Introduction
Synchronous range dynamics have been observed in the two most common native felids in North America, the bobcat (
Lynx rufus) and the Canada lynx (
Lynx canadensis). The Canada lynx range has contracted by 40% compared to its historical extent in the 18th century (
Laliberte and Ripple 2004). In contrast, the bobcat seems to be reclaiming its historical range after a decline due to overharvest in the 20th century and expanding its range northward into lynx territory (
de Vos 1964;
Lavoie et al. 2009;
Roberts and Crimmins 2010). At the turn of the 20th century, scientists noted how the ranges of these two felids seemed to be changing simultaneously (
de Vos 1964;
Hoving et al. 2003). The progression of bobcats into former lynx habitat could be a result of land clearing, since bobcats seem to prefer the more open habitat and young deciduous forests (
de Vos 1964). However, there are some exceptions to this general trend. In British Columbia, Canada, the two species’ ranges seem not to have changed since 1935 (
Gooliaff and Hodges 2018).
Some studies have discussed interactions between the two species.
Parker et al. (1983) reported that bobcats expanded into former lynx habitat in Cape Breton, Nova Scotia. However, there was no evidence of interaction between the species.
Peers et al. (2013) conducted a broadscale continental analysis and suggested that lynx and bobcats might compete for resources. In sympatry, lynx exploited a narrower range of environmental characteristics relating to climate (e.g., snow depth, minimum temperature of the coldest month) and elevation, whereas bobcats broadened their niche. In British Columbia, Canada,
Gooliaff et al. (2018) found that bobcats were restricted to the south, whereas lynx were found in the interior. The two species did, however, overlap in the southern part of the province, but lynx were generally found at higher elevations than bobcats. In another study in northern Washington, USA,
Scully et al. (2018) found that lynx avoided camera sites where bobcats were present. In general, these studies provide some evidence of spatial avoidance from a continental to population scale between these closely related felids.
In many cases snow conditions have been suggested to be important limiting factors for bobcats, essentially limiting their progression into lynx territory (
Marston 1942;
McCord 1974;
Parker et al. 1983;
Hoving et al. 2003). For example,
Parker et al. (1983) suggested that deep winter snow kept bobcats from moving into the Cape Breton highlands of Nova Scotia, Canada. Bobcats were, however, able to colonize and establish a breeding population on the lowlands of the island, where winter snow is much shallower than the highlands, immediately after the construction of a causeway to the mainland in 1955 (
Parker and Smith 1983;
Matlack and Evans 1992). Both species are similar in weight, but lynx have much larger feet and can support at least twice the weight compared with bobcats at the same snow density (
Parker et al. 1983). Accordingly, lynx likely have a competitive advantage over bobcats in catching prey in deep snow conditions (
Larivière and Walton 1997;
Nowak and Walker 1999;
Anderson and Lovallo 2003). Conversely, bobcats generally occupy areas in North America that have shallow or no snow (
Nowak and Walker 1999).
