The effects of hydrogen peroxide on mortality, escape response, and oxygen consumption of Calanus spp.
Abstract
Hydrogen peroxide (H2O2), a pesticide used in salmonid aquaculture, is released directly into the environment where nontarget organisms are at risk of exposure. We determined threshold concentrations for mortality of Calanus spp., the dominant zooplankton species in the North Atlantic, and assessed sublethal effects, focusing on the escape response and oxygen consumption rates (OCRs) as behavioral and physiological assays. One-hour exposure to 170 mg·L−1 (i.e., 10% of the recommended H2O2 treatment) was lethal to copepodite stage V (92% mortality) and adult females (100% mortality). The acute median lethal concentration (1h-LC50) was 214.1 (150.67–277.4) and 48.6 (44.9–52.2) mg·L−1 for copepodite V and adults, respectively. The 25-h LC50 was 77.1 (57.9–96.2) and 30.63 (25.4–35.8) mg·L−1 for copepodite V and adults, respectively. At concentrations of 0.5% and 1% of the recommended treatment level, Calanus spp. showed a decrease in escape performance and lower OCRs with increased concentration. At H2O2 concentrations of 5% of the recommended treatment levels (85 mg·L−1), exposed copepods showed no escape reaction response. These results suggest that sublethal concentrations of H2O2 will increase the risk of predation for Calanus spp. Furthermore, this study provides supporting evidence that theoretical “safe” values, traditionally used for predicting toxicity thresholds, underestimate the impact of H2O2 on the physiological condition of nontarget crustaceans.
Introduction
Lepeophtheirus salmonis, salmon louse, is a parasitic copepod affecting farmed and wild salmonids (Costello 2006; Torrissen et al. 2013). Addressing the economic and ecological impact of salmon lice is considered one of the most important challenges for the salmon industry. The parasite feeds on skin, causing damage associated with osmotic stress and secondary infections (Finstad et al. 2000; Johnson et al. 2004). Salmon lice infestations increase the overall cost of salmon aquaculture due to high expenses associated with delousing and the concomitant reduction in fish growth and reduced marketability due to skin lesions (Costello 2009; Liu and Bjelland 2014). In natural populations, smolts from wild salmon and trout can suffer high mortality if infested with a high density of salmon lice (Liu et al. 2011).
To control salmon lice infestations strict regulations on the number of lice per fish—0.2 adult female lice in the spring, and 0.5 adult female lice per fish for the rest of the year—have been established in Norway, and a plan for management of salmon lice is requested from each farm by the Salmon Lice Directive (www.lovdata.no/dokument/SF/forskrift/2012-12-05-1140). To meet these regulations, commercial farms rely partly on chemical therapeutants to control salmon lice populations. One of the therapeutants used in Norway to treat salmon lice and amoebic gill disease (Young et al. 2007) is hydrogen peroxide (H2O2). Hydrogen peroxide is administered by bath treatment, either directly in the net pens or using well boats (Ernst et al. 2001). After treatment, the chemical is discharged into the surrounding water. The use of H2O2 peaked in 2015 at 43 246 tons, but declined to 9277 tons in 2017 (www.fhi.no/hn/legemiddelbruk). The mechanism of action of H2O2 on salmon lice includes mechanical paralysis, inactivation of enzymes and DNA replication, and peroxidation of lipid and cellular organelle membranes by hydroxyl radicals (Cotran et al. 1989). Mechanical paralysis is caused by decomposition of H2O2 to water and O2 bubbles in the haemolymph, which causes detachment of the pre-adult and adult lice from the fish (Thomassen 1993; Aaen et al. 2014). Hydrogen peroxide is not effective on the chalimus stages.
The pelagic zooplankton, Calanus spp., is a key component in the north Atlantic food web (Melle et al. 2014) and is abundant in the coastal zone where aquaculture sites are located (Broms et al. 2009). Calanus spp. is an important grazer of primary production (Runge and de Lafontaine 1996; Heath and Lough 2007). The younger life stages are important food for juvenile fish in the nursery areas, and the adults are main prey for several pelagic fish stocks such as herring and cod (Dalpadado et al. 2000; Sundby 2000; Rullyanto et al. 2015). In the spring, egg production for Calanus spp. overlaps with the peak application of pharmaceuticals to keep the level of salmon lice below 0.2 lice females per fish. Its effect on Calanus spp. is largely unknown.
Only a few studies have examined the effect of exposing nontarget organisms to H2O2 (Burridge et al. 2014; Van Geest et al. 2014; Brokke 2015). Brokke (2015) reported that exposure to 1700 mg·L−1 H2O2 for 1 h resulted in 10% mortality in chameleon shrimp (Praunus flexuosus) and 20% in rockpool shrimp (Palaemon elegans) after a 24-h recovery period. Consequently, the median lethal concentration (LC50) values were higher than the treatment concentration for both species. In contrast the opossum shrimp (Mysid sp.) were considerably more sensitive with a LC50 of 973 mg·L−1 (i.e., lower than the recommended treatment concentration (Burridge et al. 2014)) and late copepodide stage Calanus spp. had a LC50 of 6 mg·L−1 H2O2 following a 24-h exposure, indicating a time-dependent effect (Hansen et al. 2017).
The objective of this study was to determine the threshold concentrations at which H2O2 causes mortality in Calanus spp. and to assess possible sublethal effects, focusing on the escape response as a behavioral assay and oxygen consumption rates as a physiological indicator.
