743, WGS84) in Dinosaur Provincial Park near Steveville, Alberta by GFF.
The specimen was collected under appropriate permits granted to PJC. The specimen was mechanically prepared using hand tools and consolidated using cyanoacrylate and Paraloid B-37. After initial preparation, the specimen was scanned via computed tomography (CT) using a Siemens Sensation 64 CT Scanner at the ABACUS Core Imaging Facilities in the University of Alberta Hospital Mazankowski Centre. Images were generated at 120 kV and 192.00 mAs with a pixel size of 0.443 mm and a slice increment of 1.000 mm. CT scan data were imported to Mimics 14.0 to create a mesh, which was cleaned up in Geomagic Design 64. An interactive model of this mesh is available in Supplementary Material 1. Some fragments, including the posterior portion of the acetabulum, were reassembled after CT scanning and are therefore absent in the three-dimensional model.
CT reconstruction of pneumatic spaces allowed estimation of volumetric air-space proportion (ASP). However, UALVP 56200 was preserved in two types of matrix: soft, medium-grained sandstone; and well-indurated siderite ironstone. Cavities infilled with sandstone were significantly less dense than surrounding bone, but because of the similar densities of the bone and ironstone matrix, determining the boundaries of ironstone-infilled cavities was difficult. All internal regions of low density were manually segmented in Mimics 14.0, which automatically calculates mesh volume. Subsequently, a second estimate was generated based solely on sandstone-infilled spaces, which were easier to determine unequivocally. The bone enclosing the large dorsal pneumatic spaces is broken, so the volume of this cavity was not estimated, but it would have increased the ASP considerably. The estimates presented are, therefore, conservative minimum estimates of ASP. The specimen was photographed using a Nikon D7200 with a 50 mm lens (Figs. 2A–D, F), or with a Nikon D5000 with an 18–55 mm lens (Figs. 2E, 3). Measurements were taken using digital calipers with an accuracy of 0.05 mm.
Fig. 2.
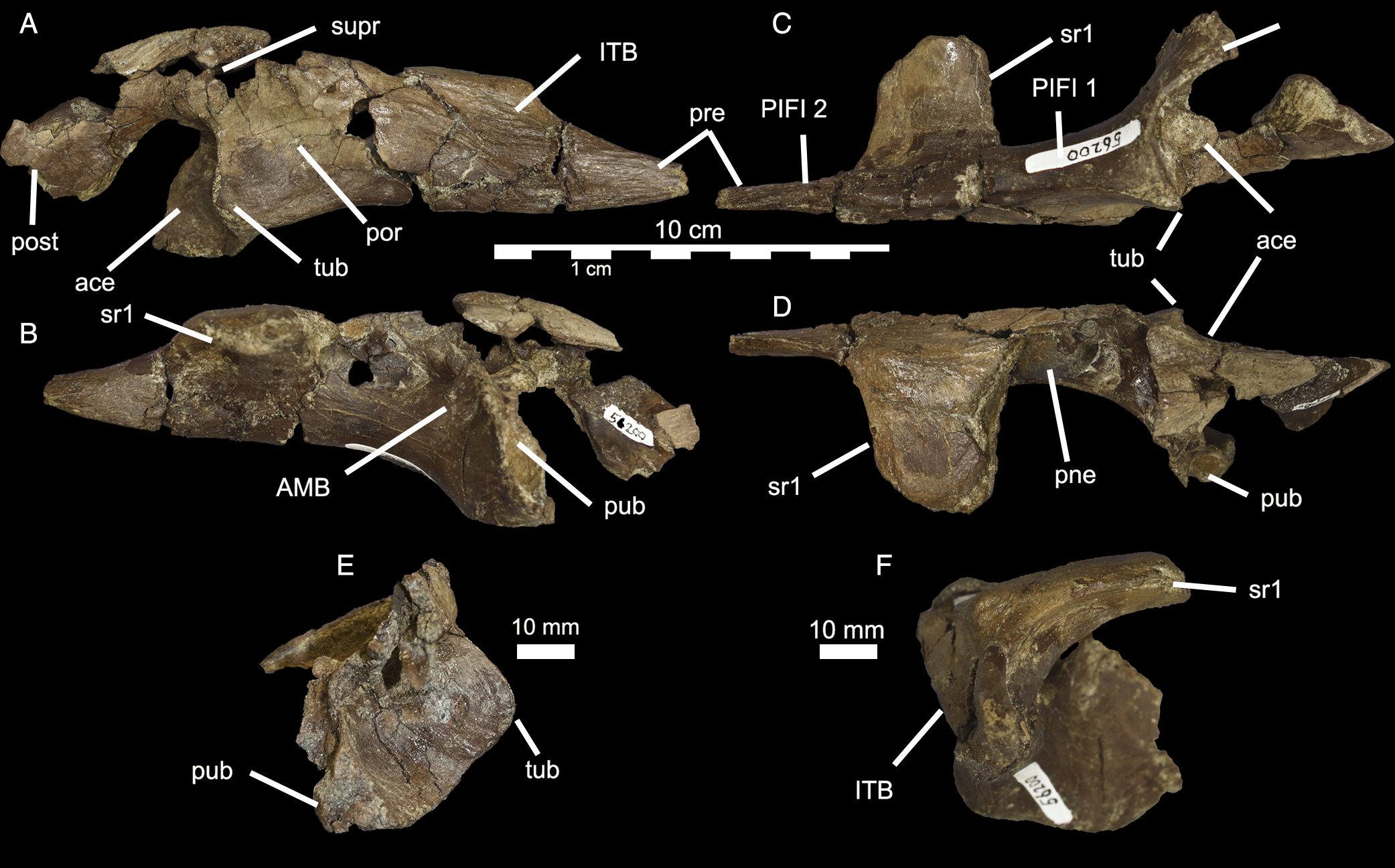
Fig. 3.
Systematic paleontology
PTEROSAURIA Kaup 1834
PTERODACTYLOIDEA Plieninger 1901
AZHDARCHOIDEA Nessov 1984
AZHDARCHIDAE Nessov 1984
Azhdarchidae indet.
Geologic and stratigraphic context
UALVP 56200 was recovered from a dense multitaxic bone bed (BB151) in the lower part of the Upper Campanian (∼ 75 ma) DPF near Steveville, Alberta. The bone bed occurs at the base of an intermittently sideritized, cross-bedded, medium-grained sandstone overlying a grey mudstone. The fossil assemblage of BB151 is composed of a variety of micro- and macrofossils including ceratopsians, champsosaurs, crocodylians, hadrosaurs, and theropods. Fossils in the bone bed are disarticulated and generally well preserved, but taphonomic signatures are conflicting. Abrasion on UALVP 56200 suggests that it was either transported a long distance or subjected to rapid flow velocities; when recovered, it was in contact with an even more weathered fragment of hadrosaur cancellous bone. In contrast, the presence of teeth in hadrosaur and crocodylian mandibulae, combined with fine preservation of small theropod elements, suggests that those elements were buried rapidly without significant weathering or transport. This taphonomic variability is likely evidence of a time-averaged assemblage of reworked skeletal material. Accordingly, the bone bed is here interpreted as a channel lag deposit; channel lags commonly host bone beds in the DPF (Eberth 2015).
