Introduction
The prevalence of type 2 diabetes (T2D) has been steadily increasing worldwide, substantially contributing to health care costs (
Guariguata et al. 2014). In Canada, T2D is one of the most common chronic diseases. According to recent statistics, the prevalence of T2D in Canada has more than doubled between 2000 and 2010 (increasing from 1.2 to 2.4 million affected), and this trend is projected to continue (
Pelletier et al. 2012;
Canadian Diabetes Association 2015). The prevalence of T2D was 8.9% in 2015 and is estimated to reach 11.4% by 2025 (
Pelletier et al. 2012;
Canadian Diabetes Association 2015). The Canadian First Nations (FNs) population is experiencing rates of T2D of epidemic proportions. The prevalence of T2D in FNs communities is 3–5 times higher than that in the general Canadian population (
Young et al. 2000;
Dannenbaum et al. 2008;
Pelletier et al. 2012). In addition, an earlier age of diabetes onset, a greater severity of the disease, and higher rates of complications of T2D are observed in on-reserve FNs compared with non-Indigenous populations (
First Nations Information Governance Centre 2012). FNs women experience higher rates of T2D than FNs men, in contrast to the pattern observed in the general Canadian population (
Pelletier et al. 2012). This is thought to be because FNs women have higher rates of obesity than FNs men, and experience high rates of gestational diabetes, which increases the risk of T2D later in life (
Millar and Young 2003).
The rapid increase in the prevalence of T2D in FNs over the last four to five decades has been influenced by a variety of risk factors including genetic, sociocultural, environmental, and lifestyle factors (
Young et al. 2000). Historically, the diet of FNs was based on traditional foods harvested from the local natural environment. This traditional food consisted of wild meat, fish and bird species, plants, and berries acquired by traditional hunting, fishing, and gathering, contributing to the intake of essential nutrients as well as physical activity and the well-being of FNs (
Kuhnlein and Receveur 2007;
Jamieson et al. 2012). FNs have been undergoing rapid lifestyle and dietary transitions, moving from a traditional high-nutrient diet toward consuming store-bought energy-dense food that is associated with increased rates of obesity and T2D (
Kuhnlein et al. 2004).
T2D is associated with numerous complications including retinopathy, neuropathy, kidney disease, and cardiovascular disease, which may lead to disability and mortality (
Naqshbandi et al. 2008). A number of modifiable risk factors for T2D are well established including overweight and obesity, poor diet, sedentary lifestyle, and smoking. Among dietary factors, fish consumption and long-chain omega-3 fatty acids (n-3 FAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been postulated to prevent T2D. n-3 FAs intake showed a favorable effect on insulin sensitivity in animal models (
Fedor and Kelley 2009). Data from an ecological study demonstrated that the prevalence of T2D was low in regions where fish consumption was high (
Nkondjock and Receveur 2003). In addition, the beneficial effects of fish consumption and n-3 FAs intake on T2D are supported by studies conducted in northern Indigenous populations. Specifically, the Inuit population has lower rates of metabolic and cardiovascular diseases (
Deering et al. 2009) than the general populations of Canadians and FNs. This is attributed to the beneficial effect of the consumption of traditional food rich in n-3 FAs (
Dewailly et al. 2001), which resulted in higher concentrations of EPA and DHA in plasma and serum phospholipids (
Proust et al. 2014). The results of epidemiological studies in general populations have been inconsistent regarding the association between fish consumption and n-3 FAs and T2D prevalence. Several prospective cohort studies reported protective effects of fish consumption and n-3 FAs intake on the risk of T2D (
Patel et al. 2009;
Nanri et al. 2011;
Villegas et al. 2011), whereas some studies suggest positive (
Kaushik et al. 2009;
Djoussé et al. 2011) or no associations (
van Woudenbergh et al. 2009). Meta-analyses reported geographical differences in the associations between fish consumption, n-3 FAs, and the development of T2D; a protective association was observed in the populations of Asian countries, and a positive association was observed in the American population (
Muley et al. 2014).
Despite its beneficial attributes, fish can be a route of exposure to environmental contaminants such as persistent organic pollutants (POP), including polychlorinated biphenyls (PCB) and dichlorodiphenyldichloroethylene (DDE). POP are lipophilic compounds that persist in the environment and, therefore, bioaccumulate and biomagnify within living organisms such as fish and mammals (
Sobek et al. 2010;
Seabert et al. 2014). Several epidemiological studies have reported a positive association between diabetes and POP including PCB, DDE, and dioxins and dioxin-like chemicals (
Lee et al. 2006;
Codru et al. 2007;
Philibert et al. 2009). These findings are supported by an experimental study that showed a link between exposure to POP and insulin resistance, visceral obesity, and glucose intolerance (
Ibrahim et al. 2011). High concentrations of POP in blood have been reported for northern Indigenous populations (
Donaldson et al. 2010). Traditional food, in particular fish, is considered the major source of exposure to POP (
Seabert et al. 2014).
