Introduction
With a rising global population that relies on a broad range of natural resources, humans increasingly impact marine environments (
Halpern et al. 2008), leading to large and rapid declines in marine biodiversity across the globe (
Johnson et al. 2017). To help prevent, or at least slow down this decline, conservation actions are taking place from local to international levels. Amongst those actions, marine protected areas (MPAs) are now widely considered as the cornerstone of most national marine conservation strategies. While MPAs can result in a broad range of benefits that extend beyond nature conservation (e.g., fisheries spill-over effect, and various ecosystem services), in this paper we adopt the definition from the International Union for the Conservation of Nature that states that the primary objective of any MPA should be nature conservation. In the last decade, the number and global coverage of MPAs increased rapidly, resulting largely from commitments by nations to meet international conservation targets, such as the Convention on Biological Diversity Aichi targets (
CBD 2011) and the United Nations sustainable development goals. This increase in the creation of new MPAs brings to light growing conflicts between society’s dependence on natural resources and the need to protect nature.
While MPAs have been shown to be effective at preventing the decline of marine biodiversity, individual MPA effectiveness varies and depends on many factors that include their size, level of protection, location, and successful funding and enforcement (e.g.,
Edgar et al. 2014). Socio-economic criteria have been recognized as fundamental determinants in the success of MPAs (
Mascia 2003). Stakeholder engagement, made possible by involving ocean users at different stages of the MPA planning process, is deemed critical to MPA design and management (
Jessen et al. 2011), helping to increase the acceptance of the MPA by local stakeholders (
Garcia-Charton et al. 2000;
Dehens and Fanning 2018), and hence increasing stakeholders’ support for the MPA (
Kessler 2004;
Pomeroy and Douvere 2008;
Ruiz-Frau et al. 2015). Meeting conservation priorities at an acceptable socio-economic cost is challenging (
Allison et al. 1998;
Devillers et al. 2015), and accommodating too many requests from stakeholders (e.g., allowing some types of activities, changing the MPA boundaries) risks compromising the MPA ability to reach its desired conservation benefits (
Kessler 2004). While some MPAs successfully balance ecosystem health and human well-being, harder trade-offs are in many cases required and can lower ecological effectiveness of MPAs (
Leslie and McLeod 2007). While any MPA planning involves some level of trade-offs, the question becomes at what point those compromises risk creating an MPA that would be ineffective in reaching its conservation outcomes.
Business interests, and the pressures exerted on the governance systems for resolving stakeholder concerns, have been shown in some cases to undermine MPA design process, and in turn, the effectiveness of MPAs, particularly in the case of weaker stakeholder support (
Oracion et al. 2005) or low institutional capacity (
Jameson et al. 2002). While the literature documents many benefits resulting from stakeholder involvement in MPA design processes (
Chess and Purcell 1999;
Chess 2000), negative consequences are rarely reported and should be better documented.
This study, which uses the Canadian context as an example, aims at better understanding how stakeholder involvement and management decisions guiding an MPA design process can negatively impact the conservation potential of an MPA.
MPA establishment in Canada started in 1997 with the
Oceans Act legislation (
Minister of Justice of Canada 1997), resulting in the creation of 13 MPAs from 2003 to 2019. Stakeholder engagement was central to the establishment of those MPAs, with some MPA design processes initiated at the request of local groups interested in protecting fish populations important to the local industry (e.g., Gilbert Bay and Eastport MPAs in the province of Newfoundland and Labrador (NL)). Under Canada’s