Description
UALVP 56200 (Figs. 2–6; Table 1) is relatively well preserved for a pterosaur element from the DPF. The specimen is mostly composed of a highly pneumatized right preacetabular process of the ilium (Fig. 2). Although it has been slightly crushed, it has retained its overall shape. The bone surface shows some signs of stage 1 weathering (sensu Behrensmeyer 1978) and part of the medial side is rounded and abraded. In the thickest regions (the dorsal margin of the ilium), the bone wall thickness is as much as 3.5 mm, but the thinnest regions, surrounding the pneumatic cavities, are less than 0.5 mm thick.
Table 1.
| Element | Measurement | Value (mm) |
|---|---|---|
| Sacral rib 1 | ||
| Anteroposterior width | 40a | |
| Dorsoventral thickness (min) | 10.75 | |
| Preacetabular blade | ||
| Length | >110.69 | |
| Shaft transverse width—min | 5.73 | |
| Shaft transverse width—max | 24.03 | |
| Height at sacral rib | 31.65 | |
| Height at acetabulum | 56.41 | |
| Height above acetabulum | 19.06 | |
| Total transverse width at anterior end of acetabulum | 44.62 | |
| Acetabulum | ||
| Anteroposterior length | 30.75 | |
| Anterior height | 30.05 | |
| Maximum height | 40.98 | |
| Depth (maximum) | 37.11 |
a
Estimated value.
The preacetabular process contacts and is fused indistinguishably with a medial plate of bone formed from the fused distal sacral ribs, a condition termed a “fenestrated sacral shield” by Naish et al. (2013). The preacetabular process is also fused to the sacral shield in Tropeognathus (Kellner et al. 2013). The sacral shield of UALVP 56200 is broken posteriorly and abraded medially, but would have contacted the first sacral rib. CT scan data reveal the suture between the sacral rib and ilium (Fig. 3).
The preacetabular process of the ilium tapers anteriorly in both lateral (Fig. 2A) and dorsal views (Fig. 2D). The lateral surface of the preacetabular process (Fig. 4A) was occupied mostly by the origin of M. iliotrochantericus (ITC; Fig. 5), but also bears a pneumatopore (Fig. 2A) and two ridges. The more ventral ridge extends to a tubercle on the anterior rim of the acetabulum, here called the preacetabular tubercle. The pneumatopore is above this ridge, 16 mm anterior to the acetabulum. The second lateral ridge extends posterodorsally from the tip of the preacetabular process. Anterodorsal to it, a series of striated ridges mark a region of muscle attachment for M. iliotibialis (ITB). Ventral to it, on the ventral edge of the anterior portion of the preacetabular process, a second muscle scar probably represents the origin of M. puboischiofemoralis internus 2 (PIFI-2). At the anterior end of the contact between the fenestrated sacral shield and the ilium, there is a rugose dorsal tubercle that probably contributed to the attachment of the ITB. The ventral ridge and preacetabular tubercle may have anchored part of M. ambiens (AMB), as suggested by Naish et al. (2013), but it seems more likely that it simply divides the enlarged origins of ITC and M. puboschiofemoralis internus 1 (PIFI-1; Fig. 5).
Fig. 4.
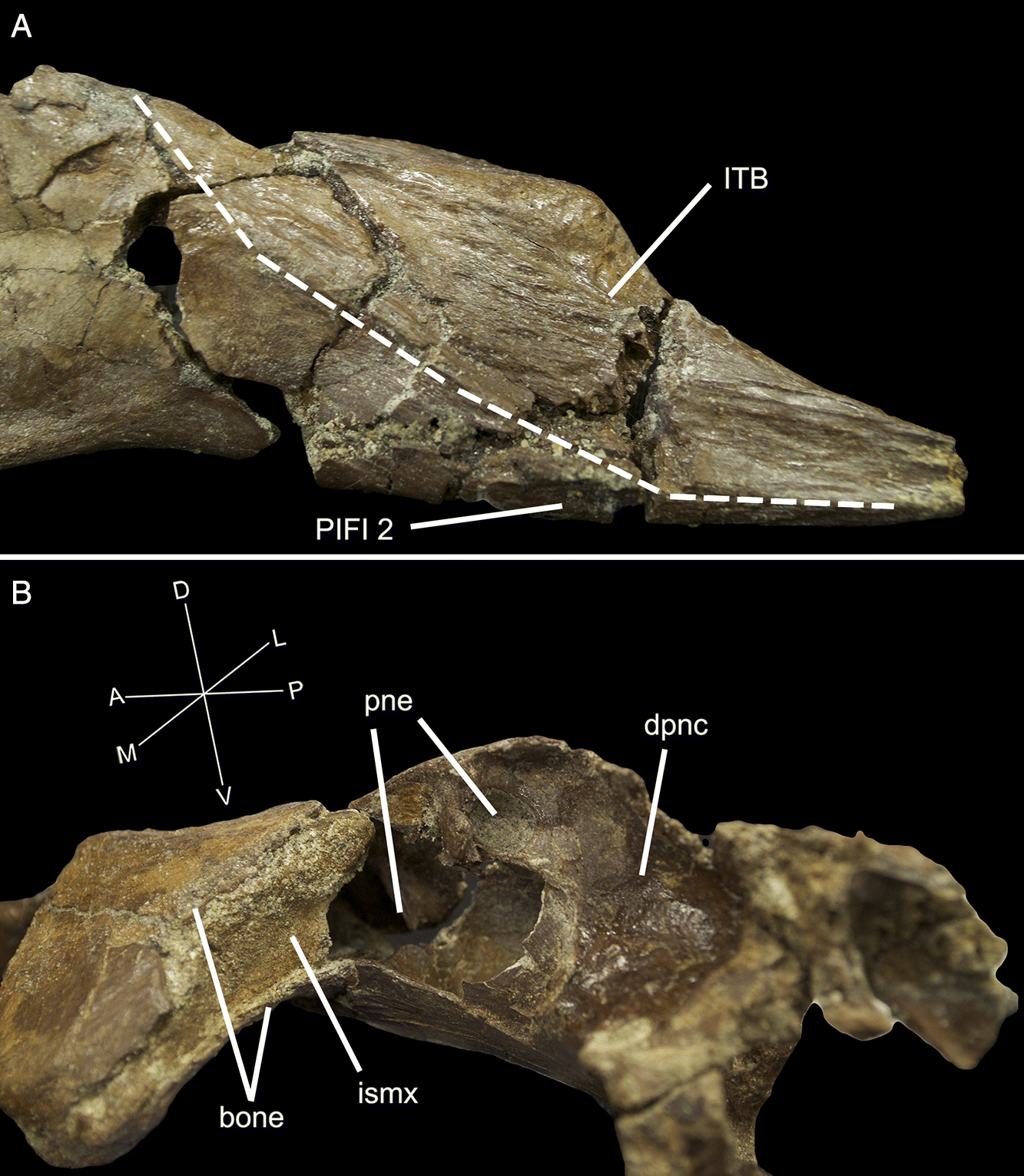
Fig. 5.