Given the increasing prevalence of T2D and potential exposure to contaminants through fish consumption in FNs, we aim to describe fish consumption patterns among First Nation adults in four Manitoba ecozones; to estimate n-3 FAs, PCB, and DDE intake from fish; and to explore the association between fish consumption and dietary DDE, PCB, and n-3 FAs intake and self-reported T2D in a representative sample of FNs adults living on reserves in Manitoba.
Methodology
Study population
We analyzed data from the First Nations Food, Nutrition and Environment Study (FNFNES) (
fnfnes.ca). FNFNES is a cross-sectional study designed to assess total diets, food-related exposure to contaminants, and food security status of FNs people living on reserves south of the 60th parallel across Canada (
Chan et al. 2012). FNFNES collected data from approximately 100 FNs communities across Canada. FNs communities were randomly sampled using a combined ecozone/cultural area framework. An ecozone is a large geographical region identified based on the distribution patterns of plants, animals, geographical characteristics, and climate (
ecozones.ca). “Culture areas” is a concept for identifying geographic areas within which Indigenous communities share a greater number of traits/cultural affinities than those outside the area (
Chan et al. 2012). The sampling was done in three stages: primary sampling was performed with the random selection of communities within each ecozone; secondary sampling was conducted with the random sampling of 125 households within each selected community; and tertiary sampling wherein one adult in each household who was self-identified as being a FNs person aged 19 and older living on a reserve was asked to participate in the study. Sample weights were calculated to permit inferences from persons included in the sample about the total population from which they were drawn, and to obtain data that reflect estimates of the population totals. Using sample weight minimizes biases arising from differences between participating and non-participating persons (
Maletta and Aires 2007). The design weight was adjusted based on the assumption that the information obtained from responding communities is representative of both responding and non-responding communities. Assuming that non-response is not related to the topic of the study (missing at random), a non-response adjustment factor was calculated within each stratum. The Bootstrap method was adopted for assessing the sampling error of the estimates produced for this study (
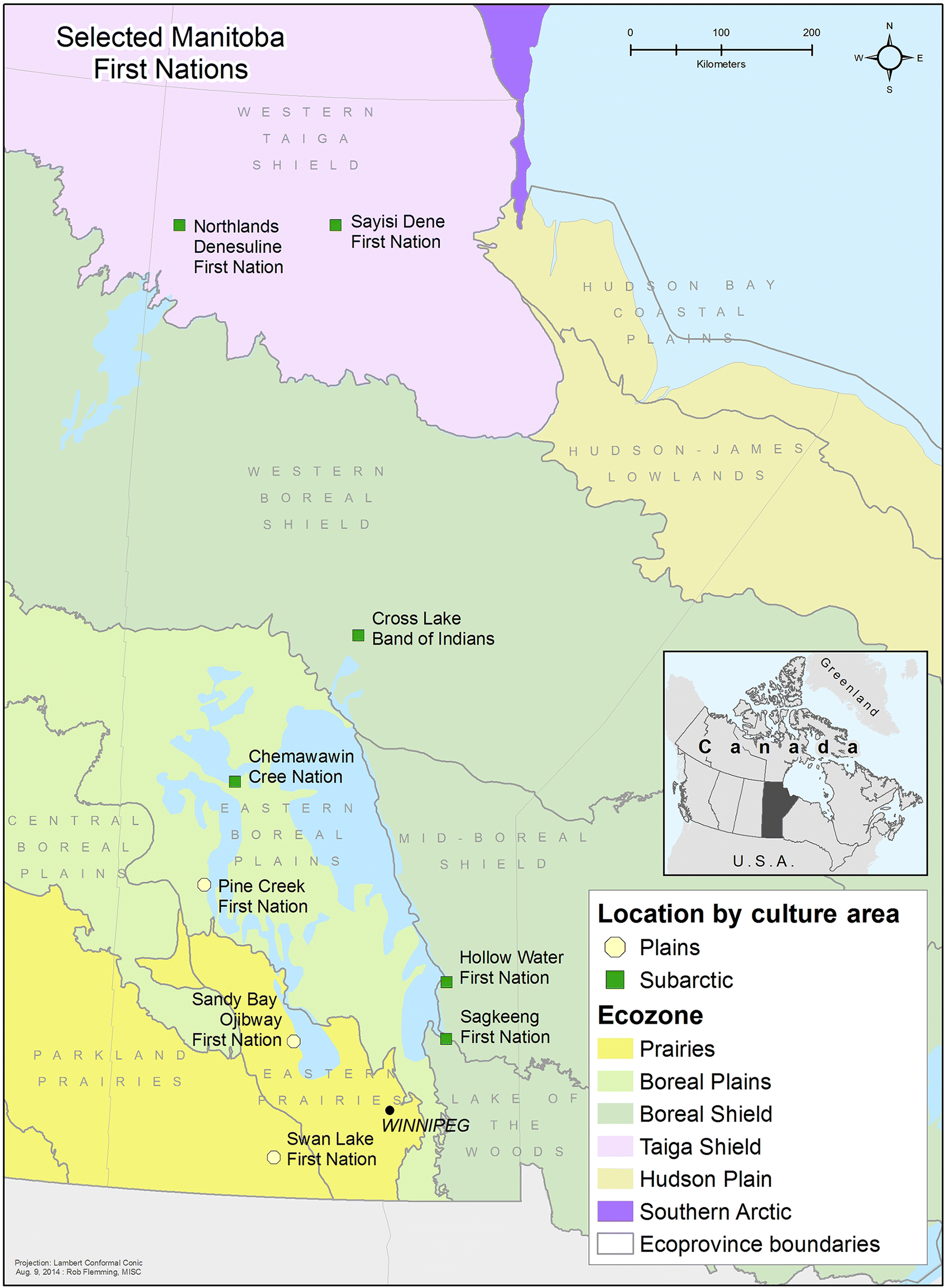
Chan et al. 2012). The current study included data collected in 2010 from nine Manitoba FNs communities across four ecozones: (1) Prairies/Plains, Prairies/Subarctic, (2) Boreal Plains, Plains/Subarctic, (3) Boreal Shield/Subarctic, and (4) Taiga Shield/Subarctic (
Fig. 1). The overall participation rate was 82%. In total, 706 participants (477 women and 229 men) aged 19 years and over were interviewed for this study.