There are few field studies of sympatric populations of bobcats and lynx, and these studies have been limited to the contact zone in western North America (
Gooliaff et al. 2018;
Scully et al. 2018). Other than anecdotes by
Parker et al. (1983) and broadscale analyses using genetics (
Koen et al. 2014a) and occurrences from museum and harvest records (
Peers et al. 2012,
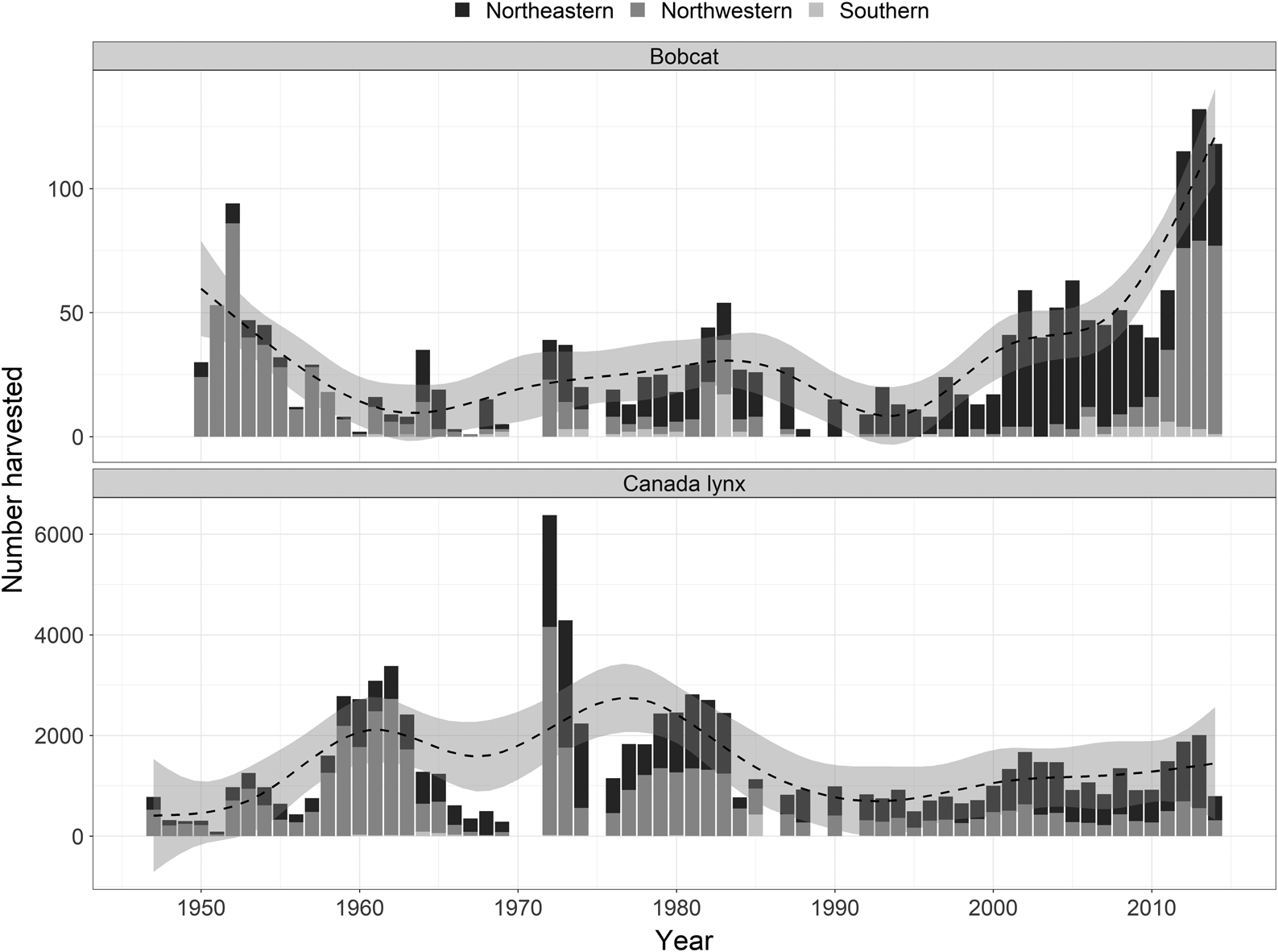
2013), regional or finer-scale field studies have not been undertaken elsewhere. In northern Ontario, Canada, the number of incidentally harvested bobcats has been increasing since the early 1990s, whereas the number of harvested lynx has been relatively stable since the mid-80s (
Fig. 1). Bobcats were first reported by trappers in northwestern Ontario at the beginning of the 20th century, while reports in the northeast near Sault Ste. Marie, Ontario, began in the 1940s (
Peterson and Downing 1952;
de Vos 1964). After the early 1940s, the number of bobcats harvested increased steadily and bobcats were harvested farther north each year (
de Vos 1964). From 1964 until present day, bobcats likely continued their progression into the province, based on summaries of annual fur harvest data (
Fig. 1). Conversely, even though the total number of harvested lynx has recently been stable, the range of the lynx has been contracting northwards (
Koen et al. 2014b). The range contraction of lynx might be associated with the current bobcat range expansion. It seems that over the past several decades, bobcats have expanded into areas that were once occupied by lynx. It is unknown whether the incursion of bobcats is the cause of the lynx range contraction or whether bobcats have spread into habitat after it has already been vacated by lynx. In any case, these dynamic processes might provide additional insight into how these closely related species coexist in a contact zone.