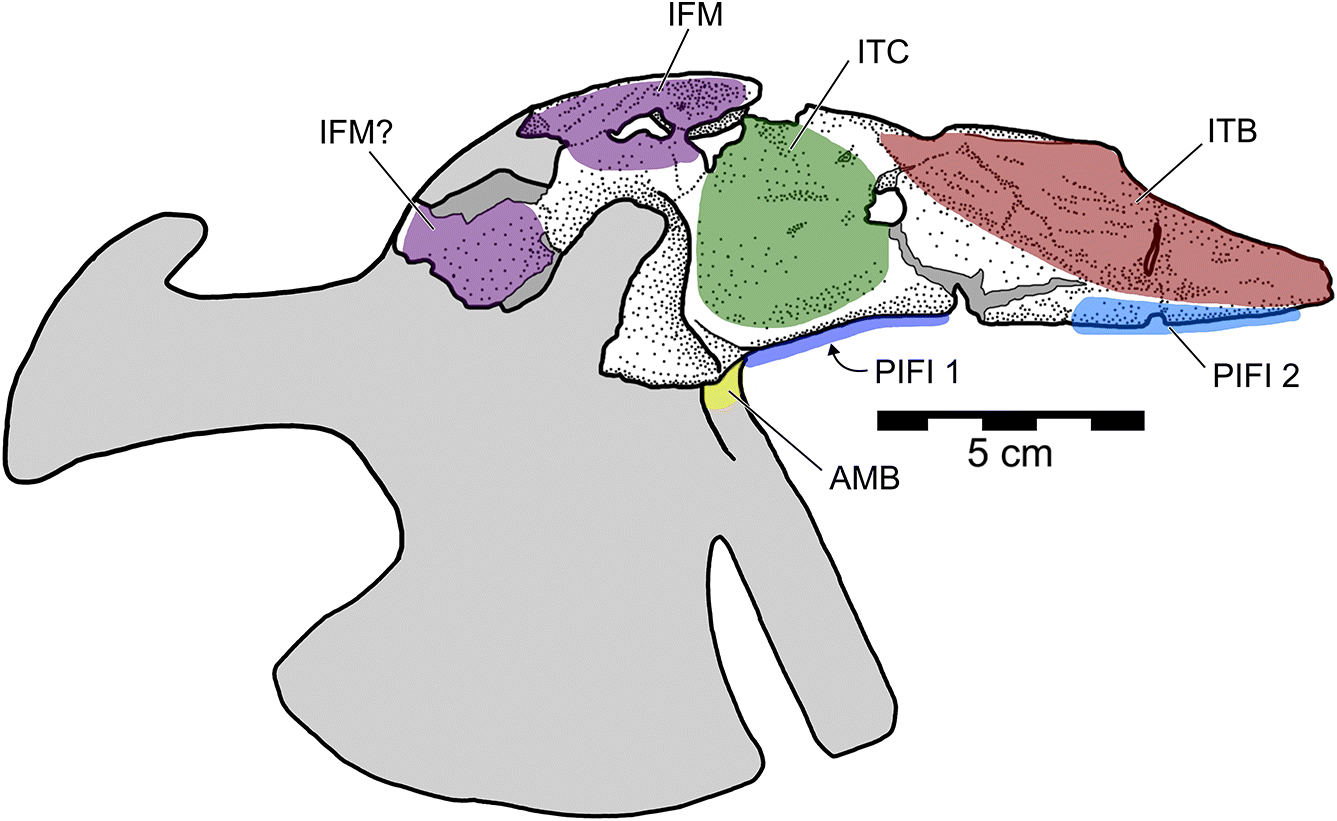
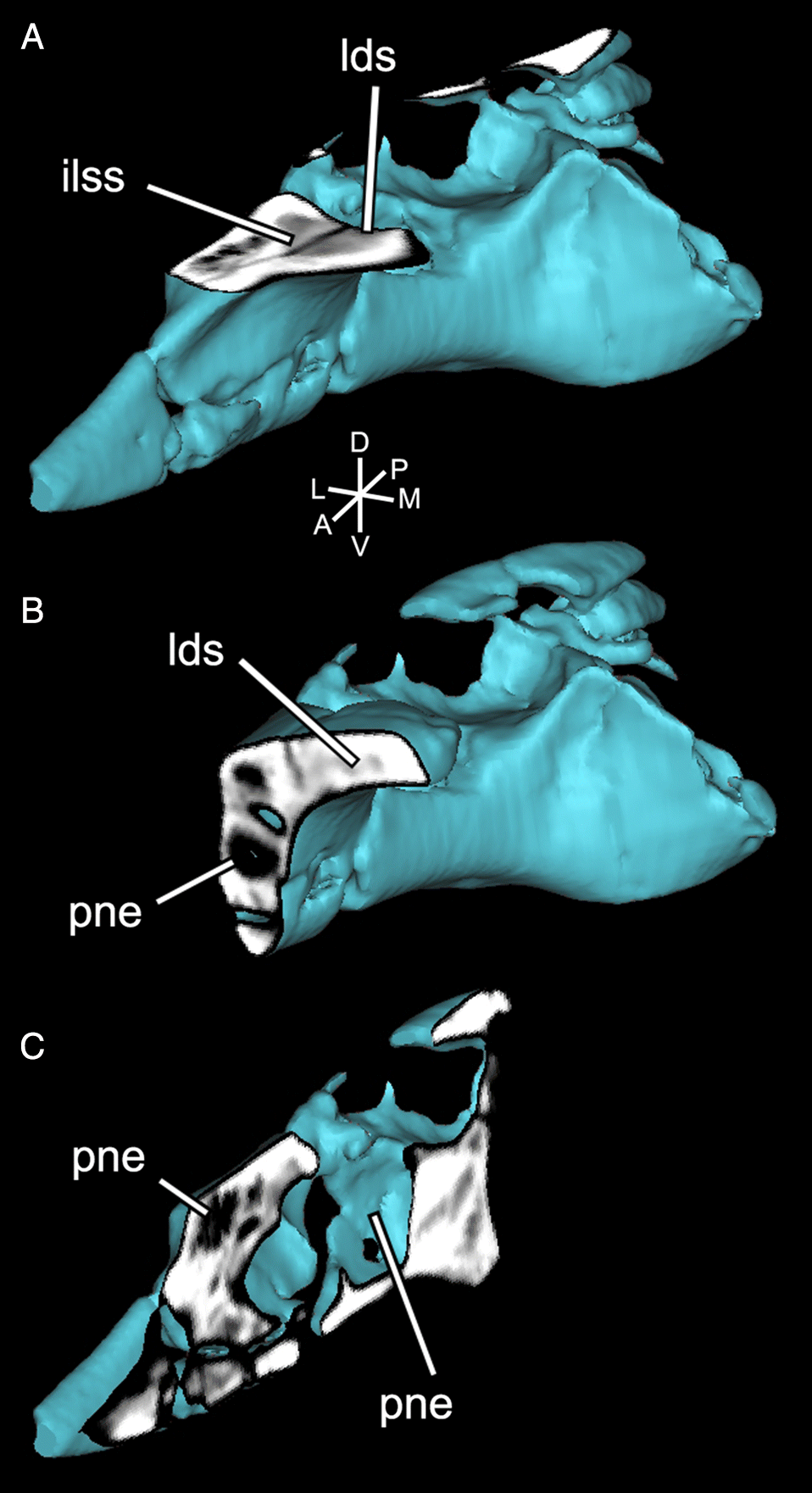
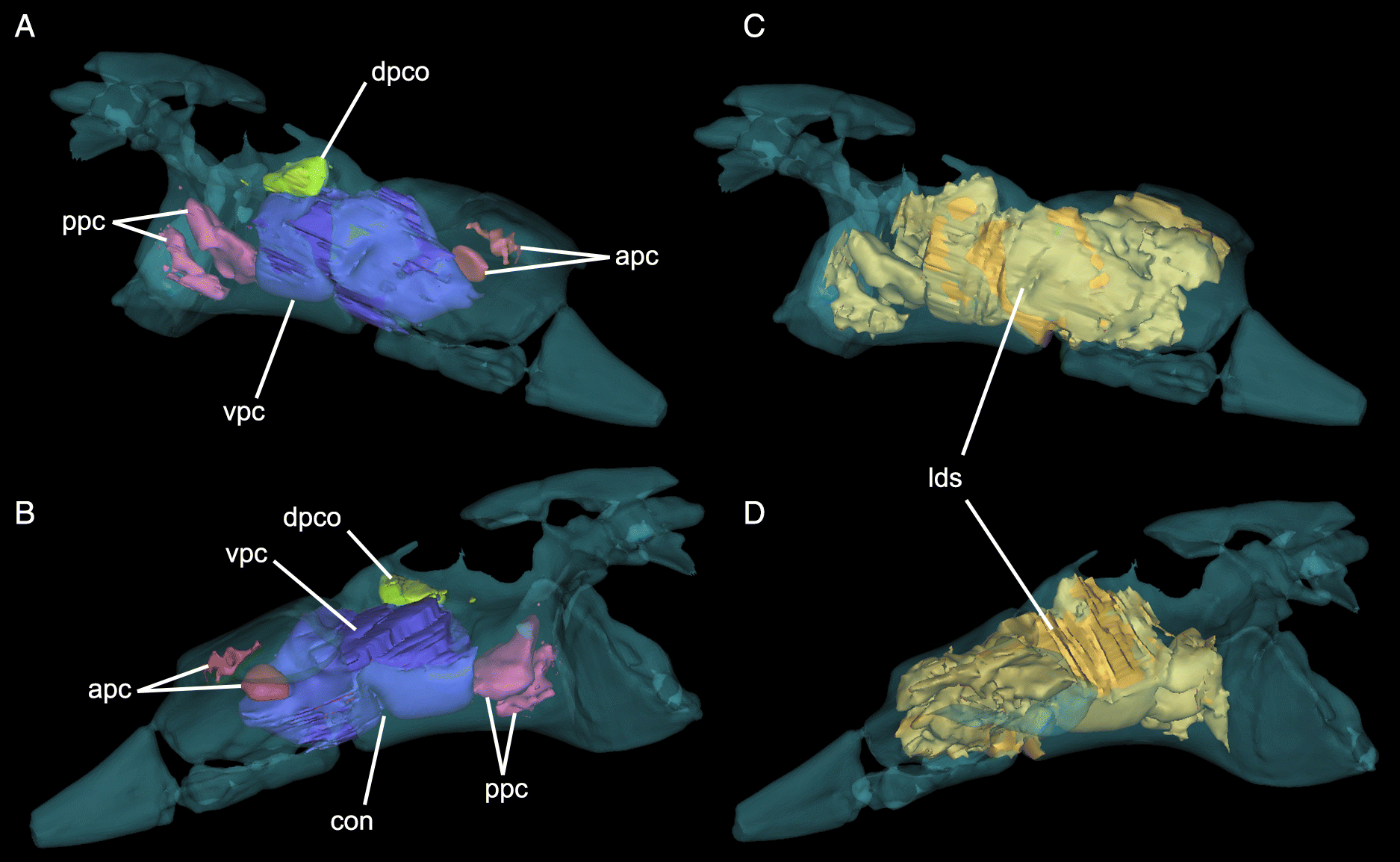
The delicate dorsomedial surface of the preacetabular process is broken, revealing numerous pneumatic cavities (Figs. 4B, 6). The most ventral of these would have been closed in life, but is broken to reveal an extensive chamber that is partially divided by an internal mediolateral ridge. This cavity is confluent with the ironstone-infilled space in the sacral shield, suggesting both elements were pneumatized (Figs. 4B, 6). Posterodorsal to the large chamber is a smaller, circular fenestra that opens into a pneumatic pocket (Figs. 3, 4B). Posterior to this, there is a deep concavity bordered ventrally by a ridge and a wide foramen. The ventral surface of the preacetabular process of the ilium is smoothly convex and has a small nutrient foramen on its lateral side about 20 mm anterior to the acetabular rim. The medial region anteroventral to the acetabulum is depressed into a deep fossa with a weakly rugose patch from which the AMB probably originated. It is likely that part of this fossa is composed of the pubis, but it is indistinguishably fused with the ilium. Directly medial to the acetabulum, a broken region of the ilium would have contacted the sacrum. Above the acetabulum, the lateral surface of the ilium is rugose and has a small foramen and anterodorsally directed striae marking the origin of M. iliofemoralis externus (IFM). This area is strongly pneumatized, with extensive open pockets that have become filled by ironstone (Fig. 4B). A small part of the postacetabular process is preserved. Its dorsal edge is thin, and ironstone infill indicates that it was extensively pneumatized. The dorsal margin of this portion is strongly convex and slopes posteroventrally, suggesting that the postacetabular blade was strongly downturned relative to the preacetabular blade. Its lateral surface is rugose, and may have anchored a head of M. iliofibularis (IFB) or possibly a posterior head of IFM.