Materials and methods
Copepods were collected from the dock at Austevoll Research Station, Institute of Marine Research, Norway (60°05′20″N, 5°15′57″E) at a depth of 20–30 m using light traps and plankton nets. The light traps (mesh size 500 μm; 0.45 m in diameter; BellaMare, San Diego, California, USA) were equipped with a white LED light and deployed overnight. A standard plankton net (mesh size, 200 μm; diameter, 30 cm) was used to collect copepods from 20 m to the surface. Copepods were collected at least 3 km away from any commercial fish farm and were transported to the laboratory at Austevoll Research Station where Calanus spp. adult females and copepodite V stages were sorted. Copepods were maintained overnight in 10 L containers at 8 °C. Seawater used in the experiments was pumped from a depth of 160 m in Bjørnafjorden and filtered through a sand filter. Copepods were tested within 24 h of capture and each copepod was tested only once.
Commercial H2O2 (Nemona, Akzo Nobel Pulp and Performance Chemicals AB, Bohus, Sweden) is 49.50% H2O2. For treating salmon, the recommended concentration for a H2O2 bath treatment is 1500–2100 mg·L−1 depending on temperature, for 20 min (https://www.felleskatalogen.no/medisin-vet/atc-register/QD08A). Typically, toxicity studies use exposure times of 24, 48, 72, and 96 h; however, this is not representative of the scenario following a bath treatment on a salmon farm. Therefore, we followed the recommendations of Burridge et al. (2014) and Van Geest et al. (2014) and limited the exposure time to 1 h.
A preliminary study was conducted to select the concentrations to be used in the main experiment. Testing was undertaken with concentrations of 1700 and 340 mg·L−1 H2O2 (∼100% and 20% of the recommended treatment concentration). These concentrations caused 100% mortality after 1 h exposure for both copepodite Vs and adult females. Thus, the concentrations chosen in this study were 170, 85, 17, and 8.5 mg·L−1, corresponding to 10%, 5%, 1%, and 0.5% of a recommended treatment dose of 1700 mg·L−1.
The copepods were randomly divided into five groups, each group consisting of 30 individuals. H2O2 was added to each of four 4 L tanks and mixed to the target concentrations. One tank contained clean seawater and served as a control. Three PVC pipes (25 cm diameter, 25 cm tall, 500 μm Nitex screen bottom) were added to each tank; each pipe contained approximately 10 individuals. The experiment was conducted in triplicate using a total of approximately 450 copepods. The temperature in the tanks was 13 °C.
Mortality
Stage V and adult female copepods were used in all experiments. The exposure time was 1 h and, after exposure, the copepods were transferred to 10 L tanks in which they were held for 24 h (recovery period). The copepods were observed under a dissecting microscope immediately after the 1-h exposure and after the 24-h recovery period. Dead individuals were counted at each time point. Individuals were considered dead if they were discolored, deformed (urosome and pleopods folded back), or if there was no movement of the antenna and pleopods after a gentle stimulus. Copepods laying on the bottom of the tank, with retracted antennae but showing uncontrolled limb motion, (e.g., twitching of the antennae) were considered immobilized. Mortality that occurred during the 1-h exposure is defined as acute mortality. Total mortality was defined as the sum of mortality during the 1-h exposure plus that after the 24-h recovery period.
Sublethal effects
The escape response of the copepods was measured as a behavioral assay and oxygen consumption rates as a physiological indicator of sublethal effects of exposure to H2O2. Exposure concentrations used in these experiments were 8.5, 17.0, and 85.0 mg·L−1 H2O2, in addition to a control. The set-up was identical to the mortality experiment. After the 1-h exposure the copepods were transferred to 10 L tanks containing filtered seawater and the behavioral responses and oxygen consumption rates (OCRs) were measured (described below). All copepods were tested within 5 h of exposure in a randomized order. The entire experiment was repeated on three consecutive days with freshly collected copepods.
Escape response
Silhouette video photography (Browman et al. 2003), was used to observe the swimming behavior of copepods. This system allows high-quality observations of small transparent organisms at high resolution and is unaffected by ambient light intensity. Two video cameras were mounted orthogonally, each camera illuminated with a 20 cm collimated beam generated by a small red (720 nm) LED light source. A glass aquarium holding the organisms was placed at their intersection of the two light paths. The two simultaneous orthogonal views allow particles in the field of view to be tracked in three dimensions. The software packages TRAKFISH, MANTRACK, and ANAPATHS (Racca Scientific Consulting and JASCO Research Ltd., Victoria, British Columbia, Canada) were used to analyze the video records (Browman et al. 2003).
Escape response was tested in a 31 L tank (25 cm × 25 cm × 50 cm) (Fig. 1). To stimulate the escape response, a siphon (a 16-gauge, stainless steel, flat-tip hypodermic needle that acted as a mimic of a suction predator) was mounted in the center of the tank, 70 mm above the bottom (Fields et al. 2012). The flow rate into the siphon was maintained at 1 mL·s−1. The velocity (V) of the water entrained by the siphon decreases exponentially with distance (r) from the siphon as: V = Q (4π × r2)−1 where Q is the volume exiting the siphon (Kiørboe et al. 1999). At 5 cm from the siphon, the flow was calculated to be 30 μm·s−1, which is below the threshold for the escape response of this species of copepod (Fields et al. 2012). As the water drained through the siphon, filtered seawater was re-introduced at the top of the tank to maintain a constant water level. This type of set-up has been used previously to initiate an escape response in other copepod species (Fields and Yen 1997). All trials (treatment and controls) were filmed for 60 min in a climate-controlled room at 13 °C (±0.5 °C). The tank contained approximately 200 copepods. Each animal was used only once.