Oceans Act, sites of interest for a future MPA are first identified officially as areas of interest (AOI). Planning for those sites then follows a process that typically takes 7–8 years on average before the MPA is officially created.
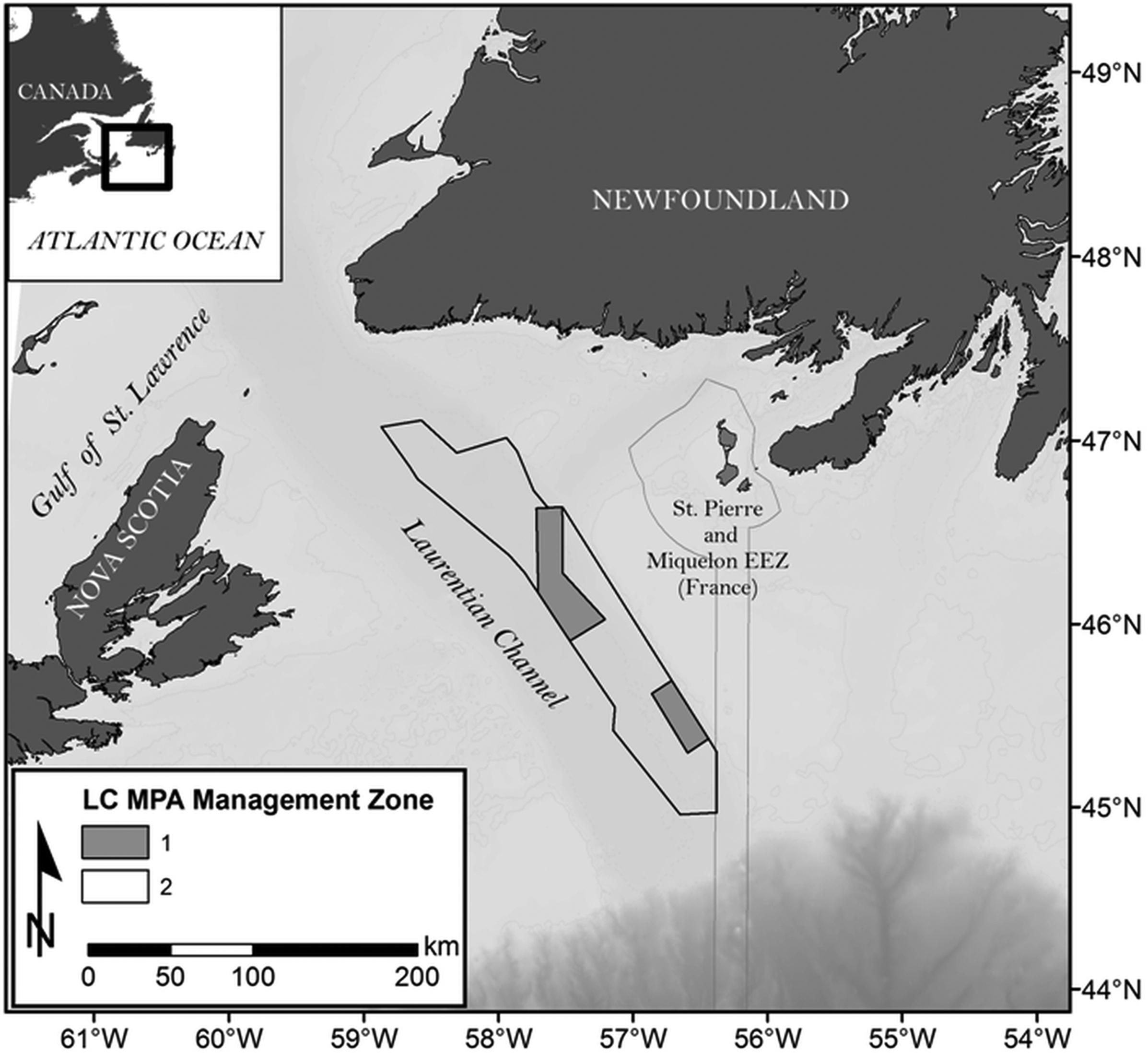
We used the Laurentian Channel (LC) MPA as an example (
Fig. 1), an area initially identified by a government scientific process as an ecologically and biologically significant area (EBSA), which became in 2019 one of Canada’s largest MPAs. With a size of about 11 619 km
2, the MPA covers part of the Laurentian channel that connects the Gulf of Saint Lawrence to the Atlantic Ocean on Canada’s east coast, with depths ranging from 139 to 485 m and a seabed largely dominated by fine sediments (
DFO 2011). The LC is thought to be a nursery ground for several species, such as porbeagle shark (
Lamna nasus), black dogfish (
Centroscyllium fabricii), and smooth skate (
Malacoraja senta). It also offers suitable habitats for sea pens (
Pennatulacea) and is a migration corridor for several marine mammal species moving into and out of the Gulf of Saint Lawrence. Through an analysis of government documents and data, we explored how stakeholder engagement impacted the MPA planning process and its potential impacts on the MPA effectiveness.
Material and methods
To help reconstruct the history of the planning process for the LC MPA, we first read 17 documents produced between 2009 and 2015 by the Department of Fisheries and Oceans (DFO), the Canadian government agency in charge of designing the LC MPA (See
Table S1, for a complete list of the documents). These public and internal documents (e.g., memos, presentations, reports) described various aspects of the MPA design process and helped identify specific stages where the proposed MPA boundaries were modified. Those stages were used in subsequent biological and socio-economic data analyses to understand the impact of boundary changes on species and on the fishing industry. Those documents also provided diverse information, such as reasons why changes were made to the AOI boundaries, data used to support those decisions, information on the position of various stakeholder groups with regards to the MPA planning, changes in species of conservation priority, etc. In the second part of our work, we conducted spatial analyses using government biological and socio-economic data to quantify the impact that MPA boundary modifications had, at each stage, in terms of potential economic losses for the fishing industry and potential conservation losses for species of interest. Conservation losses, also identified in this paper as protection levels for the species of interest, were measured as the proportion of species abundance or biomass found outside the proposed MPA when compared to the larger original MPA boundary. This conservation loss was assessed for seven species: the five species identified in the final MPA conservation objectives and two commercial species, Atlantic cod (
Gadus morhua) and redfish (
Sebastes mentella), initially listed as tentative conservation objectives but removed during the process (
DFO 2009).
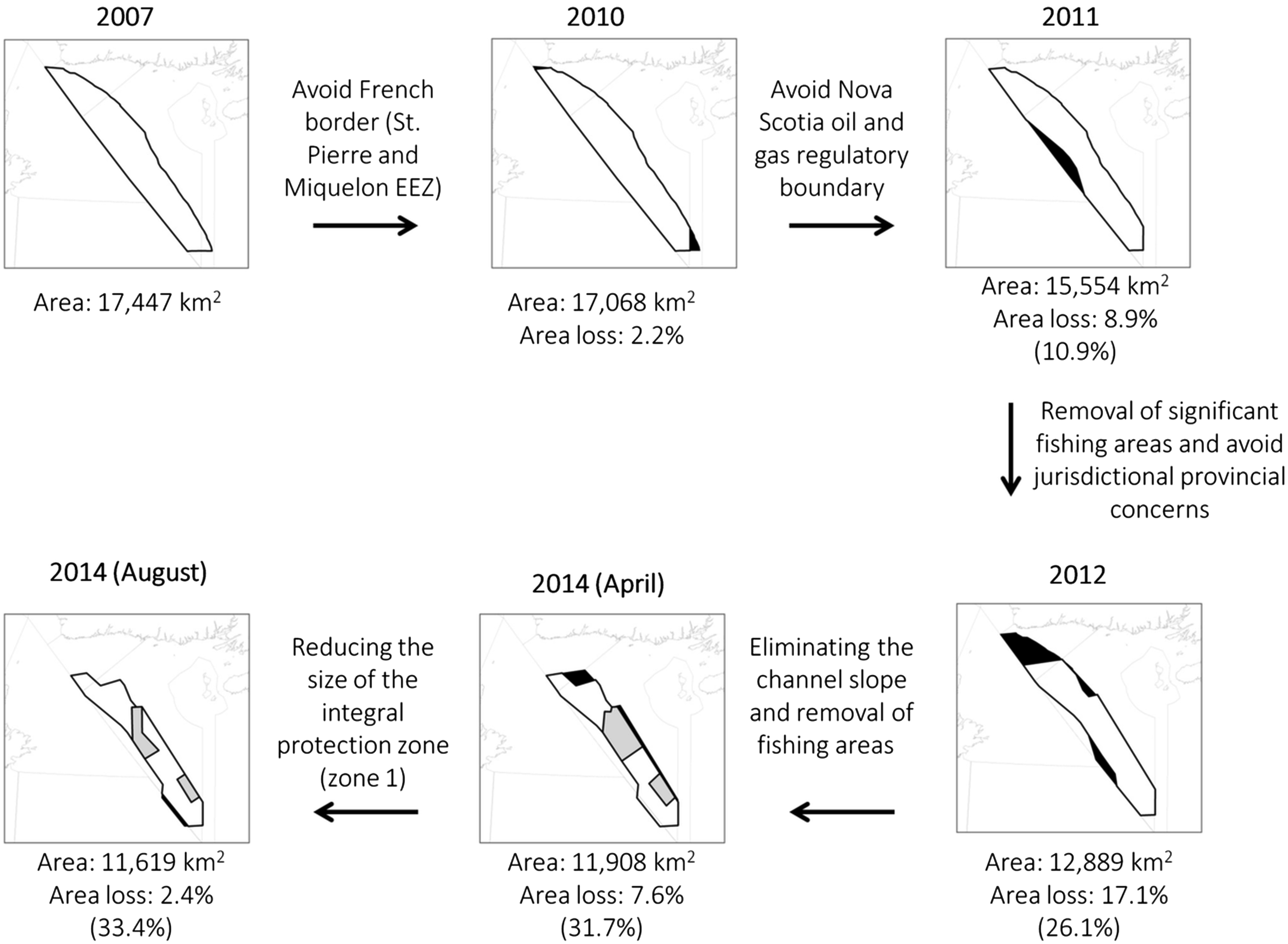
The LC MPA was first identified as an EBSA by a scientific process in 2007 (
Templeman 2007), recognizing its ecological importance for the region. Boundaries for the original LC EBSA and for the final LC MPA were provided as ArcGIS shapefiles by DFO NL Oceans Branch. Intermediate draft MPA boundaries, details on the AOI designation and the MPA planning process, and species of conservation priorities (
Table 1) were obtained from DFO documents (See
Table S1). Biological and economic data used for the analyses were provided by DFO NL Science and Oceans Branches.