We assessed home-range level occupancy of bobcats and Canada lynx on the north shore of Lake Huron in Ontario, Canada. We sought to determine to what degree the space and habitat use of these species overlap in an area of regional scale range sympatry. We hypothesized that if there was spatial segregation between the two species, then there would be a negative relationship between their occupancies. We also hypothesized that if there was habitat partitioning, then there would be measurable differences in land cover, prey, and snow conditions associated with the habitat used by each species.
Considering the geography of our study area within the northern range of the bobcat and its generalist nature, we predicted that bobcats occupy areas in the south that are predominantly human-altered (agricultural fields, pastures, urban areas, roads, etc.). Bobcats in these areas should be exposed to a higher diversity of prey species associated with anthropogenic environments and shallower, more compacted snow. In contrast, given their habitat specialist nature, lynx should occupy forest stands with coniferous cover, low human disturbance, and a low diversity of prey species but high snowshoe hare and red squirrel (Tamiasciurus hudsonicus) activity. Consequently, because of their respective habitat preferences and differing abilities to move through deep snow, we predicted that bobcat and lynx occupancy would have an inverse relationship throughout the study area. In general, we sought to record the current state of the spatial and habitat patterns in this area of dynamic range overlap to aid in evaluating causes of current and future range limits.
Materials and methods
We used snowmobile surveys to collect track occurrences of bobcats and lynx in an area of range overlap located on the north shore of Lake Huron between Sault Ste. Marie and Sudbury, Ontario, Canada, from 2016 to 2018, inclusive. Within an area of 32 832 km
2, we identified 41 survey routes and attempted to survey each one repeatedly from January until the end of March (
Fig. 2). While conducting these surveys, we estimated prey activity and snow depth and hardness. We also estimated the probability of occupancy of both species from independent occupancy models while accounting for detectability. We then compared the
n-dimensional niche of each species and investigated the potential for habitat overlap. We tested for spatial segregation and niche overlap between bobcats and lynx.
Sampling extent and survey units
Since the 1970s, bobcats have been almost exclusively caught in traplines and townships found within the Algoma and Sudbury districts of Ontario, except for a recent increase in northwestern Ontario between 2011 and 2014 (
Fig. 1). To define the extent of our study area, we calculated the minimum convex polygon (MCP) of bobcat trapping records (2000–2005). We buffered the 75% MCP by 35 km to accommodate nearby traplines or townships. We then divided our study area into 513 hexagonal sampling units of 64 km
2. The area of these units is equivalent to the home range size of a female bobcat or lynx at the fringe of its geographic distribution (
Fuller et al. 1985;
Squires et al. 2012). Ideally, we wanted a maximum of one female bobcat per sampling unit, so that we could assume that occupancy of each sampling unit was spatially independent. The study area has also historically contained abundant lynx populations (
Fig. 1).
Selecting surveying units
We attempted to sample all land use classes and their combinations. We first preselected sampling units using a clustering algorithm that was based on the associated land cover composition of each hexagon. We used the Ontario Forest Resource Inventory (
OMNRF 2015) maps to categorize each forest stand by Provincial Forest Type and seral stage. Additional areas that were not forest stands were classified as agriculture, water, wetland, and a disturbed class that included developed areas. We then extracted the proportion of each class within each hexagon and clustered these data using Affinity Propagation (
Frey and Dueck 2007). The sampling units clustered into 33 distinct groups characterized by different compositions of land cover. The algorithm produced an exemplar for each group that was the most representative unit of the group and was selected to be surveyed. In cases where the exemplar could not be surveyed due to accessibility, we instead attempted to survey the nearest unit from the same group.
Snowmobile survey routes
We surveyed 41 different routes over three winters from 2016 to 2018. In general, we attempted to map a snowmobile route to and through each selected survey unit with aerial photos, topographic maps, Google maps, and other geographic resources. After at least 48 h, but preferably 72 h, following a track-obliterating snowfall event, an observer followed the mapped route through each survey unit on a snowmobile at 20 km/h. Due to access and time constraints, the average distance surveyed on each route was 9.09 km and the length of these routes ranged from 7 to 11.6 km. However,
Squires et al. (2012) found that after 7 km of searching the probability of detection asymptotes. Only tracks found within a visible distance of the survey route were considered (∼5 m on either side of the observer).