Fig. 1.
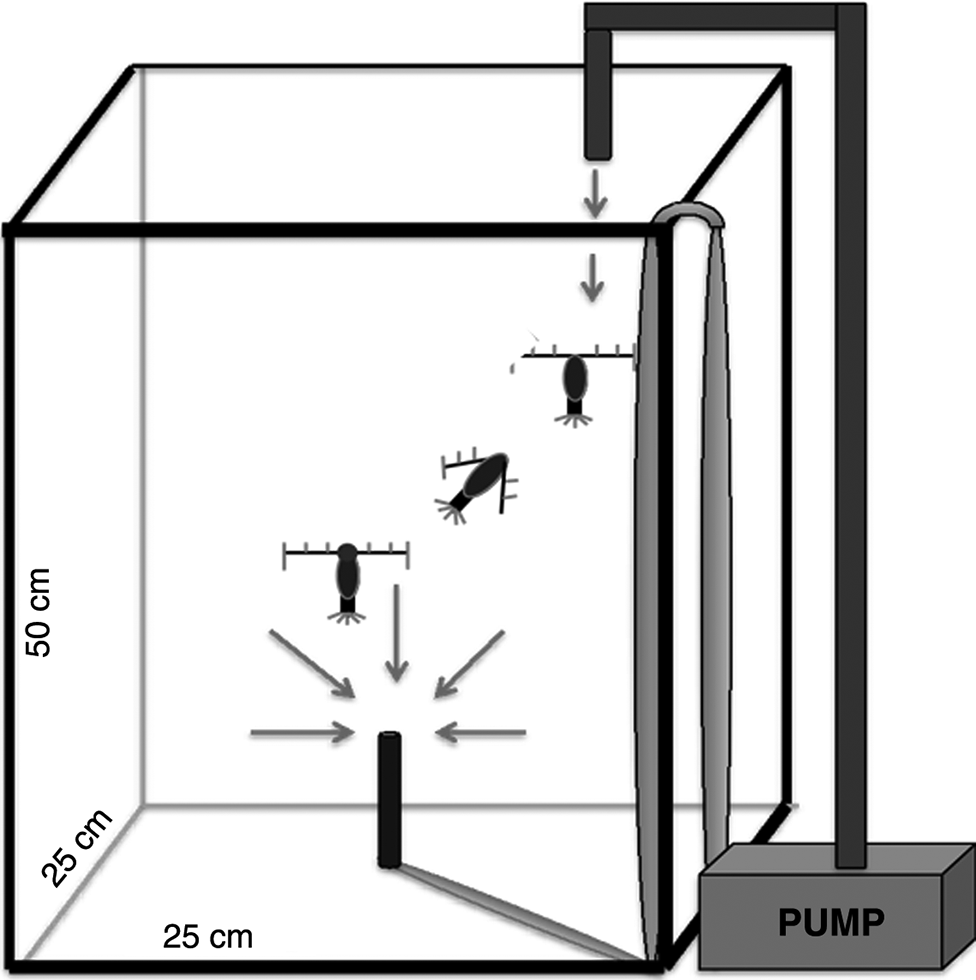
The distance from the predator at which the copepod initiates their escape (threshold distance), and how fast and how far, are the decisive factors in the ability of a copepod to avoid predation (Fields et al. 2012). An escape response involves a single or a series of jumps during which the copepod draws its antennae in to the sides of its body followed by rapid motion of the swimming legs (Strickler 1975; Fields 2000). In case of multiple sequential escape jumps, only the first escape was used. The end of the escape response was defined as the moment when the antennae returned to their original position. Copepods that initiated an escape response but were captured by the siphon were designated as unsuccessful escapes and were not included in the behavioral analysis. The threshold and magnitude of the escape response was evaluated by measuring three parameters: immediate escape response (distance of the copepod from the siphon when the first escape response was initiated), the average speed of the escape and the total distance traveled during the escape. Exposed copepods that were completely paralyzed were not included in the assessment of escape performance or oxygen consumption rate.
Oxygen consumption rates (OCRs)
Only adult female copepods were used in this experiment. Three replicate measurements were made on the same day using three individuals from each concentration and the control. Test animals were held in experimental chambers (4.3 mL) filled with filtered seawater and sealed with a ground glass top that has a small access hole (0.4 mm) to accommodate the oxygen microelectrode. The experimental chambers were stirred (10 rpm) using a glass-encased magnetic stir bar (2 mm). Dissolved oxygen concentrations were measured using a Clark-type oxygen microelectrode (Unisense; Aarhus, Denmark). The linear response of each electrode was calibrated with 0.2 μm filtered seawater bubbled for a minimum of 1 h to set the 100% dissolved oxygen calibration point (Runge et al. 2016). For the anoxic calibration, seawater was placed into a silicone tube immersed in a solution of 0.1 mol·L−1 sodium ascorbate and 0.1 mol·L−1 sodium hydroxide overnight (for over 4 h). All oxygen measurements were made at 12 °C (±0.01 °C) in a ThermoScientific water bath (Model A10B with a thermostat SC100).