Biological data
Biological data for the seven species of interest were used to estimate the abundance and biomass of each species that would be left unprotected outside of the MPA after each stage of the MPA boundary modification (i.e., from the larger original EBSA boundary to the smaller final MPA). Once outside, we assumed that those species were not protected anymore by the MPA and hence considered this as a conservation loss when compared to the initial EBSA boundaries delineated during a scientific process.
Abundance and biomass indices of the northern wolffish (
Anarchias denticulatus), black dogfish, smooth skate, Atlantic cod, and redfish were calculated based on the DFO multispecies trawl survey database (1996–2014) for the Northwest Atlantic Fisheries Organization 3Ps and 3Pn subdivisions (spring data only). Detailed information about DFO multispecies surveys in NL waters can be found in
McCallum and Walsh (1997) and
NAFO (2013). Biomass index of porbeagle shark was provided by the DFO NL Science Branch, coming from fisheries observer data (1995–2012, as presented in
Campana et al. 2015). Sea pens significant benthic areas (SiBAs) were obtained from the 2017 advisory process carried out by DFO Science to identify and delineate SiBAs (
DFO 2017); analyses of these data provide the most up-to-date perspective regarding sea pen conservation in the LC, although most MPA design decisions involving sea pens were made based on earlier studies (i.e.,
DFO 2010;
Kenchington et al. 2010). We did not include Leatherback sea turtles (
Dermochelys coriacea) in this analysis, the last of the six final conservation objectives, on the advice this species’ expert at DFO because of strong data limitations for this species that did not allow for similar quantitative analyses (Jack Lawson, personal communication).
For each stage of the MPA boundary delineation process, the total draft MPA size was calculated using Esri ArcGIS Desktop 10.3 using a Universal Transverse Mercator Zone 21 projection. The AOI was divided into 10 km × 10 km grid cells, and species’ abundance and (or) biomass were averaged for all available years (i.e., see above) and allocated to an individual grid cell. Cell size was selected to match other data obtained from DFO NL that were also used in this study. Average species abundance and (or) biomass were then summed for the entire original EBSA boundary and scaled to a value of 1, representing the species abundance or biomass for each species that was included in the original EBSA