Fig. 6.
The acetabulum is deep (Table 1), faces ventrolaterally, and is nearly circular except for a flattened anterior face. The dorsal rim of the acetabulum is shallowly rounded on its lateral surface but flat internally, forming a prominent shelf. The anterior rim protrudes far laterally because of the preacetabular tubercle, the posterior face of which is pitted with small depressions. The preacetabular tubercle partly overhangs the anterior portion of the acetabulum, reminiscent of the supraacetabular crest that dorsally overhangs the acetabulum of ornithomimid and tyrannosaurid theropods. The anteroventral corner of the acetabulum is nearly square, and the ventral and anterior rims of the acetabulum are perpendicular. The thin ventral rim encloses the acetabulum and would have projected lateral to the puboischiadic plate. Only the dorsal portion of the posterior rim of the acetabulum is preserved; in this region, it is smoothly sloped and poorly defined.
Pneumaticity
ASP was estimated as 29% air for sandstone-infilled spaces only and 45% air for all low-density regions (Fig. 6). These ratios are low compared with other estimates (mean 77%) of pterosaur pneumaticity based on long bones (Martin and Palmer 2014) and other skeletal elements (mean = 60.5%; Elgin and Hone 2013), but do not include the large unenclosed dorsal pneumatic spaces of UALVP 56200. CT scans reveal four main internal chambers, in addition to the large, broken dorsal pneumatic chamber (Supplementary Material 2). The largest of the internal chambers (ventral pneumatic cavity (vpc); Fig. 6) occupies most of the body of the preacetabular process. At its midpoint, it is constricted by a ventral lamina that divides it into two equally sized cylinders. Dorsal to this chamber, there is a small circular outpocket of the large dorsal pneumatic chamber (dpco; Fig. 6). Internal to the preacetabular tubercle, there are two small cavities (posterior pneumatic cavities (ppc); Fig. 6) that may have been joined in life. They are separated from the ventral pneumatic cavity by a thin, vertical wall of bone. They are connected to the pneumatopore near the origin of ITC, confirming its pneumatic nature (O’Connor 2006). Anterior to the ventral pneumatic cavity, two smaller pockets (anterior pneumatic cavities (apc); Fig. 6) invade the preacetabular process below the sacral rib. It is likely that these were confluent with the large ironstone-infilled chamber in the sacral rib, and, consequently, that the majority of the preacetabular process was pneumatic.
Discussion
Identity
The fragmentary and unusual nature of UALVP 56200 makes the identification of the specimen difficult. Although theropods (avian and non-avian) can have pneumatic ilia (Coria and Currie 2006; Sereno et al. 2008; Hocknull et al. 2009), the morphology of UALVP 56200 is entirely unlike any of these animals. The acetabulum is imperforate, unlike in all dinosaurs, and the supraacetabular portion of the ilium is wider than tall, instead of the plate-like condition in birds and other theropods. The preacetabular portion lacks a peduncle for the pubis, and the sacral rib contacts the dorsal surface of the ilium, rather than the medial surface. Within pterosaurs, at least two groups have pneumatized pelves: ornithocheirids (sensu Unwin 2003; Claessens et al. 2009) and azhdarchoids (Naish et al. 2013). Although unusual in its proportions, the morphology of UALVP 56200 is consistent with the pelves of these and other pterosaurs. Pterosaurs have an imperforate acetabulum, a small supraacetabular surface, and a long, ribbon-like precetabular process. Thus, we infer that UALVP 56200 constitutes the first pterosaur pelvic material from Canada.
The large size of UALVP 56200 and its provenance, both geographically and stratigraphically, strongly suggest that it is from an azhdarchid. Azhdarchid pelvic material is generally rare and fragmentary, especially in North America, and to date, no ilia have been described. The only known azhdarchid pelvis, that of Zhejiangopterus (Cai and Wei 1994), is crushed so that the morphology of the ilia cannot be discerned. Comparisons with other pterosaur groups support the exclusion of UALVP 56200 from those groups. For example, it differs from other pterodactyloid groups (ornithocheiroids sensu Unwin 2003, and ctenochasmatoids) in that the short preacetabular process is not curved, although these features are somewhat variable within these pterosaurs (Hyder et al. 2014). Furthermore, whereas most pterodactyloid preacetabular processes are spatulate and thin, UALVP 56200 tapers anteriorly and is relatively robust. The robust muscle attachment sites are unlike those of most pterosaurs, which have poorly developed muscle scars for hindlimb musculature (Molnar 1987; Wellnhofer 1988; Bennett 1990; Naish et al. 2013; Frigot 2017). The ilium is similar to those of pteranodontids, but differs in several respects. UALVP 56200 is distinct from Pteranodon longiceps (Bennett 2001a) in having a relatively thicker (minimum height 15% of length in UALVP 56200 vs. 4% in P. longiceps), nonspatulate preacetabular blade, a straight lateral margin of the preacetabular process, and more pronounced areas of muscle attachment. These features are unlikely to be allometric or ontogenetic differences, as they are not variable in P. longiceps (Bennett 2001a). Furthermore, UALVP 56200 is about equal in size to the large P. longiceps pelvis YPM 1175 (Bennett 2001a).
Azhdarchoids are one of two groups of pterosaurs that persist into the Campanian, alongside pteranodontoids. The dissimilarity of UALVP 56200 with known pteranodontoids therefore suggests that it is from an azhdarchoid, and indeed, azhdarchids are already known from the DPF (Godfrey and Currie 2005). In-clade relationships of azhdarchoids are not yet clear, but most authors recognize four distinct families: azhdarchids, chaoyangopterids, tapejarids, and thalassodromids. Of these groups, azhdarchids are the only group that persisted until the Late Cretaceous and the only group known so far in North America, and therefore, we tentatively refer UALVP 56200 to Azhdarchidae.
Comparisons
Several well-preserved azhdarchoid pelves are known, and these draw morphological ties between UALVP 56200 and azhdarchoids. In addition to the straight preacetabular process that characterizes azhdarchoids (Hyder et al. 2014), the taper of the preacetabular process and development of the preacetabular tubercle are similar to other known azhdarchoid pelves. A nearly complete pelvis (AMNH 22569) was described by Bennett (1990) and has been assigned by others to Neoazhdarchia (Hyder et al. 2014), but most of the preacetabular portion is missing. The small preacetabular portion that is present is similar to that of UALVP 56200, except that the preacetabular tubercle is relatively smaller. Furthermore, Bennett (1990) speculatively reconstructed the preacetabular process of AMNH 22569 as extending far past the first sacral rib, which is not the case in UALVP 56200.