Only 35 of these routes were used in the subsequent analysis (
Fig. 2), because for six transects, snow data were not collected, the route was only surveyed once, the route was never surveyed during good tracking conditions, or we could not assume spatial independence from other routes. For instance, we removed one survey route that ended up on the periphery of another sampling unit. Also, we removed a route that clustered our sampling in one location in our study area. The 35 routes were surveyed between two and seven times over three years, and on average each route was surveyed 4.43 times to get an estimate of detectability.
Felid tracks
We recorded the number of times a set of bobcat or lynx tracks intersected the survey route. We recorded the location of each set of tracks on a GPS unit and noted descriptors of the quality of the track and the confidence of the identification. When a felid track was encountered, the track was also photographed and measured for documentation purposes. In addition, we followed tracks of uncertain identification to confirm species identity. In general, bobcat and lynx tracks in the study area were easy to discriminate because of differences in foot size and furred foot pads (
Elbroch and McFarland 2019).
Potential prey and carrion
There is no information on the diet of the bobcats in Ontario, but bobcats from our study area are part of a subspecies that includes the northwestern Great Lakes region (
Reding et al. 2012). Studies from Minnesota and Wisconsin have reported that cervids and lagomorphs are the main prey for bobcats in this region and can on average make up 40.2% and 31.3% of the diet, respectively (
Rollings 1945;
Gilbert 2003). In addition, North American porcupine (
Erethizon dorsatum) and smaller mammals and birds can make up to 12.3%, 6.4% and 1.6% of the diet, respectively (
Rollings 1945).
The nearest study of winter lynx diet, 500 km east in Minnesota, found that snowshoe hare made up 92% of predation events (
Hanson and Moen 2015). However,
Roth et al. (2007) indicated that snowshoe hare may represent only 63% and 68% of lynx diets in Minnesota and Ontario, respectively. This suggests that although it is a specialist on snowshoe hare, the lynx might switch to alternative prey species when required (
Roth et al. 2007). As an alternative to hares, lynx might prey on red squirrels, spruce or ruffed grouse (
Falcipennis canadensis and
Bonasa umbellus, respectively) and small mammals (
Aubry et al. 2000;
Hodges 2000). In the southern boreal forest, small mammals other than squirrels only make up 3%–8%, and small birds 1%–7%, of the diet of Canada lynx (
Aubry et al. 2000).
We recorded track activity of both main and alternative prey species and potential carrion that both species would encounter in our study area. We recorded occurrences of North American beaver (Castor canadensis), eastern gray (Sciurus carolinensis) or American red squirrel, spruce or ruffed grouse, moose, North American porcupine, raccoon (Procyon lotor), striped skunk (Mephitis mephitis), snowshoe hare, wild turkey (Meleagris gallopavo), and white-tailed deer. We did not account for small rodents and passerines because of our use of snow tracking as a sampling method, which is ineffective for assessing the abundance of these groups.
Due to time constraints, we stopped counting tracks for a given species after 100 tracks were reached on each transect. To account for this data censoring, we divided these censored values by the survey route length and multiplied this value (tracks per kilometer) by the length of the shortest survey route that was censored. To account for the different survey lengths of each route and track accumulation over time, we divided these values by the survey length and then divided by the number of days since the last snowfall. Therefore, these values were the rate of track accumulation of each species per kilometer per day since the last snowfall.
Statistical modelling
The observation of tracks on survey routes may be influenced by detectability. Under-detecting occupancy could be caused by animals not yet having moved through the area, tracks missed because of poor conditions, or tracks missed because of observer error. Therefore, when investigating influences on occupancy of an area it is suggested to account for detectability, since it may influence the parameter estimation of habitat predictors (
MacKenzie et al. 2006). We modelled both bobcat and lynx occupancy using a single-season occupancy model based on a zero-inflated binomial distribution; covariates were modelled using a logit link (
MacKenzie et al. 2006;
Royle and Dorazio 2008). The predictors of these occupancy models were separated into two classes, observation-level and site-level covariates. Observation-level covariates may vary between sampling occasions (snow conditions, temperature, etc.), whereas site-level covariates are characteristics of a sampling location that remain somewhat constant (land use, land cover, prey density, etc.).