boundary. Both abundance and biomass data were used as proxies to quantify potential losses in protection for the northern wolffish, black dogfish, smooth skate, cod, and redfish. Since no abundance data were available for the porbeagle shark, analyses were conducted using biomass data only. Because of differences in estimation methods, changes in sea pens protection levels were estimated by calculating the proportion of protected SiBA surface with respect to the surface protected within the initial EBSA boundaries. SiBA used at this stage refers to the areas identified in 2016 (
Kenchington et al. 2016;
DFO 2017).
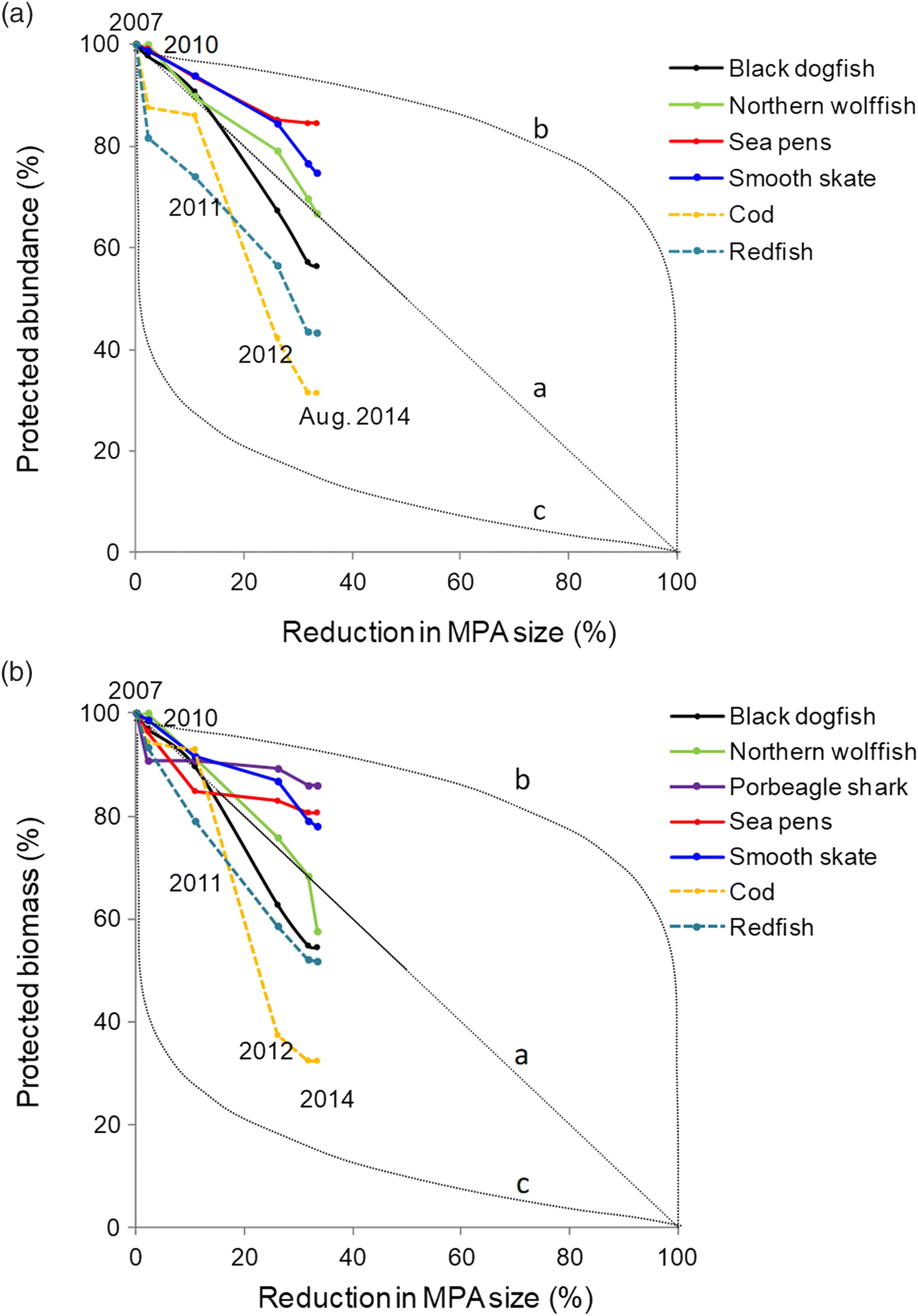
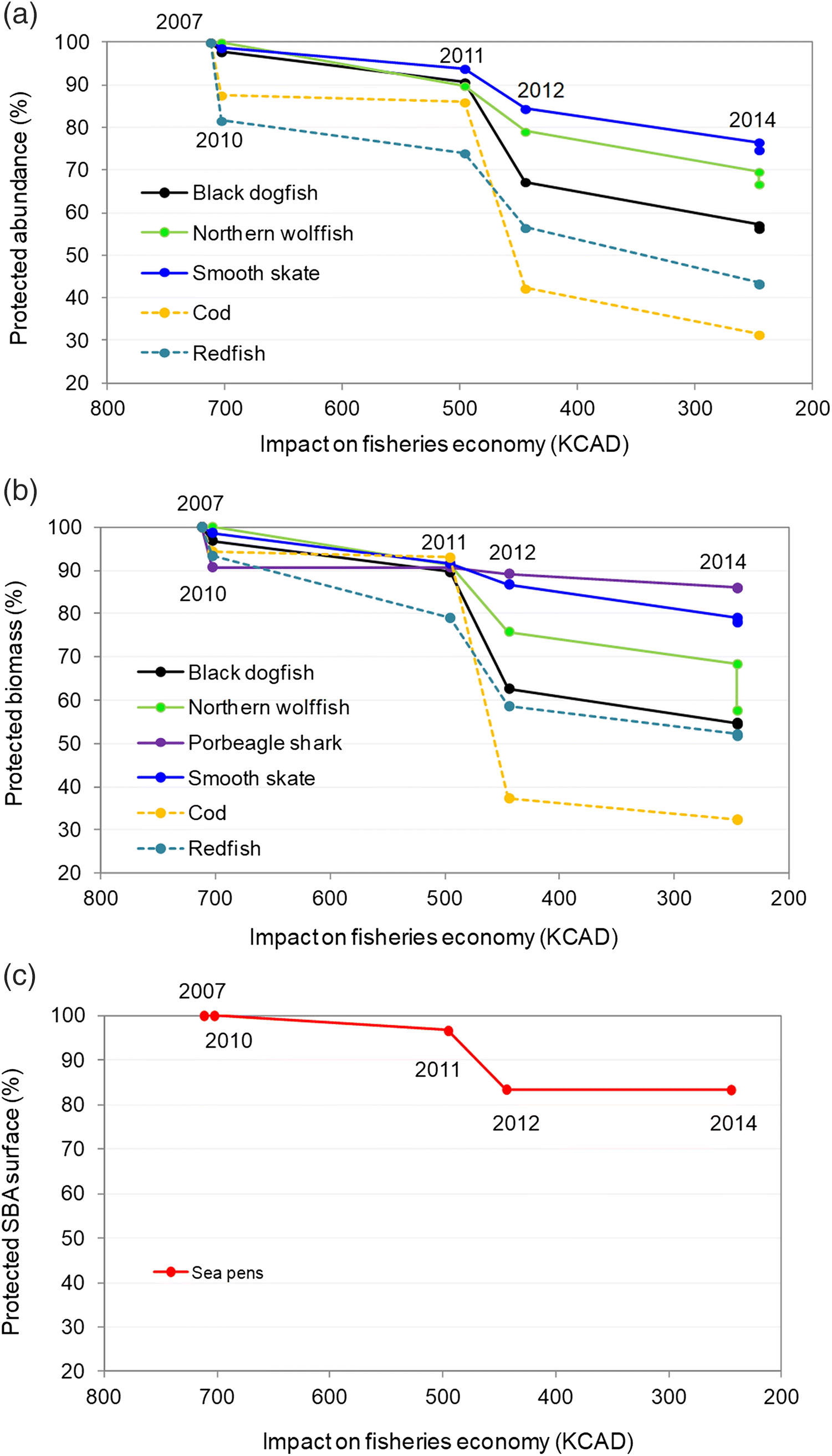
We quantified decreases in species biomass and (or) abundance coverage after each MPA boundary change (i.e., each stage in the MPA planning). We then proposed three conservation loss scenarios to help interpret species loss trends. Those scenarios linked decreases in MPA size with decreases in species protection levels at each stage. A reduction in species biomass and (or) abundance that would be proportional to the reduction in size of the MPA was represented in our results by line “a” (i.e., proportional loss scenario). Under this scenario, reducing an MPA size of 30% would for instance result in a decrease of 30% in the protection for a given species, something expected for instance if the species were distributed homogeneously in the area. Line “b” (i.e., marginal loss scenario) represents a scenario where large reductions in the MPA size would only have a small impact on the species protection (adapted from
Tear et al. 2005). Such a scenario, preferable for conservation, could result from removing areas from the draft MPA where species are less present. The line “c” (i.e., disproportional loss scenario) is the reverse, with small reductions in the MPA size resulting in large impact on species protection. This scenario would suggest that areas removed during the planning process included larger density of species of conservation interest. The basic underlying assumptions were that (
i) the original EBSA spatial extent, informed by science, provided sufficient coverage to meet the conservation objectives and (
ii) reductions in the MPA size were mostly associated with removal of conservation objectives (i.e., species) from the process and (or) to accommodate trade-offs with different stakeholder sectors, but the reduction in size of the area did not result from a need to better match conservation objectives.