Naish et al. (2013) described a small, nearly complete azhdarchoid pelvis (Vectidraco daisymorrisae) from the Early Cretaceous of the Isle of Wight, which provides important comparative material. Overall, the morphology of Vectidraco is similar to that of UALVP 56200, but there are important differences, including the scale. The preserved length of the entire pelvis of Vectidraco is 40 mm (Naish et al. 2013), which is just over one-third the length of the preacetabular process of UALVP 56200 (>110 mm). The anterior end of the preacetabular blade of Vectidraco is missing, and although it tapers anteriorly in lateral view, it does not taper transversely. The origin of ITB in Vectidraco is not as well developed, nor is it rugose, but Naish et al. (2013) described a sharp lateral ridge, which is present in UALVP 56200 separating the origins of ITC and PIFI-1. The preacetabular tubercle is relatively smaller in Vectidraco and does not protrude as far laterally. Although the postacetabular process of Vectidraco is pneumatized, Naish et al. (2013) did not describe any preacetabular pneumaticity, which is extensive in UALVP 56200. It is likely that the difference in degree of pneumatization is tied to body size (O’Connor 2009), but it may also indicate differences in pelvic air sac organization.
Frigot (2017) recently provided a description of the reconstructed pelvic myology of Vectidraco, which allows a reference for UALVP 56200. Although all of the muscular origins in UALVP 56200 are relatively larger than those of Vectidraco, the starkest contrasts are the ITB, ITC, and IFM. The origin of ITB in UALVP 56200 is large and rugose, and occupies the anterodorsal portion of the preacetabular process. Frigot (2017) reconstructed the ITB of Vectidraco as originating along a small lateral strip of the preacetabular process, but it is situated more dorsally in UALVP 56200. Frigot (2017) did not reconstruct the ITC in Vectidraco, but based on the inferences of Costa et al. (2014a), it appears to occupy much of the lateral surface of the preacetabular process in UALVP 56200 and would have played a major role in femoral abduction and hip flexion. Similarly, an expanded supraacetabular portion of the ilium of UALVP 56200 is marked by a muscular origin, which we infer to be an anteriorly expanded head of IFM. In Vectidraco, IFM is restricted posteriorly, and no muscle originates dorsal to the acetabulum (Frigot 2017).
To assess the relationships of UALVP 56200, it was incorporated into the phylogenetic matrix (25 taxa, 23 characters) of Naish et al. (2013). UALVP 56200 could be coded for only three characters, and with only one multistate character (50 character states total), the matrix was at the threshold of being resolvable (the minimum number of character states for a matrix of 25 taxa is 50). The analysis produced 1230 most parsimonious trees, and the strict consensus tree had only one node resolved: a trichotomy of the “Toolebuc pterosaur”, Dsungaripterus weii, and Coloborhynchus spielbergi. UALVP 56200 was within an unresolved polytomy of the remaining 22 taxa. The results were, therefore, uninformative regarding the affinities of UALVP 56200 and are not displayed here.
Body size
Estimations of body size and wingspan are difficult based on the material preserved in UALVP 56200. Few pelves have been used to estimate size in pterosaurs, but the dimensions of the acetabulum may provide an adequate proxy for body size and allow other dimensions to be estimated. Based on the acetabulum of Vectidraco (7 mm × 7 mm; Naish et al. 2013) and Anhanguera (22.7 mm × 14.9 mm; Wellnhofer 1988), UALVP 56200 (30.75 mm × 40.98 mm) would have been a very large animal. Naish et al. (2013) estimated the wingspan of Vectidraco as 750 mm, and Wellnhofer (1988) suggested that the pelvis of Anhanguera corresponded to a 4.5 m wingspan. Based on this range of proportions, UALVP 56200 could have had a wingspan between 3.2 and 7.0 m. However, wingspan in pterosaurs is likely positively allometric to compensate for the cubic increase in mass for a linear increase in dimension, so that flight capability can be maintained. Therefore, the wingspan of UAVLP 56200 was probably towards the higher part of this range and may even have exceeded it. Because of the considerable error in these estimated body sizes, UALVP 56200 cannot be confidently associated with any of the size morphs proposed by Godfrey and Currie (2005), although the estimated range overlaps with their “intermediate” morph (6 m wingspan). In any case, it highlights the varying sizes of DPF azhdarchids, whether ontogimorphs or separate taxa. It is likely that differently sized azhdarchids in the DPF partitioned niches according to their size (Vremir et al. 2013).
Locomotion
Recent studies (Witton and Naish 2008, 2015; Naish and Witton 2017) have suggested that azhdarchids were pterosaurs adapted for increased terrestrial locomotion. They envision azhdarchids as “terrestrial stalkers”, using their tall frame and long neck to ambush unsuspecting prey. The prominent muscular attachment sites on UALVP 56200 further support this assertion. The origin of ITB occupies more than half the preacetabular length. Its rugose surface indicates that it was a relatively strong hip flexor. Bennett (2001a) noted that in Pteranodon, the origin of ITB is not demarcated in most specimens, and it was, therefore, probably not as well developed, which is expected in a mostly pelagic animal. The ITB inserted onto the cnemial crest of the tibia and would have functioned as a major limb extensor. The origins of ITC and IFM are also large for a pterosaur and would also have anchored sizable muscles on UALVP 56200. The expanded areas of the ITB, ITC, and IFM suggest that there were exceptionally powerful muscles for hip flexion and limb extension. Although it is conceivable and likely that more powerful limb extension aided in takeoff, previous work suggests that large pterosaurs were quadrupedal on land (Costa et al. 2014b) and, thus, may have relied on forelimb-dominated quadrupedal launch strategies (Habib 2008). Furthermore, assuming that other pterosaurs of similar size without increased pelvic musculature were capable of launch, it seems unlikely that takeoff was a strong selection pressure for increasing hindlimb musculature. Instead, it is more likely that the larger muscle mass and stronger hip flexion of UALVP 56200 was related to increased participation of the hindlimbs in locomotion. In terrestrial locomotion, strong limb extensors and hip flexors would have quickly propelled the hindlimb forward, possibly increasing stride speed. Despite the absence of most of the postacetabular process and associated flexor muscle attachment sites, strong retractors probably complemented the strong limb extensors.