Observation-level covariates
In our analysis, the observation-level covariates described the general meteorological conditions during which the snowmobile surveys were undertaken and the quality of the tracking conditions. We accounted for tracking conditions using the number of days since the last snowfall. We also thought that the minimum temperature of the previous night would be a good indicator of whether bobcats or lynx would be active, since we noticed while we were live trapping and tracking these species with GPS collars that they were less active during nights that were below −20 °C. We also thought that the different lengths of the survey routes might influence detectability. We did not account for the year of the survey as an observation-level covariate, because this would have suggested that there was detectability bias between years. We consider this unlikely, because the same observer surveyed for snow tracks during the entire study. Also, the addition of a time index such as year in our occupancy model would have served as a surrogate for observation-level covariates, but the post-hoc interpretation of this time index would have been complicated, because it is generally uninformative and not biologically relevant (e.g.,
Howe et al. 2013).
Site-level covariates
The site-level covariates included the potential habitat characteristics that bobcats and lynx avoided or were associated with within areas where they occurred. We accounted for three types of site-level covariates related to prey activity, snow conditions, and land cover. We generated two different types of prey indices. First, we estimated abundance of snow tracks of different prey species or species groups. We included the average track accumulation of prey species that were found on the survey route, but we only kept prey or carrion species that had an average track accumulation >1 track per 10 km in a day. Although we tracked the abundance of all potential prey we could observe using these methods, for our occupancy analysis, we focused on prey types that we considered particularly important for bobcats and lynx: hare, deer, grouse, and squirrel. As a second type of prey index, we estimated prey richness by counting the number of different prey or carrion species found on each route over the three winters (including rare species). However, we had to remove the effect of sampling effort from the richness measure, which in this case was the total distance surveyed on each survey route. We fit the total number of kilometers surveyed on each route to the number of prey species found using ordinary least squares regression. We then predicted the number of prey species that would be found on a 7 km route and added the residuals of each survey route to this value.
We accounted for the average snow conditions measured during the last two winters of the study (2017 and 2018) by calculating the average snow depth and snow hardness of each route over these two years. During each sampling occasion we measured snow depth and hardness three times at points evenly distributed across the survey route. Snow depth was measured with a metal meter stick and visually verified by digging away snow if necessary. Snow hardness was the depth that a 150 g plastic ball fell through the snow when dropped from a height of 1 m above the surface of the snow. Consequently, a small value indicated compacted snow and a large value indicated soft snow.
We included the proportion of different land cover types found within a 1 km buffer of the survey route. Land cover covariates are the proportion of land occupied by anthropogenic disturbances (agricultural and rangeland, roads, highways, railways, urban areas, mines, etc.), wetlands (wetlands, lakes, and rivers), and the proportion of land occupied by coniferous, deciduous, and mixed forest, and their associated seral stage (immature or mature forest).
Occupancy analysis
We gathered many habitat predictors that were related to aspects of the niche of bobcats and lynx that have been investigated in the past. Unfortunately, many of these predictors were collinear, which may cause unstable parameter estimates, inflated standard error, and potentially biased inference (
Dormann et al. 2013). Consequently, we used principal component analysis to create orthogonal latent predictors that represented habitat gradients found across our survey routes. We first selected significant principal components using the Auer–Gervini method (
Auer and Gervini 2008) with the R package “PCDimension” (
Coombes and Wang 2018). We then selected latent predictors that we could easily relate to measured habitat characteristics. We used a Pearson’s correlation coefficient to identify the habitat characteristics responsible for the major variation of each principal component or latent predictor. We discarded latent predictors that did not have an absolute Pearson’s correlation >0.5 against any habitat descriptor. We further limited these latent predictors to those that described more than one habitat condition. This left us with a reduced set of latent predictors that described several major habitat gradients that might explain bobcat and lynx occupancy across our survey routes.