Ethics
Individual participation in the project was voluntary and based on informed written consent after an oral and written explanation of each project component. This survey was conducted following the “Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans” and in particular Chapter 9, pertaining to research involving the FNs, Inuit, and Métis Peoples of Canada. The study was approved by the ethics review boards at the University of Northern British Columbia, the University of Ottawa, the Université de Montreal, and Health Canada.
Data collection
Data were collected using an “in-person” household interview. A trained interviewer completed the survey with the participants; the survey was conducted in the participant’s household and consisted of several questionnaires including a 24 h recall questionnaire; a traditional food frequency questionnaire (FFQ); and a socio-demographic, health, and lifestyle (SHL) questionnaire. The multi-pass technique with three stages was used to record the 24 h recall. In the first stage, a quick list of all foods and beverages consumed during the prior 24 h was built; in the second, a detailed description of the consumed foods and beverages (brands, amount eaten, etc.) was recorded; in the third, the recall was reviewed (
Raper et al. 2004). A subsample of 20% of the participants were invited to complete a second 24 h recall for later analysis using the SIDE SAS (version 9.2) sub-routine to partially adjust for intra-individual variations that allows for a better approximation of the usual diet. To estimate corresponding intake quantities, three-dimensional food and beverage models were used. The FFQ was developed based on a comprehensive list of locally harvested traditional foods that was representative for each participating community. In Manitoba, the FFQ consisted of 153 traditional food items including 30 fish species. All participants provided information on traditional food consumption during the four seasons of the past year. Age- and gender-specific portion sizes of each traditional food item were determined from the 24 h recall data.
The SHL Questionnaire collected information on socio-demographic characteristics, lifestyle choices, and self-perceived health status. Body mass index (BMI) was calculated as weight (in kilograms) divided by the square of height (in meters). BMI categories were as follows: normal weight was characterized as a BMI <25 kg/m2, overweight was categorized as a BMI of 25 kg/m2 or higher but <30 kg/m2, and obesity was categorized as a BMI ≥30 kg/m2. Physical activity data were self-reported, and the study participants were asked to describe their physical activities based on the provided descriptions, as follows: (a) I am usually sitting and do not walk around very much; (b) I stand or walk around quite a lot, but I do not have to carry or lift things very often; (c) I usually lift or carry light loads or I have to climb stairs or walk up hills often; (d) I do heavy work or carry heavy loads.
Assessment of T2D
T2D was defined as a self-reported diagnosis of T2D via the SHL questionnaire. In addition, information on the type of diabetes (type 1, 2) and the onset of the disease (i.e., how many years ago individuals were diagnosed with T2D) was collected. In this study, only those participants who reported being diagnosed with T2D were coded as cases of T2D (
Huerta et al. 2009;
Schneider et al. 2012). Participants with type 1 diabetes were grouped with those who did not have diabetes in the analysis.
Self-reported estimates of T2D in the FNFNES survey were validated by comparing the estimates with those reported by the Regional Health Survey (RHS), based on data collected between 2008 and 2010. The RHS is the only FNs-governed national health survey in Canada (
AMC 2012). The weighted prevalence of diabetes reported by the FNFNES in Manitoba was 22%, which was similar to the 21% reported by the RHS in Manitoba (
AMC 2012).
Fish sampling for contaminant content
Fish samples collected for contaminant analyses were representative of all fish species consumed by members in each community. The fish sampling strategy was as follows: each community was to identify the most commonly consumed fish, identify the fish species that are of greatest concern from a nutrition and environmental perspective, and identify those fish species known to accumulate higher concentrations of contaminants (
Chan et al. 2012). A total of eight fish species (bass, perch, pike, sturgeon, suckers, trout, walleye, and whitefish) were collected during the fall of 2010 (September through November). The collected fish samples were analyzed for POP including total PCB and DDE at Maxxam Analytics, formerly CANTEST, in Burnaby, British Columbia.