Socio-economic data
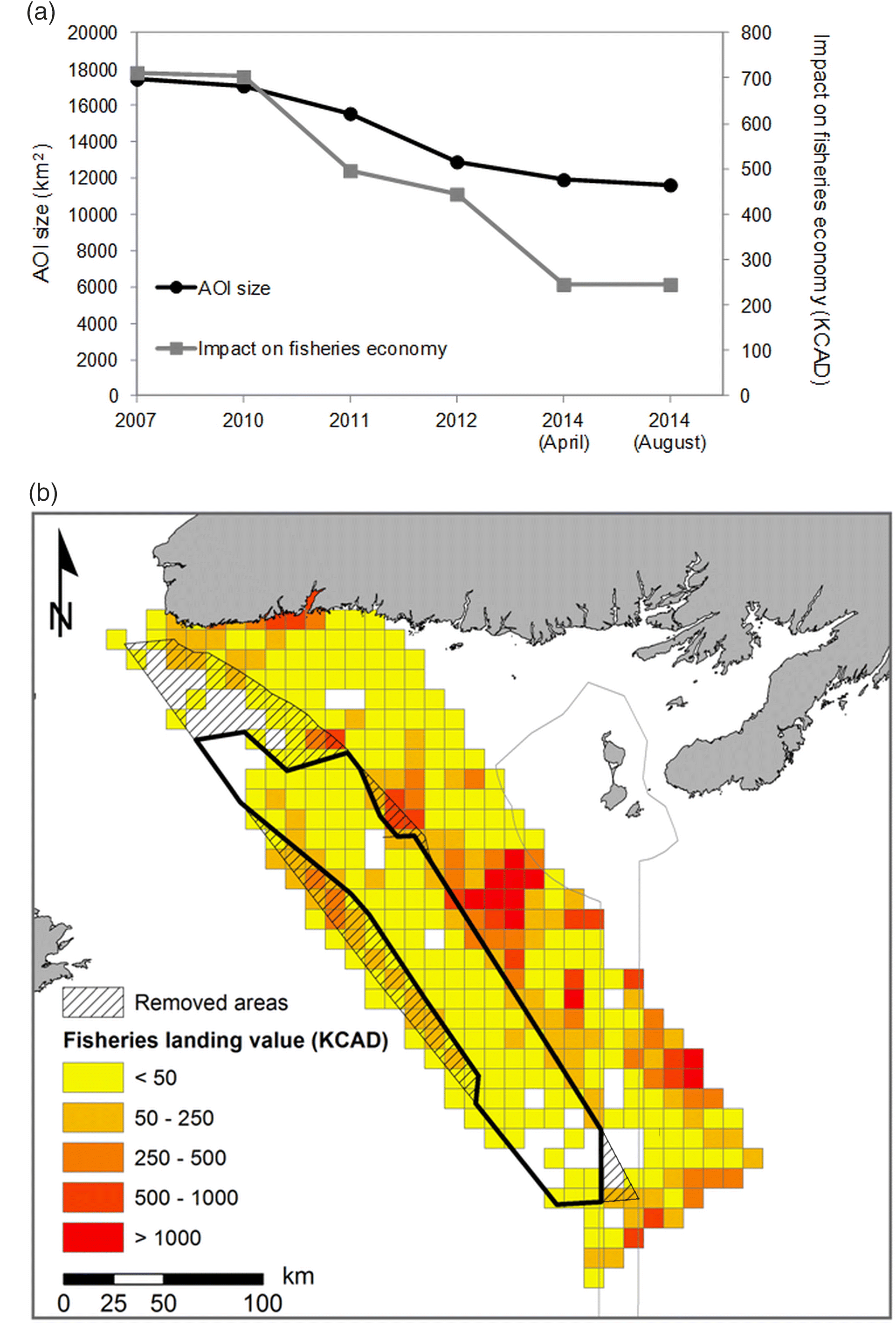
Socio-economic data were analysed to better understand the potential economic impacts each MPA planning stage could have on the fisheries industry. As the LC MPA bans all industrial fishing activities, we used past fish landing data to assess potential losses in terms of future economic opportunities for the industry. Similar to the analyses performed using biological data, we calculated decreases in the total fish landings at each stage of the MPA boundary delineation.
The mean annual fisheries landed value was calculated using 10 km × 10 km gridded data provided by DFO NL Oceans Branch for the 2004–2013 period. The total economic value of the fisheries landings that have historically been generated within the proposed MPA boundaries was estimated at each stage of the boundary delineation process by summing the value of all the cells having their centroid within the proposed MPA boundary. This mean annual fisheries landed value was used to estimate the economic impact the MPA would have after a ban on industrial fishing activities in the area that would result from the creation of the MPA (including shrimp trawlers, purse seiners, potters, longliners, gill-netters, and bottom otter trawlers). Then, the Spearman correlation coefficient was calculated to assess the significance of the relationship between fisheries landed values and species protection values (i.e., abundance and (or) biomass of species within the MPA boundary) at each stage of the planning process. We designed our analyses to separately explore the species that remained conservation objectives until the end of the planning process, and two species of commercial interest (i.e., cod and redfish) that were removed from the conservation objective list during the planning process. This helped explore the premise that the boundary modification process was primarily driven by the removal of areas from the AOI characterised by high concentrations in commercial species, something also documented in the documents consulted in this study.