Oxygen concentration in the chambers was measured every 2 s for 1.5 h. The oxygen concentration never decreased below 20% saturation. Control chambers, without copepods, were measured to determine background levels of microbial and algal respiration. The oxygen consumption was computed as the difference between the beginning and end of the incubation and then corrected with the values obtained from the control chambers. Activity level of the copepods was assessed under a dissecting microscope before and after the OCR analysis to ensure that all of the copepods were alive.
Statistical analysis
Statistical analysis was conducted using the software R (R Studio, version 3.4.3). The concentration of H2O2 that caused 50% mortality (LC50), and their 95% confidence intervals (CI), were calculated for each stage using a generalized linear model with binomial error structures and probit links according to Finney (1971). Pesticide concentrations were log10 transformed to linearize the data. At sublethal concentrations, changes in the escape performance variables and OCR as a function of H2O2 concentration were tested using a linear regression with a significance level (p) of 0.05 after significance between replicates within treatments was tested using ANOVA.
Results
Mortality
No mortality was recorded in any of the control groups. After 1-h exposure to 170 mg·L−1 H2O2, the acute mortality was 38.0% ± 0.08% for copepodite stage V and 97.0% ± 0.03% for adult Calanus spp. females (Table 1); all of the surviving animals in this treatment were immobilized. During the 24-h recovery period, the mortality increased to 92.0% ± 0.01% for copepodite stage V and 100% for adult females. At 85 mg·L−1, total mortality was 34% ± 0.09% (copepodite stage V) and 89% ± 0.17% for adult females. At 17 mg·L−1 total mortality was 30% ± 0.12% and 14% ± 0.03% for stage V and adults, respectively. No delayed mortality or immobilization was observed when exposed to 8.5 mg·L−1 in either stage V or adults. Based on the total mortality rates, the acute 1h-LC50 and total 25h-LC50 values with 95% CIs were calculated (Table 2).
Table 1.
| H2O2 (mg·L−1) | Acute mortality (%) | Total mortality (%) | ||
|---|---|---|---|---|
| Copepodite V | Adult | Copepodite V | Adult | |
| 0 | 0 | 0 | 0 | 0 |
| 8.5 | 0 | 0 | 0 | 0 |
| 17 | 14 ± 0.03 | 14 ± 0.09 | 30 ± 0.12 | 14 ± 0.03 |
| 85 | 14 ± 0.12 | 74 ± 0.08 | 34 ± 0.09 | 89 ± 0.17 |
| 170 | 38 ± 0.08 | 97 ± 0.03 | 92 ± 0.01 | 100 |
| 340a | 100 | 100 | — | — |
| 1700a | 100 | 100 | — | — |
a
From the preliminary study.
Table 2.
| Hydrogen peroxide (mg·L−1) | ||||
|---|---|---|---|---|
| Copepodite V | 1h-LC10 | 29.3 (18.8–39.7) | 25h-LC10 | 10.9 (6.5–15.3) |
| 1h-LC50 | 214.1 (150.7–277.4) | 25h-LC50 | 77.1 (57.9–96.2) | |
| 1h-LC90 | 1566.1 (673.75–2458.6) | 25h-LC90 | 545.6 (284.8–806.5) | |
| Adults | 1h-LC10 | 17.2 (14.6–19.8) | 25h-LC10 | 11.6 (9.1–14.2) |
| 1h-LC50 | 48.3 (44.9–52.2) | 25h-LC50 | 30.6 (25.4–35.8) | |
| 1h-LC90 | 135.2 (121.5–148.9) | 25h-LC90 | 80.7 (60.6–100.8) |
Escape response
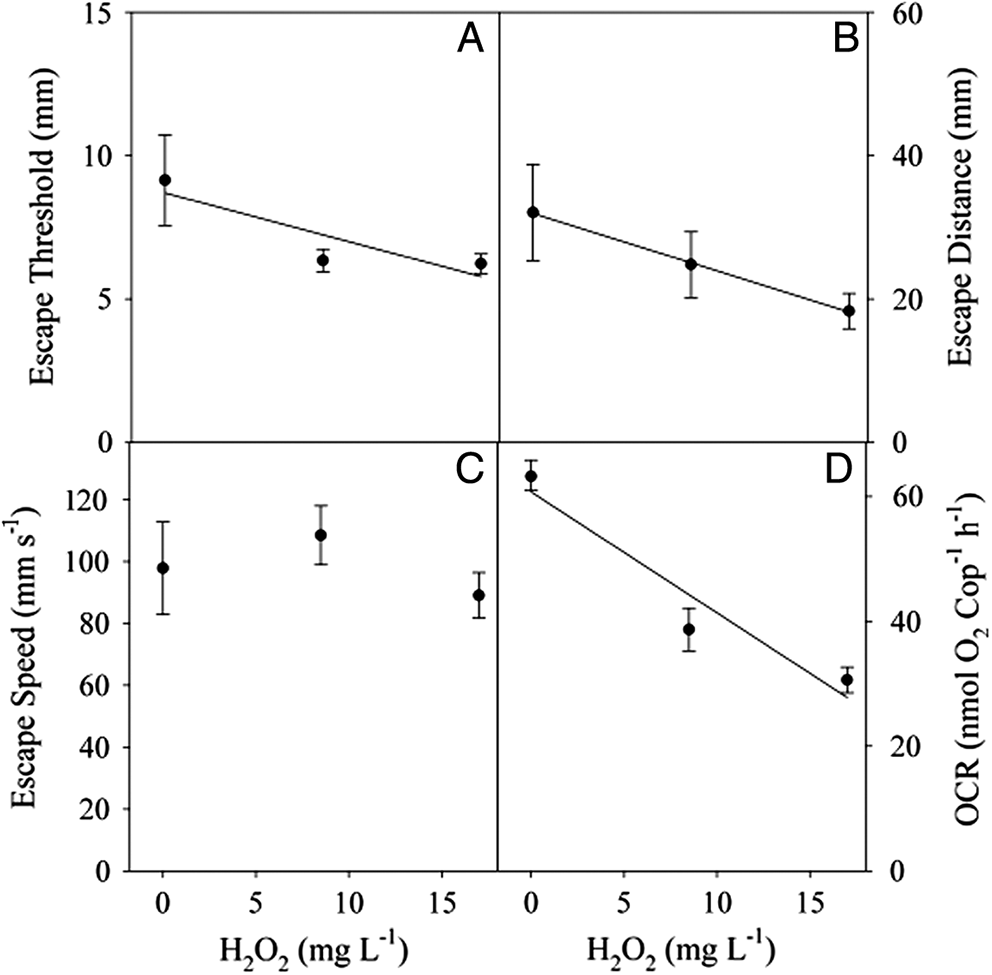