Replicate samples (n = 3–5) from each fish species from each community were homogenized to provide a homogeneous pooled sample for subsequent analysis. A total of 41 pooled fish samples were analyzed. If required, a moisture value was determined gravimetrically after drying a portion of the blended sample at 105 °C overnight. A modified US Environmental Protection Agency 1668C Method was used for extraction. Briefly, six grams of tissue was spiked with C-13 and deuterium-labeled extraction internal standard and homogenized in dicloromethane (DCM), followed by Soxhlet extraction and filtered through anhydrous sodium sulphate. The extract was evaporated to 6 mL, and 5 mL of it was injected onto the gel permeation chromatography column where a fraction of the eluent was collected, concentrated, and solvent exchanged to acetone:hexane (1:1). Further cleanup was performed by eluting this extract through multilayered silica gel and then alumina column chromatography. The final extract was concentrated and solvent exchanged to isooctane. Analysis was performed for DDE and PCB using gas chromatography mass spectrometry (GC-MS) in selective ion monitoring mode with an EI source (Agilent 7890 GC coupled to a 5975 mass spectrometer, Agilent Technologies, Santa Clara, California) based on US Environmental Protection Agency Method 680. All results were calculated by isotope dilution. Laboratory control samples and blank samples were measured for quality assurance/quality control. A total of 27 PCB congeners (PCB-1, 3, 4, 15, 19, 37, 54, 77, 81, 104, 105, 114, 118, 123, 126, 155, 156, 157, 167, 169, 188, 189, 202, 205, 206, 208, and 209) were measured and the sum was used to estimate total PCB concentrations.
Estimation of fish, dietary POP (DDE, PCB), and long-chain omega-3 FAs intake
Daily fish intake (g/d) was estimated using data from the FFQ by totaling the number of days in the past four seasons when fish consumption was reported (for each fish species). Then, the total number of days of reported fish intake was multiplied by the age- and gender-specific portion size of fish (g) estimated from dietary data generated by the 24 h recalls for each age and sex group, and divided by 360 d (in this study, a year included four seasons of 90 d each).
Total dietary PCB and DDE intake was calculated as follows: first, the amounts of PCB and DDE in each fish species (ng/g) were multiplied by the total amount of each fish species eaten per day (in grams); second, the amounts of PCB and DDE from all fish species consumed per day were totaled (g/d) and divided by the body weight of each participant (ng/kg of body weight per day).
Community-specific data of POP content in fish species were used to calculate total PCB and DDE intake for each participant. Ecozone-specific concentrations of DDE and PCB content in fish species were used for the communities that were located within a particular ecozone if community-specific data were not available. Validation of the dietary assessments was performed through correlation analysis between mercury exposure from traditional food estimated using the FFQ and mercury concentrations in hair measured in FNs participants. Dietary mercury intake was correlated with mercury levels in hair.
The Canadian File of Nutrients (
Health Canada 2015) was used to determine the concentrations of n-3 FAs in all fish species reported by FNs in Manitoba. In this analysis, n-3 FAs refers to the combined EPA and DHA intake from consuming fish. The data are expressed as mg of EPA + DHA per gram of raw fish (mg/g). Raw values were used as fish tissue EPA + DHA concentrations may vary with cooking method. Using the raw values allows the comparison of our results with other studies. The total amount of EPA + DHA consumed by each participant based on fish intake (g/d) was calculated as follows:
The most consumed fish species in Manitoba were walleye, northern pike, lake whitefish, and lake trout. To define fish consumption patterns, the consumption of commonly consumed fish species as well as their contribution to dietary n-3 FAs, DDE, and PCB intake were described across four ecozones in Manitoba.
Statistical analyses
Descriptive statistics include the calculation of means with standard deviations (SDs) for continuous variables, and proportions for categorical variables. Medians (interquartile range) were calculated for skewed variables. Geometric means (95% CI) were estimated for dietary DDE and PCB intake. Student’s
t tests, analysis of variance (ANOVA), and χ
2 tests were used to test if differences between groups were statistically significant. Sub-group stratified analyses by gender, age groups, and ecozones were performed to describe the study population by diabetes status. Fish consumption was categorized into four groups: no or <1/month, 1/month, 2–3/month, and ≥1/week to examine dose–response relationship between fish consumption and T2D prevalence. We chose 150 g as the fish portion size in this analysis because this amount represents two servings (75 g each) of fish per week as recommended by Canada’s Food Guide – FNs, Inuit and Métis (
Health Canada 2007). Pearson’s correlation coefficients were investigated among all continuous predictors. Collinearity was observed between fish consumption with n-3 FAs intake, and n-3 FAs intake with PCB and DDE.