Discussion and conclusions
MPAs are often created at sites of conservation value identified by earlier scientific studies. This was the case for the LC MPA that was selected from a set of candidate sites identified by a scientific process for their ecological and biological significance (i.e., the EBSAs). Such context is also common internationally, as efforts led by the United Nations (UN) in the past decade have aimed at identifying UN EBSAs that can be used as a basis for MPA selection (
Dunn et al. 2014). Although conservation science encourages prioritizing the protection of areas facing the highest threats (
Margules and Pressey 2000), the LC was instead explicitly selected out other potential EBSA sites for its lower level of human use in an attempt to minimize conflicts with stakeholders, and hence ease the MPA design process. Such an approach is fairly common internationally, described by
Devillers et al. (2015) as leading to the creation of “residual MPAs” (i.e., MPAs that are located in areas of low economic interest but often fail to protect areas in more urgent need of protection). While many EBSAs can benefit from the protection provided by an MPA, MPAs may not be the most appropriate management tool to protect species that led to the designation of an EBSAs (e.g., MPAs may be a less appropriate tool to protect some pelagic species). Government documents reviewed do not suggest that the adequacy of this specific management tool for the LC EBSA has played a role in the selection of the LC EBSA out of the other candidate sites.
Our study found that the LC MPA is one-third smaller than the original EBSA identified by science. Size reductions are typical in MPA planning processes in response to socio-economic or political constraints (
Pastoors et al. 2000;
MPA News 2006). In the LC MPA case, establishing an MPA smaller than the original EBSA can be expected. Many EBSAs are defined as large areas of ecological significance that may not require protecting the entire area, depending on the features and activities occurring within it. The documents analysed do not suggest that changes to the MPA boundaries were motivated by a desire to increase (or maintain) the ecological effectiveness. The impact of reductions in size resulting from an MPA planning process on the capacity of the MPA to protect species of interest is however rarely assessed by scientific studies. Such assessment can however be critical to ensure the ecological effectiveness of future MPAs. An ongoing formal scientific assessment of the proposed MPA design criteria (e.g., boundaries, level of protection) throughout the entire process could have helped confirm if the various stages of the proposed MPA still allow for an appropriate protection of the species of conservation priority. The review of the documents indicates that successive changes made to the proposed MPA boundaries and the creation of zones were solely motivated by a desire to accommodate industry users’ concerns and done in the absence of any formal scientific advice on the impacts those changes could have on the conservation priorities and the overall MPA ecological effectiveness.
As demonstrated by our analyses, the gradual reduction in size of the MPA has affected some species more than others. The two species that were the most impacted by reduction of the MPA size are cod and redfish, species of commercial interest in this region that were removed from the list of conservation priorities during the process. Those reductions in species abundance and biomass are the direct outcome of the removal of areas that had high concentrations of those two species. Documents also suggest that the removal of those areas important for the fishing industry may have played a role in the decision of dropping these species from the conservation priorities. The abundance and (or) biomass of the five species captured by the final MPA conservation objectives all declined due to the size reduction of the MPA. While some species were less impacted (e.g., porbeagle shark and sea pens, with less of 20% decline), black dogfish and the biomass of northern wolffish showed declines that are larger than the “proportional loss” scenario (i.e., below line “a” on
Fig. 4). Such differences can be explained by the geographic distribution of those species in the region, and their affiliation with specific habitats or substrates. For instance, sea pens that tend to be found on soft sediments in the centre of the LC, areas characterized by smaller fishing effort, were less impacted than some species found in higher abundance on the slope of channel that are better fishing grounds for the industry.
While the future ecological effectiveness of the LC MPA cannot be simply derived from our results, the analytical approach proposed in this paper can be used as a rapid assessment tool by managers and scientists that could prompt more extensive scientific assessments. Species the most impacted by the MPA size reduction could have a higher priority when conducting a formal scientific assessment of the impacts of the proposed changes to the MPA plan. While a reduction of the total area protected can clearly impact the ability of the MPA to protect species, such an impact would not necessarily be detected in a formal assessment of the MPA effectiveness due to the way conservation objectives are worded. Several LC MPA conservation objectives were designed to “protect” species (i.e., corals, black dogfish, smooth skate, and porbeagle sharks). It could be argued that reductions made to the size of the MPA would still ensure a protection of those species, hence resulting in the MPA meeting its objectives, whatever the reduction in size of the MPA is. This highlights the importance of setting conservation objectives that meaningfully reflect and contribute to address the regional conservation challenges as, in this case, meeting the conservation objectives could be very different from protecting effectively the species in this region.