Biological replicates within each treatment were not significantly different (ANOVA p < 0.5) so they were pooled for further statistical analysis. Copepods from the control, 8.5 and 17.0 mg·L−1 H2O2 treatments successfully escaped the siphon 94% of the time. There was a significant difference in the escape performance between the control copepods and those exposed to 8.5 or 17.0 mg·L−1. The escape threshold decreased significantly with increased concentration (y = 8.24 − 0.14x; R2 = 0.13; p = 0.015) (Fig. 2A). Every 10 mg·L−1 increase in concentration caused a 1.4 mm decrease in distance from the siphon at which the copepod initiated the escape reaction. Similarly, once the copepod initiated the escape response the distance that Calanus spp. traveled decreased with increasing H2O2 (y = 31.84 − 0.8x; R2 = 0.103; p = 0.031) (Fig. 2B). Every 10 mg·L−1 increase in concentration caused an 8-mm decrease in distance traveled away from the siphon. However, there was no significant difference in the escape speed between the control and treatment levels up to 17 mg·L−1 (p = 0.32) (Fig. 2C). At higher concentrations (85 mg·L−1), none of the copepods made a successful escape reaction from the siphon or even initiated an escape response. Although alive, the copepods exposed to concentrations of 85 mg·L−1 showed limited swimming ability and were most often lying on the bottom of the tank.
Fig. 2.
Oxygen consumption rate (OCR)
OCR was measured at H2O2 concentrations at which copepods showed a behavioral response to the siphon (0, 8.5, and 17 mg·L−1 H2O2 treatments). OCR decreased significantly in the H2O2 treatments relative to controls (y = 60.75 − 1.93x; R2 = 0.886; p = 0.005). At 12 °C, OCR for Calanus spp. decreased from 64 nmol O2 ind−1·h−1 in the control group to 24 nmol O2 ind−1·h−1and 39 nmol O2 ind−1·h−1 in 8.5 and 17.0 mg·L−1 H2O2 respectively (Fig. 2D). At 85 mg·L−1 H2O2, the copepods were still alive but were unresponsive; their OCR was not significantly different from that of controls (ANOVA, p > 0.5).
Discussion
The recommended concentration of H2O2 used to treat salmon lice (1700 mg·L−1) causes acute mortality in wild-captured Calanus spp. A 1-h exposure to the recommended treatment concentration, and to 20% of the recommended concentration (340 mg·L−1), caused 100% mortality in both copepodite stage V and adult females. As the concentration of H2O2 is decreased, the mortality in Calanus spp. also decreased. At the lowest concentration tested (8.5 mg·L−1) no acute or delayed mortality was observed. The acute mortality (recorded immediately after exposure), was lower than the total mortality recorded after a 24-h recovery period. These results suggest that it is important to include a 24-h recovery period in these types of experiments to obtain an accurate estimate of mortality.
Adult Calanus spp. showed higher mortality to H2O2 exposure than copepodite stage V. Similar stage-specific differences in sensitivity to H2O2 exposure have been reported for other copepod species (Acartia sp.; Van Geest et al. 2014), including salmon lice (L. salmonis; Mitchell and Collins 1997) and their eggs (Aaen et al. 2014; Bravo et al. 2015). We found no available data on the sensitivity of Calanus spp. eggs or nauplii to H2O2. However, given the abundance of ovigerous copepod females present during the spring period when H2O2 application is the highest (Grefsrud et al. 2018), this should be tested in future.
The immobilization of the copepods observed in this study was expected as it is one of the effects of H2O2 on salmon lice. Bubbles of O2 gas in the haemolymph are the primary cause of sea lice detachment from the host following treatment with H2O2 (Cotran et al. 1989; Treasurer et al. 2000; Aaen et al. 2014). However, the formation of gas bubbles observed in salmon lice (Bruno and Raynard 1994) was not reported in Acartia hudsonica, Metacarcinus edwardsii, or in Calanus spp. (Van Geest et al. 2014; Gebauer et al. 2017; Hansen et al. 2017), so the mechanism of immobilization observed in Calanus spp. is unclear.