Simple logistic regression models were used to explore the relationships between an outcome (T2D) and each individual primary predictor of interest (fish intake (categorical), PCB, DDE (continuous), n-3 FAs (continuous and categorical)), as well as all potential confounders (age, gender, BMI, total energy intake (obtained from 24 h recall), smoking, physical activity, household size (number of people per household), and years of education).
Multiple logistic regression models adjusted for potential covariates were developed to investigate the associations between each of the following factors and the prevalence of T2D: total fish intake, dietary POP (PCB and DDE), and n-3 FAs (EPA + DHA) intake. Independent variables such as DDE, PCB, and n-3 FAs did not fit a normal distribution and were normalized using the natural logarithmic function. POP concentrations below the limit of quantification were imputed with half the limit of detection (LOD) of PCB and DDE to avoid errors in the analysis. The LOD of DDE is 0.0005 μg/g and the LOD of PCB is 0.0003 μg/g (
Chan et al. 2012).
Three models were developed to analyze the association between the frequency of fish consumption (four categories) and T2D prevalence. Adjustment variables were selected based on well-established risk factors for T2D reported in the literature including age, sex, body mass index (BMI), smoking, physical activity, total energy intake, education, and household size. Covariates were added to the models gradually to evaluate their relative contribution to the association between the predictors of interest and the outcome variable. Model 1 was adjusted for age, gender, and BMI; Model 2 was additionally adjusted for physical activity, total energy intake, smoking, household size, and education; Model 3 was controlled for covariates in Model 2 and DDE/PCB intake to eliminate their possible effect on T2D. Age, BMI, energy intake, number of people per household, and years of education were treated as continuous variables, whereas gender, smoking, and physical activity were categorical variables. Fat, saturated fat, carbohydrate, fruit, and vegetable intake were also considered as covariates but were removed from the models because they did not change the value of the association between T2D and fish consumption.
Logistic regression models were developed to analyze the relationships between log-transformed dietary DDE, PCB, and n-3 FAs intake and T2D prevalence, both overall and stratified by age group (<45 years; ≥45 years). We chose the age of 45 as the division between the groups as it was close to the median age of the study population, across different regions of Canada.
The models were tested for interactions between predictors (
x 1,
x 2) by including the product “
x 1 ×
x 2” as an additional predictor in a model containing
x 1 and
x 2:
No interaction was detected.
Results with a p-value of <0.05 were considered statistically significant. All statistical analyses were performed using weighting variables to obtain representative estimates at the regional level. JMP version 11 and R software were used to conduct the statistical analyses.
Sensitivity analyses were conducted to explore differences in dietary characteristics and physical activity between responders who were recently diagnosed with T2D (≤5 years ago) and those who have had T2D for a long period of time (>5 years) (
Table S1). In addition, analyses of dietary and lifestyle practice by dieting status were performed between participants with and without T2D (
Table S2).
Results
This study included 706 participants (477 women and 229 men). Participant ages ranged from 19 to 96 years, with a mean age of 43.4 years. Descriptive characteristics of Manitoba participants by diabetes status are presented in
Table 1. The prevalence of diagnosed T2D was 22%. When standardized to the 2015 Canadian population, the prevalence of T2D was 28.4%. Participants with T2D were more likely to be older, male, to have a higher BMI, and to be less physically active and less educated than those without T2D. Some differences in dietary intake were noted between diabetic and non-diabetic participants: individuals with T2D reported significantly lower intakes of total energy, total fat, and carbohydrate. Fruit and vegetable consumption was very low among all Manitoba participants (9.9 g/d), and was comparable between diabetic (10.2 g/d) and non-diabetic participants (9.8 g/d).
Overall, 82% of participants reported the consumption of fish at least once in the prior year. The mean intake of total fish in the study population was 9.2 g/d (median 2.7 g/d; range 0–230 g/d). Individuals with T2D consumed 11.6 g/d of fish, whereas those without T2D reported eating 8.5 g/d of fish. The intake of long-chain n-3 FAs (EPA + DHA) was slightly higher in participants with T2D than those without the disease (69 mg/d vs. 42 g/d, respectively). The mean intake of DDE and PCB was very low in both groups. Significant differences in fish consumption were found between men and women (16.7 g/d vs. 5.2 g/d, respectively).
Table 2 presents the characteristics of participants by age group (<45 years vs. ≥45years). The prevalence of T2D was higher in older responders (36.8%) than younger participants (10%). Mean BMI was comparable between the two groups, whereas physical activity was lower in older subjects. In addition, individuals aged 45 years and over reported lower total energy, fat, and carbohydrate intake, but greater fruit, vegetable, and fish consumption.
Table 3 shows the characteristics of the participants by frequency of fish intake. More frequent fish consumption was associated with lower prevalence of self-reported T2D, older age, being male, and higher physical activity.