The morphology of the acetabulum is also important for understanding locomotion, as recognized by many previous authors (Wellnhofer 1988; Bennett 2001b). Bennett (1990) noted that the acetabulum of the neoazhdarchian AMNH 22569 faces laterally, allowing the femur to be positioned below the pelvis. In UALVP 56200, the deep acetabulum faces ventrolaterally, consistent with a parasagittal gait. This is congruent with purported azhdarchid tracks (Haenamichnus) from Korea (Hwang et al. 2002), which indicate a relatively parasagittal gait in that pterosaur. Costa et al. (2014b) reconstructed a 10° upturn to the pelvis in the resting position of Anhanguera. In UALVP 56200, this arrangement would allow the femur to dissipate upwards force into the strongest part of the acetabulum. The dorsal shelf above the acetabulum of UALVP 56200 would have provided a strong weight-bearing surface to support the femur. The anterior border of the acetabulum is reinforced by the preacetabular tubercle, which would have helped disperse reactive forces when pressure was put on the limb while it was extended. These forces would be generated if the pelvis was rotated vertically as the animal reared up, or as the leg pushed off the substrate, but are unlikely to have been generated during flight. The large preacetabular tubercle may, therefore, have been an adaptation for countering increased pelvic load as weight is placed on the hindlimb. Together, the increased hindlimb musculature and structural reinforcement of the acetabulum likely indicate that azhdarchids engaged in more terrestrial locomotion involving the hindlimbs than other pterosaurs.
Conclusions
UALVP 56200 is the first recorded azhdarchid pelvis from North America and provides a wealth of new information. The anatomy of UALVP 56200 is suggestive of a relatively large pterosaur with well-developed hindlimb musculature. The ilium is extensively pneumatized, but the acetabulum is structurally reinforced. The strong musculature and reinforcement of the pelvis support previous assertions that azhdarchids are adapted for increased terrestrial locomotion.
Institutional abbreviations
AMNH, American Museum of Natural History; TMP, Royal Tyrrell Museum of Palaeontology; UALVP, University of Alberta Laboratory of Vertebrate Paleontology; YPM, Yale Peabody Museum.
Acknowledgements
GFF is funded by Vanier Canada, NSERC, Alberta Innovates, and the Dinosaur Research Institute. EMS is funded by the University of Southampton (GSNOC), an NSERC PGS-D, and a Sir James Lougheed Award of Distinction. PJC is supported by NSERC (grant #2016-04674). GFF thanks M Rhodes (University of Alberta) for useful discussions on pelvic musculature.
References
Averianov AO. 2014. Review of taxonomy, geographic distribution, and paleoenvironments of Azhdarchidae (Pterosauria). ZooKeys, 432: 1–107.
Behrensmeyer AK. 1978. Taphonomic and ecologic information from bone weathering. Paleobiology, 4(2): 150–162.
Bennett SC. 1990. A pterodactyloid pterosaur pelvis from the Santana Formation of Brazil: implications for terrestrial locomotion. Journal of Vertebrate Paleontology, 10(1): 80–85.
Bennett SC. 2001a. Osteology and functional morphology of the Late Cretaceous pterosaur Pteranodon: Part I. General description of osteology. Palaeontographica Abteilung A, 260: 1–112.
Bennett SC. 2001b. Osteology and functional morphology of the Late Cretaceous pterosaur Pteranodon. Part II. Size and functional morphology. Palaeontographica Abteilung A, 260: 113–153.
Brown CM, Evans DC, Campione NE, O’Brien LJ, and Eberth DA. 2013. Evidence for taphonomic size bias in the Dinosaur Park Formation (Campanian, Alberta), a model Mesozoic terrestrial alluvial-paralic system. Palaeogeography, Palaeoclimatology, Palaeoecology, 372: 108–122.
Buffetaut E, Grigorescu D, and Csiki Z. 2003. Giant azhdarchid pterosaurs from the terminal Cretaceous of Transylvania (western Romania). In Evolution and paleobiology of pterosaurs. Edited by E Buffetaut, and J-M Mazin. Geological Society, Mayfair, London, Special Publications 217. pp. 91–104.
Cai Z, and Wei F. 1994. Zhejiangopterus linhaiensis (Pterosauria) from the Upper Cretaceous of Linhai, Zhejiang, China. Vertebrata Palasiatica, 32: 181–194.
Carroll N. 2015. Reassignment of Montanazhdarcho minor as a non-azhdarchid member of the Azhdarchoidea. Journal of Vertebrate Paleontology, Programs and Abstracts, 2015: 104.
Chiappe LM, and Witmer LM. 2002. Mesozoic birds. University of California Press, Berkeley and Los Angeles, California.
Claessens LPAM, O’Connor PM, and Unwin DM. 2009. Respiratory evolution facilitated the origin of pterosaur flight and aerial gigantism. PLoS ONE, 4(2): e4497.
Coria RA, and Currie PJ. 2006. A new carcharodontosaurid (Dinosauria, Theropoda) from the Upper Cretaceous of Argentina. Geodiversitas, 28(1): 71–118.
Costa FR, Rocha-Barbosa O, and Kellner AWA. 2014a. Myological reconstruction of the pelvic girdle of Anhanguera piscator (Pterosauria: Pterodactyloidea) using three dimensional virtual animation. Revista Brasileira de Paleontologia, 17(1): 11–22.
Costa FR, Rocha-Barbosa O, and Kellner AWA. 2014b. A biomechanical approach on the optimal stance of Anhanguera piscator and its implications for pterosaur gait on land. Historical Biology, 26(5): 582–590.
Currie PJ, and Jacobsen AR. 1995. An azhdarchid pterosaur eaten by a velociraptorine theropod. Canadian Journal of Earth Sciences, 32: 922–925.
Currie PJ, and Russell DA. 1982. A giant pterosaur (Reptilia: Archosauria) from the Judith River (Oldman) Formation of Alberta. Canadian Journal of Earth Sciences, 19: 894–897.
Eberth DA. 2015. Origins of dinosaur bonebeds in the Cretaceous of Alberta, Canada. Canadian Journal of Earth Sciences, 52: 655–681.
Elgin RA, and Hone DWE. 2013. Pneumatization of an immature azhdarchoid pterosaur. Cretaceous Research, 45: 16–24.
Frigot R. 2017. Pelvic musculature of Vectidraco daisymorrisae and consequences for pterosaur locomotion. In New perspectives on pterosaur palaeobiology. Edited by DWE Hone, MP Witton, and DM Martill. Geological Society, Mayfair, London, Special Publications 455, 11 p.
Godfrey SJ, and Currie PJ. 2005. Pterosaurs. In Dinosaur Provincial Park: a spectacular ancient ecosystem revealed. Edited by PJ Currie, and EB Koppelhus. Indiana University Press, Bloomington, Indiana. pp. 292–311.
Habib MB. 2008. Comparative evidence for quadrupedal launch in pterosaurs. Zitteliana, B28: 161–168.