The distance from the predator at which a copepod initiates an escape reaction, and the strength of the escape reaction, are decisive factors in the copepod’s ability to avoid predation (Fields and Yen 1997). Calanus spp. that were not exposed to H2O2 (control) showed escape performance metrics consistent with earlier studies on copepods (Fields et al. 2012). At sublethal levels, exposure to H2O2 had measurable effects on the escape reaction of Calanus spp. At a concentration of 85 mg·L−1, approximately 25% of the exposed adult copepods survived a 1-h exposure, yet none of these survivors made a successful escape from the siphon. Many of the copepods exposed to 17 mg·L−1 were partially immobilized and sank to the bottom of the aquarium during exposure, unlike copepods exposed at 8.5 mg·L−1 at which no immobilization was observed. Calanus spp. showed a decrease in the threshold distance at which they initiated their escape reaction with increased H2O2 concentration and after the copepods initiated the escape response, they traveled a significantly shorter distance from the siphon. These behavioral results suggest that Calanus spp. exposed to sublethal concentrations of H2O2 (at 1% of the recommended treatment levels) will be more susceptible to predation because of an impaired escape response. These results are consistent with the findings of Van Geest et al. (2014) who observed immobilization of A. hudsonica after 15 min of exposure to concentrations of ≥10 mg·L−1.
Exposed copepods also experienced reduced OCR in response to increased concentrations of H2O2. The lower metabolic activity is a likely cause of the decreased distance traveled during the escape. In addition, the lower metabolic activity may impact the repetitive escape reaction of copepods. The escape reaction of copepods is energetically costly (Strickler 1975) and the strength of the response decreases with increased escape frequency (Fields 2000). A lower OCR will decrease the ability of the copepod to perform multiple escape reactions and thereby further increase their predation risk (Fields 2000).
At the highest sublethal concentrations tested (85 mg·L−1; 5% of treatment levels), Calanus spp. were unable to swim and sank to the bottom of the tank. These individuals exhibited no response to the predator mimic. Paradoxically, the respiration rates of the copepod at these higher levels of exposure were similar to levels measured in the controls. These results suggest a change in the mode of action of the H2O2 (Rand 1995). At higher concentration (above 17 mg·L−1), H2O2 may produce narcosis, causing partial paralysis. The risk of predation for these copepods with no escape reactions is extremely high. The data show that some of these animals may recover normal escape behavior; however, since predation can have a significant effect on the population dynamics of invertebrates (Pangle et al. 2007), sublethal effects on escape behavior potentially have important ecological implications for the affected population. The results of this study provide supporting evidence that theoretical “safe” values, traditionally used for predicting toxicity thresholds, underestimate the impact of H2O2 on the physiological condition of nontarget crustaceans. This warrants additional research.
Our results indicate that the No Observable Effect Concentration for Calanus spp. is between 8.5 and 17 mg·L−1. This is considerably higher than the concentration reported for another calanoid copepod species, A. hudsonica, for which the sublethal concentration level of 2.6–10.0 mg·L−1 (EC50) was determined based on feeding rate measurements (Van Geest et al. 2014). This suggests that the impact of H2O2 on copepods is species specific. Copepods as a group may be more sensitive to H2O2 than other planktonic crustaceans. For example, Gebauer et al. (2017) reported a LC50 for the mola rock crab larvae (M. edwardsii) of 1642 mg·L−1, two orders of magnitude higher than thresholds for Calanus spp.
While it is clear that even 0.5% of the standard treatment concentration of H2O2 has a detrimental effect on Calanus spp., the dispersal and dilution processes that affect the effluent plumes after treatments at aquaculture sites are still unclear (Ernst et al. 2001). Development and testing of dispersion models, including field studies to verify the models, will be important to evaluate the broader impact of H2O2 on the organisms living around salmon farms.
Acknowledgements
This work was funded by the Norwegian Institute of Marine Research project “Fine scale interactions in the plankton” (Project #81529) led by H.I.B. Thanks to all staff at IMR Austevoll for their assistance with these experiments.
References
Aaen SM, Aunsmo A, and Horsberg TE. 2014. Impact of hydrogen peroxide on hatching ability of egg strings from salmon lice (Lepeophtheirus salmonis) in a field treatment and in a laboratory study with ascending concentrations. Aquaculture, 422: 167–171.
Bravo S, Silva MT, Agusti C, Sambra K, and Horsberg TE. 2015. The effect of chemotherapeutic drugs used to control sea lice on the hatching viability of egg strings from Caligus rogercresseyi. Aquaculture, 443: 77–83.
Brokke KE. 2015. Mortality caused by de-licing agents on the non-target organisms chameleon shrimp (Praunus flexuosus) and grass prawns (Palaemon elegans). M.Sc. thesis, University of Bergen, Bergen, Norway.
Broms C, Melle W, and Kaartvedt S. 2009. Oceanic distribution and life cycle of Calanus species in the Norwegian Sea and adjacent waters. Deep Sea Research Part II: Topical Studies in Oceanography, 56(21–22): 1910–1921.
Browman HI, St-Pierre JF, Skiftesvik AB, and Racca RG. 2003. Behaviour of Atlantic cod (Gadus morhua) larvae: an attempt to link maternal condition with larval quality. In The big fish bang. Proceedings of the 26th Annual Larval Fish Conference. Edited by HI Browman and AB Skiftesvik. Institute of Marine Research, Bergen, Norway. pp. 71–95.