Description of fish consumption patterns and long-chain n-3 FAs and DDE/PCB intake in four Manitoba ecozones (Prairies/Plains, Boreal Plains, Boreal Shield, and Taiga Shield) are summarized in
Table 4. Overall, the consumption of 30 different types of fish was reported by Manitoba FNs participants. The most consumed fish species across all Manitoba ecozones were walleye, lake whitefish, northern pike, and lake trout, which constituted 78% of the total fish intake. Geographical differences in the amount and type of fish consumed were noted in four ecozones. The highest total fish intake was reported by participants living in the northern Taiga Shield/Subarctic ecozone with a mean intake of 25.6 g/d (median, 13.5 g/d) compared with only 11.4 g/d (median, 3.7 g/d) in the Boreal Shield, 5.8 g/d (median, 2.2 g/d) in the Prairies/Plains, and 5.9 g/d (median, 3.3 g/d) in the Boreal Plains. Walleye was the most consumed fish species of all fish consumed, with the proportion of consumption varying from 60% to 69% across four ecozones. The lowest total fish intake and the greatest number of consumed fish species were reported by participants in the Prairies/Plains ecozone. Lake trout was the primary contributor to DDE and PCB intake. Nevertheless, lake trout and lake whitefish were the main sources of EPA and DHA. There were differences in the prevalence of T2D across the Manitoba ecozones. In the Taiga Shield, only 5% of individuals reported being diagnosed with T2D, whereas in the other three regions reported prevalence ranged from 18% to 31%. The mean BMI, physical activity, and total energy intake were comparable among participants across the four Manitoba ecozones.
Table 5 presents the multiple logistic regression analysis of the association between the frequency of fish consumption and T2D prevalence. Dose–response associations were observed across the frequency of fish intake and T2D prevalence. Model 1 was adjusted for age, gender, and BMI only. Model 2 was additionally adjusted for other covariates such as physical activity (categorical), total energy intake (continuous), smoking (yes/no), household size (continuous), and years of education (continuous). Model 3 was further controlled for DDE/PCB intake to examine whether the relationship between fish intake and T2D prevalence was mediated by DDE and PCB in fish. The results from all the models showed statistically significant inverse associations between fish consumption of 2–3 portions per month with an odds ratio (OR) = 0.51 (95% CI: 0.28–0.91) and ≥1/week with an OR = 0.40 (95% CI: 0.19–0.82) and T2D prevalence when compared with no consumption or consumption of less than one portion of fish per month.
Multiple logistic regressions of log-transformed DDE, PCB, and EPA + DHA intakes with the prevalence of T2D both overall and stratified by age group are presented in
Table 6. DDE, PCB, and EPA + DHA variables were not normally distributed, and so were normalized using the natural logarithmic function. Two models were developed. Model 1 was adjusted for potential covariates: age, BMI, gender, energy intake, physical activity, smoking, household size, and years of education. In Model 2, DDE and PCB were additionally adjusted for EPA + DHA intake, whereas EPA + DHA was controlled for DDE/PCB intake. There were no associations between DDE and PCB intake with T2D prevalence in both the overall and stratified by age group analyses. However, an inverse association between EPA + DHA intake and T2D prevalence was found among younger participants in the model controlled for DDE/PCB intake, and in both models among older individuals. When EPA + DHA intake was stratified by tertiles (
Table 7), the highest intake of EPA + DHA showed statistically significant protective effects on T2D prevalence with OR = 0.48 (95% CI: 0.30–0.77) compared with the reference group.
Table 8 presents concentrations of POP and n-3 FAs (EPA + DHA) in the four most consumed fish species in Manitoba. The concentration of DDE and PCB was not detected in walleye across the four Manitoba ecozones. In northern pike, PCB and DDE were detected only in the Boreal Shield. Lake whitefish contained low concentrations of DDE ranging from 0.65 to 1.61 ng/g, and PCB ranging from 0.17 to 0.33 ng/g. Lake trout had the highest POP concentrations across the four regions compared with other species (DDE: 7.65–15.8 ng/g; PCB: 7.41–11.06 ng/g). There were no statistically significant differences in PCB and DDE concentrations in the four most consumed fish species (ANOVA,
p = 0.99 for PCB, and
p = 0.94 for DDE) among the four ecozones. For n-3 FAs content, whitefish and lake trout had significantly higher concentrations of EPA + DHA (1.24 g/100 g and 0.84 g/100 g, respectively) compared with walleye (0.31 g/100 g) and northern pike (0.27 g/100 g of fish).
In addition, we assessed dietary and lifestyle behavior in individuals with and without T2D to examine if participants diagnosed with T2D tend to change their diets. The first sensitivity analysis aimed to compare diet and lifestyle practice between participants recently diagnosed with T2D (0–5 years) and those who have T2D for a long period of time (>5 years) (
Table S1). The analysis showed no statistically significant differences in physical activity, macronutrient intakes, and fish consumption between the two groups.
The second sensitivity analysis explored diet and lifestyle in participants with and without T2D, which may be associated with self-reported dieting status (
Table S2). The analysis revealed that there were no differences in macronutrient intakes between individuals with and without T2D. However, dieting participants had higher mean BMI, reported a slightly lower smoking rate, and higher physical activity compared with non-dieting subjects. Fish and associated n-3 FA consumption was comparable between the two groups.