Habib MB. 2010. 10,000 miles: maximum range and soaring efficiency of azhdarchid pterosaurs. Journal of Paleontology, 30: 99A–100A.
Hocknull SA, White MA, Tischler TR, Cook AG, Calleja ND, Sloan T, et al. 2009. New mid-Cretaceous (latest Albian) dinosaurs from Winton, Queensland, Australia. PLoS ONE, 4(7): e6190.
Hwang K-G, Huh M, Lockley MG, Unwin DM, and Wright JL. 2002. New pterosaur tracks (Pteraichnidae) from the Late Cretaceous Uhangri Formation, southwestern Korea. Geology Magazine, 139(4): 421–435.
Hyder ES, Witton MP, and Martill DM. 2014. Evolution of the pterosaur pelvis. Acta Palaeontologica Polonica, 59(1): 109–124.
Kaup JJ, 1834. Versuch einer Eintheilung der Saugethiere in 6 Stämme und der Amphibien in 6 Ordnungen. Isis, 3: 311–315.
Kellner AWA, Campos DA, Sayão JM, Saraiva AAF, Rodrigues T, Oliveria G, et al. 2013. The largest flying reptile from Gondwana: a new specimen of Tropeognathus cf. T. mesembrinus Wellnhofer, 1987 (Pterodactyloidea, Anhangueridae) and other large pterosaurs from the Romualdo Formation, Lower Cretaceous, Brazil. Annals of the Brazilian Academy of Sciences, 85(1): 113–135.
Martin EG, and Palmer C. 2014. Air space proportion in pterosaur limb bones using computed tomography and its implications for previous estimates of pneumaticity. PLoS ONE, 9(5): e97159.
Molnar RE. 1987. A pterosaur pelvis from western Queensland, Australia. Alcheringa, 11: 87–94.
Naish D, Simpson M, and Dyke G. 2013. A new small-bodied azhdarchoid pterosaur from the Lower Cretaceous of England and its implications for pterosaur anatomy, diversity and phylogeny. PLoS ONE, 8(3): e58451.
Naish D, and Witton MP. 2017. Neck biomechanics indicate that giant Transylvanian azhdarchid pterosaurs were short-necked arch predators. PeerJ, 5: e2908.
Nessov LA. 1984. Pterosaurs and birds of the Late Cretaceous of Central Asia. Palaontologische Zeitschrift, 1: 47–57. [Russian].
O’Connor PM. 2006. Postcranial pneumaticity: an evaluation of soft-tissue influences on the postcranial skeleton and the reconstruction of pulmonary anatomy in archosaurs. Journal of Morphology, 267: 1199–1226.
O’Connor PM. 2009. Evolution of archosaurian body plans: skeletal adaptations of an air-sac-based breathing apparatus in birds and other archosaurs. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 311A: 629–646.
Padian K. 1984. A large pterodactyloid pterosaur from the Two Medicine Formation (Campanian) of Montana. Journal of Vertebrate Paleontology, 4(4): 516–524.
Padian K, and Smith M. 1992. New light on Late Cretaceous pterosaur material from Montana. Journal of Vertebrate Paleontology, 12(1): 87–92.
Padian K, de Ricqlès AJ, and Horner JR. 1995. Bone histology determines identification of a new fossil taxon of pterosaur (Reptilia: Archosauria). Comptes Rendu Academie de Sciences de Paris, 320: 77–84.
Plieninger F. 1901. Beiträge zur Kenntnis der Flugsaurier. Palaeontographica, 48: 65–90.
Sereno PC, Martinez RN, Wilson JA, Varricchio DJ, Alcober OA, and Larsson HCE. 2008. Evidence for avian intrathoracic air sacs in a new predatory dinosaur from Argentina. PLoS ONE, 3(9): e3303.
Sullivan RM, and Fowler DW. 2011. Navajodactylus boerei, n.gen., n.sp. (Pterosauria, ?Azhdarchidae) from the Upper Cretaceous Kirtland Formation (Upper Campanian) of New Mexico. New Mexico Museum of Natural History and Science Bulletin, 53: 393–404.
Unwin DM. 2003. On the phylogeny and evolutionary history of pterosaurs. In Evolution and palaeobiology of pterosaurs. Edited by E Buffetaut, and J-M Mazin. Geological Society of London, Special Publications 217, London, pp. 139–190.
Vremir M, Kellner AWA, Naish D, and Dyke GJ. 2013. A new azhdarchid pterosaur from the Late Cretaceous of the Transylvanian Basin, Romania: implications for azhdarchid diversity and distribution. PLoS ONE, 8(1): e54268.
Wellnhofer P. 1988. Terrestrial locomotion in pterosaurs. Historical Biology, 1: 3–16.
Witton MP, and Naish D. 2008. A reappraisal of azhdarchid pterosaur functional morphology and paleoecology. PLoS ONE, 3(5): e2271.
Witton MP, and Naish D. 2015. Azhdarchid pterosaurs: water-trawling pelican mimics or “terrestrial stalkers”? Acta Palaeontologica Polonica, 60(3): 651–660.
Supplementary material
Supplementary Material 1 (PDF / 1.02 MB)
- Download
- 1.02 MB
Supplementary Material 2 (PDF / 19.99 MB)
- Download
- 19.99 MB
Information & Authors
Information
Published In

FACETS
Volume 2 • Number 1 • January 2017
Pages: 559 - 574
Editor: David C. Evans
History
Received: 14 November 2016
Accepted: 19 May 2017
Version of record online: 11 July 2017
Corrected: 23 February 2018
Copyright
© 2017 Funston et al. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Data Availability Statement
All relevant data are within the paper and in the Supplementary Material.
Key Words
Sections
Subjects
Authors
Author Contributions
All conceived and designed the study.
All performed the experiments/collected the data.
All analyzed and interpreted the data.
All contributed resources.
All drafted or revised the manuscript.
Competing Interests
The authors have declared that no competing interests exist.
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Gregory F. Funston, Elizabeth Martin-Silverstone, and Philip J. Currie. 2017. The first pterosaur pelvic material from the Dinosaur Park Formation (Campanian) and implications for azhdarchid locomotion. FACETS.
2(): 559-574. https://doi.org/10.1139/facets-2016-0067
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
Cited by
1. Geochemical extraction of ceratopsian remains from ironstoneCitation for this article: Cross, E. G., Surette, C., & Matheson, C. (2023) Geochemical extraction of ceratopsian remains from ironstone.
Journal of Vertebrate Paleontology
. https://doi.org/10.1080/02724634.2023.2282650
2. Morphology and taxonomy of
Quetzalcoatlus
Lawson 1975 (Pterodactyloidea: Azhdarchoidea)
3. Correction: The first pterosaur pelvic material from the Dinosaur Park Formation (Campanian) and implications for azhdarchid locomotion