Bruno DW, and Raynard RS. 1994. Studies on the use of hydrogen peroxide as a method for the control of sea lice on Atlantic salmon. Aquaculture International, 2(1): 10–18.
Burridge LE, Lyons MC, Wong DKH, MacKeigan K, and VanGeest JL. 2014. The acute lethality of three anti-sea lice formulations: AlphaMax®, Salmosan®, and Interox® Paramove™ 50 to lobster and shrimp. Aquaculture, 420: 180–186.
Costello MJ. 2006. Ecology of sea lice parasitic on farmed and wild fish. Trends in Parasitology, 22(10): 475–483.
Costello MJ. 2009. The global economic cost of sea lice to the salmonid farming industry. Journal of Fish Diseases, 32(1): 115–118.
Cotran RS, Kumar V, and Robbins SL. 1989. Pathological basis of disease. 4th edition. W.B. Saunders, Toronto, Ontario.
Dalpadado P, Ellertsen B, Melle W, and Dommasnes A. 2000. Food and feeding conditions of Norwegian spring-spawning herring (Clupea harengus) through its feeding migrations. ICES Journal of Marine Science: Journal du Conseil, 57(4): 843–857.
Ernst W, Jackman P, Doe K, Page F, Julien G, MacKay K, et al. 2001. Dispersion and toxicity to non-target aquatic organisms of pesticides used to treat sea lice on salmon in net pen enclosures. Marine Pollution Bulletin, 42(6): 432–443.
Fields DM. 2000. Characteristics of the high frequency escape reactions of Oithona SP. Marine and Freshwater Behaviour and Physiology, 34(1): 21–35.
Fields DM, and Yen J. 1997. The escape behavior of marine copepods in response to a quantifiable fluid mechanical disturbance. Journal of Plankton Research, 19(9): 1289–1304.
Fields DM, Shema SD, Browman HI, Browne TQ, and Skiftesvik AB. 2012. Light primes the escape response of the calanoid copepod, Calanus finmarchicus. PLoS ONE, 7(6): e39594.
Finney DJ. 1971. Probit analysis. 3rd edition. Cambridge University Press, Cambridge, UK.
Finstad B, Bjørn PA, Grimnes A, and Hvidsten NA. 2000. Laboratory and field investigations of salmon lice [Lepeophtheirus salmonis (Krøyer)] infestation on Atlantic salmon (Salmo salar L.) post-smolts. Aquaculture Research, 31(11): 795–803.
Gebauer P, Paschke K, Vera C, Toro JE, Pardo M, and Urbina M. 2017. Lethal and sub-lethal effects of commonly used anti-sea lice formulations on non-target crab Metacarcinus edwardsii larvae. Chemosphere, 185: 1019–1029.
Grefsrud ES, Glover K, Grøsvik BE, Husa V, Karlsen Ø, Kristiansen T, et al., (Editors). 2018. Risikorapport norsk fiskeoppdrett 2018. Fisken og havet, særnr. 1-2018. Havforskningsinstituttet, Bergen, Norway [online]: Available from hi.no/publikasjoner/andre_publikasjoner/risikovurdering_miljovirkninger_av_norsk_fiskeoppdrett/nb-no.
Hansen BH, Hallmann A, Altin D, Jensen BM, and Ciesielski M. 2017. Acute hydrogen peroxide (H2O2) exposure does not cause oxidative stress in late-copepodite stage of Calanus finmarchicus. Journal of Toxicology and Environmental Health, Part A, 80(16–18): 820–829.
Heath MR, and Lough RG. 2007. A synthesis of large-scale patterns in the planktonic prey of larval and juvenile cod (Gadus morhua). Fisheries Oceanography, 16(2): 169–185.
Johnson SC, Bravo S, Nagasawa K, Kabata Z, Hwang JS, Ho JS, et al. 2004. A review of the impact of parasitic copepods on marine aquaculture. Zoological Studies, 43(2): 229–243.
Kiørboe T, Saiz E, and Visser A. 1999. Hydrodynamic signal perception in the copepod Acartia tonsa. Marine Ecology Progress Series, 179: 97–111.
Liu Y, and Bjelland H. 2014. Estimating costs of sea lice control strategy in Norway. Preventive Veterinary Medicine, 117(3–4): 469–477.
Liu Y, Olaussen JO, and Skonhoft A. 2011. Wild and farmed salmon in Norway—a review. Marine Policy, 35(3): 413–418.
Melle W, Runge J, Head E, Plourde S, Castellani C, Licandro P, et al. 2014. The North Atlantic Ocean as habitat for Calanus finmarchicus: environmental factors and life history traits. Progress in Oceanography, 129(Part B): 244–284.
Mitchell AJ, and Collins C. 1997. Review of the therapeutic uses of hydrogen peroxide in fish production. Aquaculture Magazine, 23(3): 74–79.
Pangle KL, Peacor SD, and Johannsson OE. 2007. Large nonlethal effects of an invasive invertebrate predator on zooplankton population growth rate. Ecology, 88(2): 402–412.
Rand G. 1995. Fundamentals of aquatic toxicology: effects, environmental fate, and risk assessment. CRC Press, Boca Raton, Florida. ISBN 1-56032-091-5.
Rullyanto A, Jónasdóttir SH, and Visser AW. 2015. Advective loss of overwintering Calanus finmarchicus from the Faroe–Shetland Channel. Deep Sea Research Part I: Oceanographic Research Papers, 98: 76–82.