Discussion
The prevalence of self-reported T2D in Manitoba FNs adults aged 19 years and older was 22%, which is similar to the estimate reported by the Manitoba First Nation RHS (21%) in individuals aged ≥18 years (
AMC 2012). These rates are more than three times higher than those in the general Canadian population (6.8% in individuals aged ≥20 years) (
Pelletier et al. 2012). In this study, more men than women reported having T2D (26% vs. 20%). The average amount of traditional food consumed by the participants of this study was 45 g/person per day, and 82% of participants reported eating fish (
Chan et al. 2012).
In this cross-sectional study, a dose–response relationship between fish intake and self-reported T2D was found. Statistically significant inverse associations between the frequency of fish consumption and self-reported T2D were observed in the multivariable-adjusted models when compared with no fish intake or less that one portion per month. Fish intake of one portion per week and more may decrease the OR for T2D by 60%. Similarly, the highest intake of n-3 FAs was associated with a 52% decrease in T2D prevalence compared with no n-3 FAs intake. The stronger protective effect of fish consumption on T2D prevalence than that of n-3 FAs intake might be attributed to other nutrients contained in fish such as protein and vitamin D, which have been reported to decrease the risk of T2D by a favorable effect on glucose metabolism (
Pittas et al. 2007). The protective effect of n-3 FAs was also found in the analyses stratified by age group (>45 years vs. ≥45 years), particularly in the models adjusted for DDE and PCB intakes.
Our findings are consistent with a number of previous population-based prospective cohort studies on the relationships between fish consumption and n-3 FAs intake on the one hand and T2D prevalence on the other, conducted among different populations (
Patel et al. 2009;
Nanri et al. 2011;
Villegas et al. 2011).
Patel et al. (2009) reported that consumption of one or more portion of fish per week decreased the OR for diabetes by about 25% compared with less than one portion per week in analyses adjusted for a number of risk factors for diabetes.
Nanri et al. (2011) found that fish intake in men was significantly associated with a decreased risk of T2D with the OR values of T2D prevalence for the highest compared with the lowest quartile of intake being 0.73 (95% CI: 0.54–1.00) for total fish intake and 0.68 (95% CI: 0.5–0.92) for small and medium fish.
Villegas et al. (2011) found that the relative risks for quintiles of fish intake were 1.00, 0.96, 0.84, 0.8, and 0.89 (
p = 0.003). Recent meta-analyses of prospective studies found that in the overall analysis, fish consumption and dietary n-3 FAs intake were not associated with lower risk of T2D (
Wallin et al. 2012;
Wu et al. 2012;
Zheng et al. 2012). However, geographical differences in the associations were demonstrated with a protective effect of fish and n-3 FAs consumption on T2D in Asian countries (
Nanri et al. 2011;
Villegas et al. 2011) and positive associations in the US population (
Kaushik et al. 2009). This heterogeneity may originate from several sources including genetic differences or gene–diet interactions (
Dedoussis et al. 2007;
Lee et al. 2011). The heterogeneity may also originate from differences in the type and amount of fish consumed as well as the concentrations of contaminants in fish species that were not considered in previous studies (
Lee and Jacobs 2010). In fact,
Wallin et al. (2015) reported that dietary contaminants in fish may influence the relationship between fish consumption and T2D prevalence. Limited data on the association between fish consumption and T2D prevalence are available for the Indigenous population. A cross-sectional study in Canadian FNs communities reported that the consumption of whitefish and trout may have reduced the risk of diabetes, whereas exposure to contaminants (DDE, PCB) increased the risk of T2D (
Philibert et al. 2009).
The positive associations between n-3 FAs intake and T2D prevalence could reflect beneficial effects on dyslipidemia characterized by low HDL-cholesterol and high triglyceride levels. There is evidence that n-3 FAs can decrease triglyceride levels and improve glucose tolerance and insulin sensitivity (
Ebbesson et al. 2005), but have no effect on HDL-cholesterol levels (
Hartweg et al. 2009).
Tørris et al. (2014,
2017) reported that fish consumption was associated with a lower risk of metabolic syndrome. Experimental studies reported that fish or fish oil supplements may improve insulin secretion and insulin sensitivity (
Akinkuolie et al. 2011).
Dietary DDE and PCB intake showed no association with T2D prevalence in the model controlling for several covariates; however, there were positive, but not statistically significant, associations in the models controlling for EPA + DHA intake in the total population and older individuals. Several previous studies have suggested a positive association between serum DDE and PCB concentrations and T2D prevalence (
Codru et al. 2007;
Philibert et al. 2009;
Turyk et al. 2009). Statistically significant positive associations between dietary DDE and PCB intake and T2D prevalence were confirmed in Ontario FNs where dietary POP exposure was higher than in Manitoba FNs (
Marushka et al. 2017). In the current study, the weak association between exposure to DDE and PCB and T2D prevalence may be explained by very low dietary DDE and PCB intake via fish; however, it was slightly higher among older participants than younger ones as they consumed higher amounts of fish.