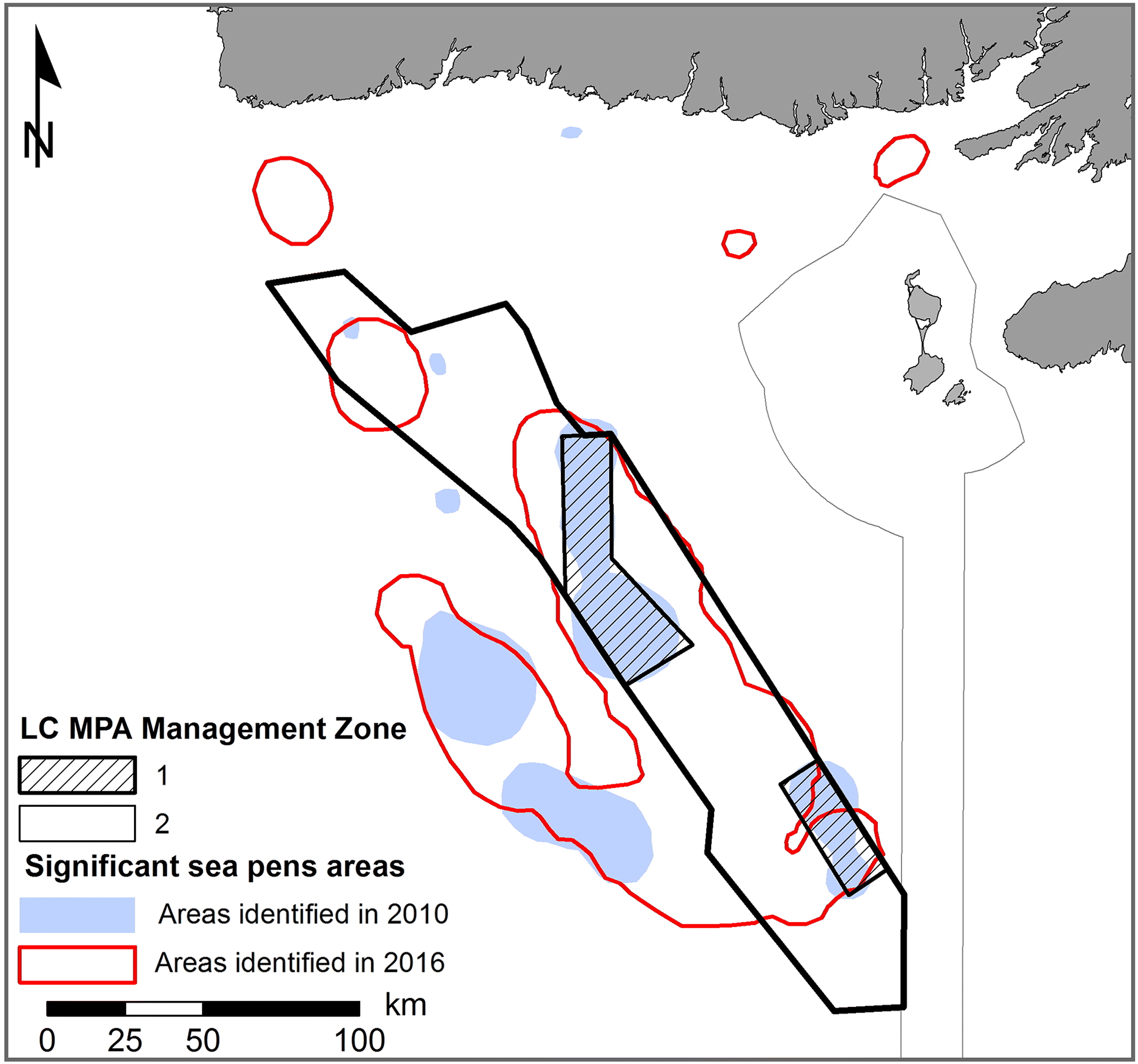
An interesting example of how a paucity of science input can influence MPA design can be illustrated with sea pens in the LC. Now the flagship species of the LC MPA, sea pens were not even listed as a species of interest when the LC EBSA was first selected (
Templeman 2007). By the time the LC AOI was formalized in 2010, scientific knowledge had advanced and identified sea pens as important species in the area (
DFO 2010;
Kenchington et al. 2010), leading to their inclusion among the conservation priorities. The location of sea pen habitats identified in 2010 also led to the delineation of management zones with different protection levels within the MPA (
Fig. 6). However, while negotiations with stakeholders led to the MPA zoning and to a size reduction of Zone 1, scientific knowledge improved and the most recent science advice (
Kenchington et al. 2016;
DFO 2017) indicates that most of the LC MPA emerges as a significant sea pen habitat. This may bring to question the current usefulness of the proposed zonation. If lower protection zones were justified by the absence of sea pen habitats, the most recent scientific advice would then suggest that the higher protection zone should have been be vastly expanded to protect other areas of known sea pen presence. Part of this apparent contradiction is associated with the timelines of the decisions, the availability of new scientific studies, and the challenge to integrate new scientific knowledge along the planning process, highlighting issues that could have been identified by formal scientific assessments during the design process and (or) an a posteriori science assessment of the final MPA design. A more regular integration of scientific advice through the entire MPA planning process would help ensure that the MPA design remains appropriate in light of the most recent scientific knowledge. Similarly, a mechanism should exist to update regulations in light of new scientific knowledge during the life of the MPA, something known as adaptive management that has rarely been used in practice for Canadian MPAs (
Mills et al. 2015).
The LC case study also raises more general concerns about the way science and stakeholder engagement are incorporated in some MPA design processes. While stakeholder engagement should clearly be an essential component of any MPA design (
Kessler 2004;
Pomeroy and Douvere 2008), it should be carefully integrated with scientific advice at all stages of the planning process to reduce the risks of establishing “residual reserves” (
Devillers et al. 2015). Stakeholders’ requests must be carefully implemented in conservation planning to better assess the trade-offs and the impacts that these requests can have on potential conservation effectiveness. As summarized by
Klein et al. (2008), a successful MPA design should incorporate stakeholders’ interests without compromising biodiversity conservation goals. Such an approach was adopted when revising the Great Barrier Reef MPA network in 2002–2003 by using a systematic conservation planning approach (i.e., using the software tool Marxan). Such an approach ensured that the proposed MPA network design would still meet the quantitative conservation objectives after stakeholders’ consultations were conducted (
Fernandes et al. 2005). Such an approach appears relatively rare in conservation planning, where systematic approaches are more the exception than the rule (
McIntosh et al. 2017). Other successful models exist for ensuring that scientific criteria remain central to the MPA planning process. For instance, the creation of a committee called the “Blue Ribbon Task Force” helped ensure that the scientific principles guiding the California State MPA network design were still satisfied once the process was informed by stakeholder groups (
Gleason et al. 2013;
Kirlin et al. 2013).
Our study provides, to the best of our knowledge, the first quantitative demonstration that integrating stakeholders’ interests in MPA planning, when not supported by clear initial quantitative targets or guided along all stages by a formal scientific evaluation, can negatively impact conservation outcomes. Such process can lead to the establishment of residual MPAs (i.e., MPAs are created where there is lower economic interest) and (or) increase the risk of compromising MPAs ecological effectiveness. In the LC case study, it is unclear if the MPA will be effective at protecting black dogfish while >40% of this species’ abundance and biomass originally covered by the EBSA is now located outside the boundaries of the MPA.