We chose the models with the lowest Akaike information criterion corrected for small sample sizes (AICc). If there were any models within 2 ΔAICc from the top model we used model averaging. We then further investigated the relationship between probability of occupancy (
Ψ) and detection (
ρ) for each species and habitat gradients. Next, we predicted the probability of detection of both species during each survey to calculate the average probability of detection, and we also predicted the probability of detection of the two species over all three observation-level covariates. We predicted the probability of occupancy over the range of each predictor found in the top models to investigate their importance. We also predicted the probability of occupancy of each species over our study area to investigate the spatial relationship and potential habitat overlap between bobcats and lynx. When investigating the importance of a covariate over
Ψ and
ρ, we fixed other covariates to the median value found on our survey routes. We fit all occupancy models with the package “unmarked” (
Fiske and Chandler 2011) in R version 3.5.1 (
r-project.org). We used the function “occu” within the unmarked package.
Results
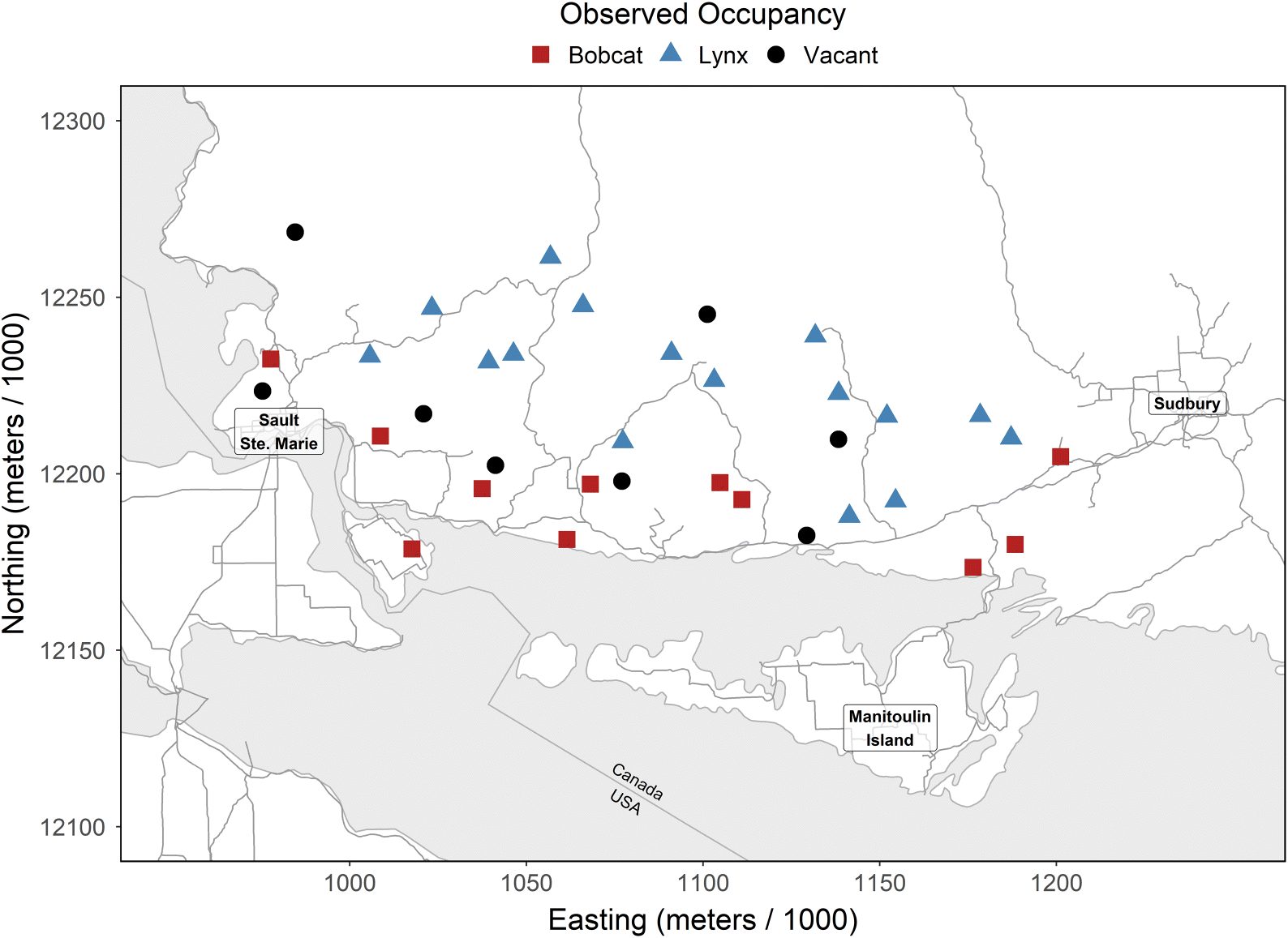
Of the 35 routes used in the analysis, lynx tracks were found on 16 routes, bobcat tracks were found on 11 routes, and neither species was found on eight routes. We never found both species on the same survey route. Of the 155 surveys, lynx tracks were found on 39 surveys and bobcat tracks were found on 19 surveys. Lynx track activity ranged from 0 to 4 crossings per survey and bobcat ranged from 0 to 3 crossings. Bobcats were generally found near the shores of Lake Huron, whereas lynx were found in areas slightly farther north or inland (
Fig. 2).