The percentage of participants consuming fish differed across Manitoba regions depending on variety and availability of fish species. For example, 90%–94% of participants reported eating fish at least once in the prior year in the Boreal Plains, Plains/Subarctic, and Taiga Shield/Subarctic ecozones, in contrast with 80% of responders from the Boreal Shield/Subarctic ecozone and only 68% of participants living in the Prairies/Plains and Prairies/Subarctic ecozones. Even though most of the participants reported eating fish in the prior year, the daily mean intake of fish was estimated to be relatively low except in the Taiga Shield/Subarctic region. Walleye, northern pike, lake whitefish, and lake trout were the most consumed species among 30 types of fish, constituting almost 80% of the total fish intake.
Regional differences in the prevalence of T2D were found across four Manitoba ecozones, with the lowest observed T2D rate in the Taiga Shield/Subarctic region (5%). Dietary characteristics and lifestyle practice were analyzed and compared among individuals living in the four Manitoba regions. No statistically significant differences in dietary intake were observed; however, participants living in the Taiga Shield/Subarctic region consumed much more fish than those from the other regions (25.6 g/d vs. 11.4 g/d, 5.8 g/d, 5.9 g/d). In addition, they reported a higher consumption of whitefish and lake trout, which contain high levels of n-3 FAs.
The DDE and PCB concentrations varied among the four most consumed fish species. DDE and PCB were not detectable in walleye and northern pike, whereas lake trout had the highest concentrations. Elevated concentrations of POP in lake trout were also documented in other studies (
McGoldrick and Murphy 2015). The concentrations of DDE and PCB in fish species were comparable across four Manitoba ecozones.
This study has several limitations. As the design of our study is cross-sectional, we cannot state the causal relationship between fish and n-3 FAs consumption and T2D prevalence. Second, the reliance on self-reporting for the calculation of the prevalence of T2D could have resulted in an underestimation. We validated our estimates of T2D prevalence by comparing them with the self-reports presented by the RHS, which is a representative study for FNs living on reserves conducted over the same period of time (2008–2010) as our data collection. The RHS collected more comprehensive information on T2D prevalence that included data on the type of treatment people used to control their diabetes, how frequently people checked their blood sugar levels, complications of diabetes experienced, whether individuals adopted a healthier lifestyle including diet and exercise, attendance of a diabetes clinic, and getting diabetes education. A study among Cree FNs living in northern Quebec reported that 4.5% of participants had undiagnosed diabetes based on glucose levels that indicates diabetes (≥7 mmol/L), but no mention of diabetes in their medical charts (
Bobet 2013). Third, self-reported dietary intake estimates may lead to some degree of measurement error of the intake level. Some previous studies reported that overweight and obese individuals tend to underestimate their energy, fat, and carbohydrate intake (
Heitmann and Lissner 1995), whereas other scientists reported that both obese and non-obese subjects tend to underreport between-meal snack foods (
Poppitt et al. 1998).
Bailey et al. (2007) reported that weight status, education, and smoking status are characteristics consistently associated with underreporting. The authors suggested that these factors should be used to control for errors in statistical models when examining relationships with diet and health (
Bailey et al. 2007). In the current study, the results were controlled for lifestyle confounders including total energy intake, body mass index, physical activity, smoking, education level, and crowding. Other potential covariates such as dietary fat, saturated fat, carbohydrate, fruit, and vegetable intake were also considered. However, because they did not change the estimates of the association between T2D prevalence and fish consumption, they were not included in the models. Further, lifestyle and dietary habits associated with both fish consumption and T2D prevalence may influence the results. To examine differences in dietary and lifestyle behavior between diabetic and non-diabetic individuals, several sensitivity analyses were performed. The analyses revealed that no statistical differences in habits as well as fish consumption, dietary n-3 FAs, and POP intake were found (
Supplemental Material 1). Finally, physical activity level was self-reported and therefore may not be entirely accurate. Finally, we estimated PCB and DDE exposure from fish consumption and so the total PCB and DDE intake may be even higher. Future studies may need to verify this using biomonitoring data. To our knowledge, this is the first study investigating the relationships between fish consumption, dietary intake of n-3 FAs and POP, and T2D prevalence in Manitoba FNs living on reserves. The strength of our study is a large sample size, which is representative of all FNs adults living on reserves in Manitoba. POP concentrations in locally harvested fish were measured in this study. The individual total dietary PCB and DDE intake was calculated based on community-specific data of POP content in fish species. Also, we were able to control our results for a number of risk factors for T2D such as age, gender, BMI, smoking, physical activity, energy intake, and education level. Several epidemiological studies reported a positive association between n-3 FAs serum levels and T2D prevalence (
Virtanen et al. 2014). We observed the same positive relationship between dietary n-3 FAs intake and T2D prevalence which is useful in developing a dietary advisory for fish consumption.