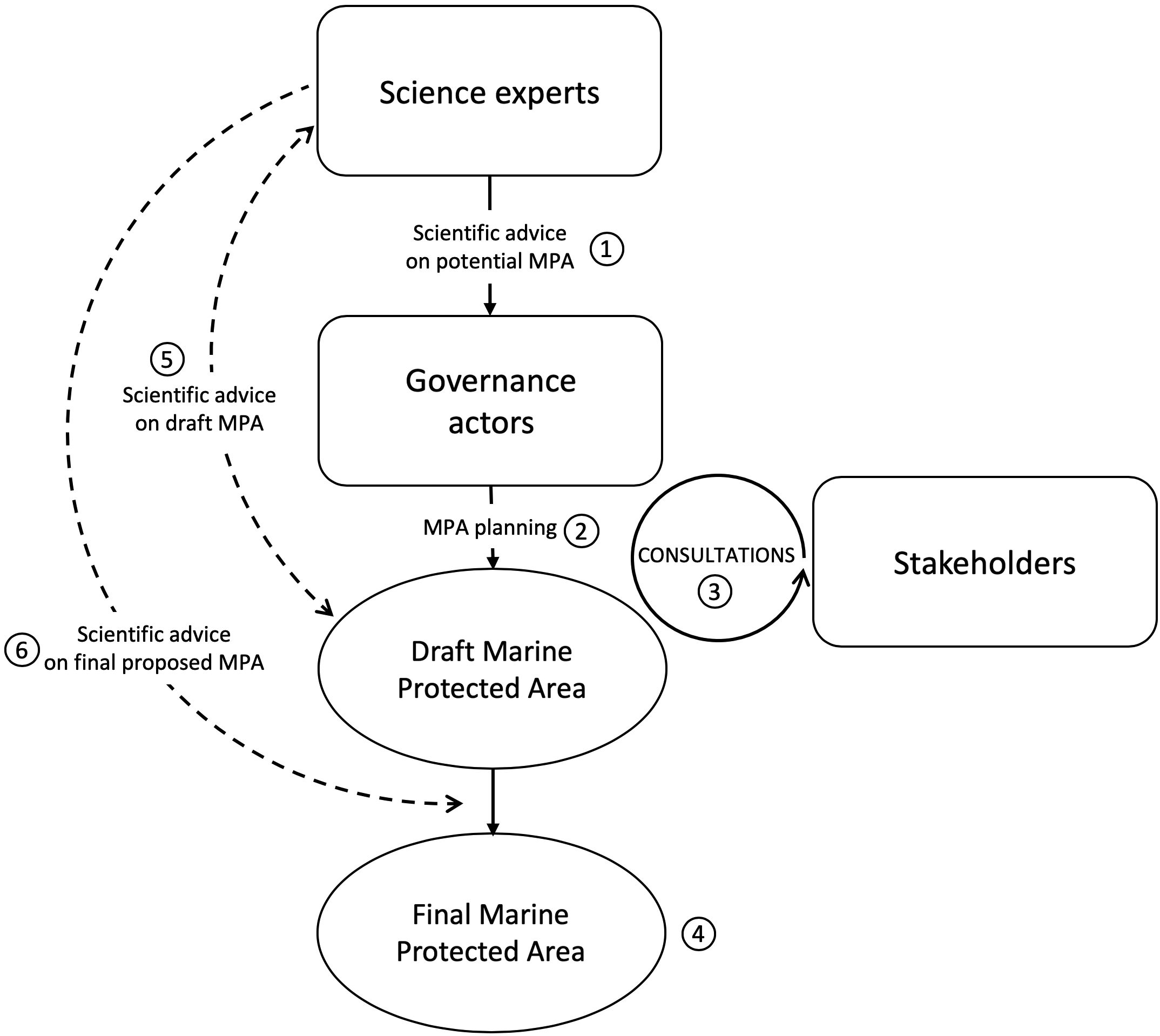
In the LC case, formal science advice by DFO scientists played a limited role during the planning process (2011–2014) (
Fig. 7 summarizes some of this process). As far as the available documents indicate (i.e., meeting minutes; see
Table S1), the advisory committee provided little advice on changes made to the MPA boundaries and levels of protection, and the scientists involved in the committee were not requested to assess the consequences of such changes on the potential MPA effectiveness, an exercise that would have required formal data analyses conducted by species experts. Our study suggests that many important choices made during the MPA design (e.g., boundary and zoning) seem to have largely responded to socio-economic and political priorities to minimize conflicts with stakeholders and to obtain their support. Such a process potentially jeopardizes how defensible the final proposed MPA is to ensure an effective protection of the species it is being designed to protect. While many studies highlighted the importance of evidence-based decision making in conservation (see
Walsh et al. (2014) for an overview or
Lemieux et al. (2018) for a discussion in the Canadian context), recent studies pointed out that “decision-makers themselves may not seek or use evidence to make their decisions, even when it is available” (
Gardner et al. 2018), an attitude called “evidence complacency” by
Sutherland and Wordley (2017). Reasons for such attitude towards evidence can be diverse, but in the context of the LC they could be related to time constraints related to the process, to a perception that additional evidence is not required, or a perception that it will impact the ability to reach an agreement with the stakeholders. A more thorough scientific process used to validate intermediate MPA designs would have required time and resources from DFO Science branch, delayed the progress of the DFO Ocean branch in their planning, but may also have advised against some changes (e.g., exclusion of some areas of importance to the fisheries industry), risking to jeopardize a potential agreement between DFO management and the different stakeholders.
Our study illustrates some of the complex trade-offs that exist in an MPA design process, a challenge discussed in the literature (
Halpern et al. 2013), highlights the need for ongoing and formal assessments of the potential MPA effectiveness as the process advances and provides a novel and simple approach to quantify some consequences of those trade-offs (i.e.,
Fig. 4). The use of a systematic approach to conservation planning similar to what was done for the Great Barrier Reef MPA network could have helped guide the process using clear quantitative conservation targets (e.g.,
Margules and Pressey 2000), minimizing the risks of compromising the MPA effectiveness. The LC MPA example points to some of the risks of engaging stakeholders in the absence of a continuous scientific supporting process.
When carefully implemented, MPAs were shown to be effective tools supporting the global efforts toward biodiversity conservation. Often, MPAs will restrict industrial activities and lead to lost economic opportunities for different user groups. Clearly, attempting to manage those different and at times diverging priorities is essential for minimizing the risks related to the establishment of residuals MPAs. If there is one lesson to be learned from this study, it is that any MPA planning processes should integrate regular scientific advice with stakeholder inputs in a formal and structured way. As the MPA design process develops, this could result in more transparent MPA planning processes that would help ensure the MPA ecological effectiveness, while minimizing its impacts on the different user groups. Doing so would not only prevent potential negative consequences emerging after the MPA has been established, but would also provide a natural mechanism for public engagement and the construction of a broader consensus towards the MPA process.