Runge JA, and de Lafontaine Y. 1996. Characterization of the pelagic ecosystem in surface waters of the northern Gulf of St. Lawrence in early summer: the larval redfish-Calanus-microplankton interaction. Fisheries Oceanography, 5(1): 21–37.
Runge JA, Fields DM, Thompson CRS, Shema SD, Bjelland RM, Durif CMF, et al. 2016. End of the century CO2 concentrations do not have a negative effect on vital rates of Calanus finmarchicus, an ecologically critical planktonic species in North Atlantic ecosystems. ICES Journal of Marine Science: Journal Du Conseil, 73(3): 937–950.
Strickler JR. 1975. Swimming of planktonic Cyclops species (Copepoda, Crustacea): pattern, movements and their control. In Swimming and flying in nature. Edited by TY-T Wu, CJ Brokaw, and C Brennen. Springer, Boston, Massachusetts. pp. 599–613.
Sundby S. 2000. Recruitment of Atlantic cod stocks in relation to temperature and advection of copepod populations. Sarsia, 85(4): 277–298.
Thomassen JM. 1993. A new method for control of salmon lice. In Fish farming technology. Edited by H Reinertsen, LA Dahle, L Jørgensen, and K Tvinnereim. Balkema, Rotterdam, the Netherlands. pp. 233–236.
Torrissen O, Jones S, Asche F, Guttormsen A, Skilbrei OT, Nilsen F, et al. 2013. Salmon lice—impact on wild salmonids and salmon aquaculture. Journal of Fish Diseases, 36(3): 171–194.
Treasurer JW, Grant A, and Davis PJ. 2000. Physical constraints of bath treatments of Atlantic salmon (Salmo salar) with a sea lice burden (Copepoda: Caligidae). Contributions to Zoology, 69: 129–136.
Van Geest JL, Burridge LE, Fife FJ, and Kidd KA. 2014. Feeding response in marine copepods as a measure of acute toxicity of four anti-sea lice pesticides. Marine Environmental Research, 101: 145–152.
Young ND, Crosbie PBB, Adams MB, Nowak BF, and Morrison RN. 2007. Neoparamoeba perurans n. sp., an agent of amoebic gill disease of Atlantic salmon (Salmo salar). International Journal for Parasitology, 37(13): 1469–1481.
Information & Authors
Information
Published In

FACETS
Volume 4 • Number 1 • June 2019
Pages: 626 - 637
Editor: Sophia Johannessen
History
Received: 27 February 2019
Accepted: 9 September 2019
Version of record online: 19 December 2019
Copyright
© 2019 Escobar-Lux et al. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Data Availability Statement
All relevant data are within the paper.
Key Words
Sections
Subjects
Plain Language Summary
Pesticides that remove salmon lice harm other tiny crustaceans found near fish farms
Authors
Author Contributions
RHE-L, DMF, HIB, RMB, ABS, and CMFD conceived and designed the study.
RHE-L, DMF, SDS, and RMB performed the experiments/collected the data.
RHE-L, DMF, and CMFD analyzed and interpreted the data.
HIB, A-LA, ABS, and OBS contributed resources.
All drafted or revised the manuscript.
Competing Interests
The authors have declared that no competing interests exist.
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Rosa H. Escobar-Lux, David M. Fields, Howard I. Browman, Steven D. Shema, Reidun M. Bjelland, Ann-Lisbeth Agnalt, Anne Berit Skiftesvik, Ole B. Samuelsen, and Caroline M.F. Durif. 2019. The effects of hydrogen peroxide on mortality, escape response, and oxygen consumption of Calanus spp.. FACETS.
4(1): 626-637. https://doi.org/10.1139/facets-2019-0011
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
Cited by
1. Analysis of spatial conflicts of large scale salmonid aquaculture with coastal fisheries and other interests in a Norwegian fjord environment, using the novel GIS-tool SEAGRID and stakeholder surveys
2. Interspecific differences in oxidative DNA damage after hydrogen peroxide exposure of sea urchin coelomocytes
3. Drug and pesticide usage for sea lice treatment in salmon aquaculture sites in a Canadian province from 2016 to 2019
4. Calcium peroxide (CaO2) granules enclosed in fabrics as an alternative H2O2 delivery system to combat Microcystis sp.
5. Effect of sea lice chemotherapeutant hydrogen peroxide on the photosynthetic characteristics and bleaching of the coralline alga Lithothamnion soriferum
6. Pelagic ecosystem dynamics between late autumn and the post spring bloom in a sub-Arctic fjord
7. Higher sensitivity to hydrogen peroxide and light stress conditions of the microcystin producer Microcystis aeruginosa sp PCC7806 compared to non-producer strains.
8. The High Seas Solution
9. Interactions between finfish aquaculture and American lobster in Atlantic Canada
10. Outdoor disinfectant sprays for the prevention of COVID-19: Are they safe for the environment?
11. Mind the Depth: The Vertical Dimension of a Small‐Scale Coastal Fishery Shapes Selection on Species, Size, and Sex in Wrasses
12. The Acute and Delayed Mortality of the Northern Krill (Meganyctiphanes norvegica) When Exposed to Hydrogen Peroxide
13. Short-term exposure to hydrogen peroxide induces mortality and alters exploratory behaviour of European lobster (Homarus gammarus)
14. The impact of anti-sea lice pesticides, azamethiphos and deltamethrin, on European lobster (Homarus gammarus) larvae in the Norwegian marine environment
15. Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human- and climatically-altered world