The survey routes were dominated by deciduous mature forest (
Fig. S1). However, lynx occupied areas with a higher average proportion of coniferous forest and bobcats were on average more frequently found in areas with a higher proportion of anthropogenic disturbance and near wet areas (wetlands, rivers, or lakes). On the survey routes, we found track evidence of beaver, squirrel, grouse, moose, porcupine, raccoon, skunk, hare, turkey, and deer (
Fig. S2). Bobcats and lynx occupied areas with similar beaver, squirrel, grouse, moose, and snowshoe hare track activity. However, sites occupied by bobcats had skunk, porcupine, and turkey, whereas sites occupied by lynx did not. Also, routes with bobcats had on average higher track activity of white-tailed deer and raccoon. In addition, areas with bobcat activity had higher average prey richness compared to areas where lynx tracks were found (
Fig. S3). Finally, the snow conditions of survey routes where lynx tracks were found were like those of routes on which bobcat tracks were found (
Fig. S4).
Principal component analysis
We performed a principal component analysis on 14 of 20 of the habitat variables (
Fig. S5). We only included snowshoe hare, squirrel, deer, and grouse as prey in the analysis, since track accumulation was generally higher for these prey items across the study area, and we suspected that these species were particularly important resources for bobcats and lynx. The Auer–Gervini method indicated that up to six principal components were likely signals and not noise in the data. However we further investigated and found that only four principal components best described these 14 habitat predictors and the remaining axes were difficult to interpret, because we could not easily link them back to the habitat variables (i.e., there was a low correlation) or only a single habitat characteristic dominated the loadings of the principal component (e.g., components 5–6). The four principal components that we included each explained over 9% of the variance, and all four combined explained 69.3% of the variance of the habitat predictors. These latent predictors described four orthogonal habitat gradients found across our 35 survey routes that we used as predictors in our subsequent bobcat and lynx occupancy models (
Table 1).
Occupancy models
Models with more than three site-level covariates did not converge. This was most likely due to a lack of degrees of freedom on the site-level of the hierarchical model. Therefore, we only investigated up to three covariate combinations and consequently 15 models for both bobcat and lynx.
The average probability of detection for lynx was 1.59 times higher than for bobcats in the study area over all three winters (0.29/0.46;
Fig. S6). After we averaged the top models, we found very different effects of observation-level covariates on detection (
Fig. S7). We found that the number of days since the last snowfall did not seem to influence bobcat detectability but had a positive effect on lynx detectability. The temperature of the previous night had no effect on detectability of either species. Finally, the length of the survey route was positively associated with the detectability of bobcats but did not influence lynx detectability.
For both species, we found that the top models contained both principal components 2 and 4 (
Table 1 and
Fig. S8). For bobcats, we found that a single top model had a ΔAICc of >2 higher than the remaining models, so we did not have to perform any model averaging. The top model for lynx was the same, but an additional model was within 2 ΔAICc units of this model. This model contained the solitary effect of principal component 2. We first averaged the lynx models and then we investigated the effect of principal components 2 and 4 on the probability of detection and occupancy of both species.
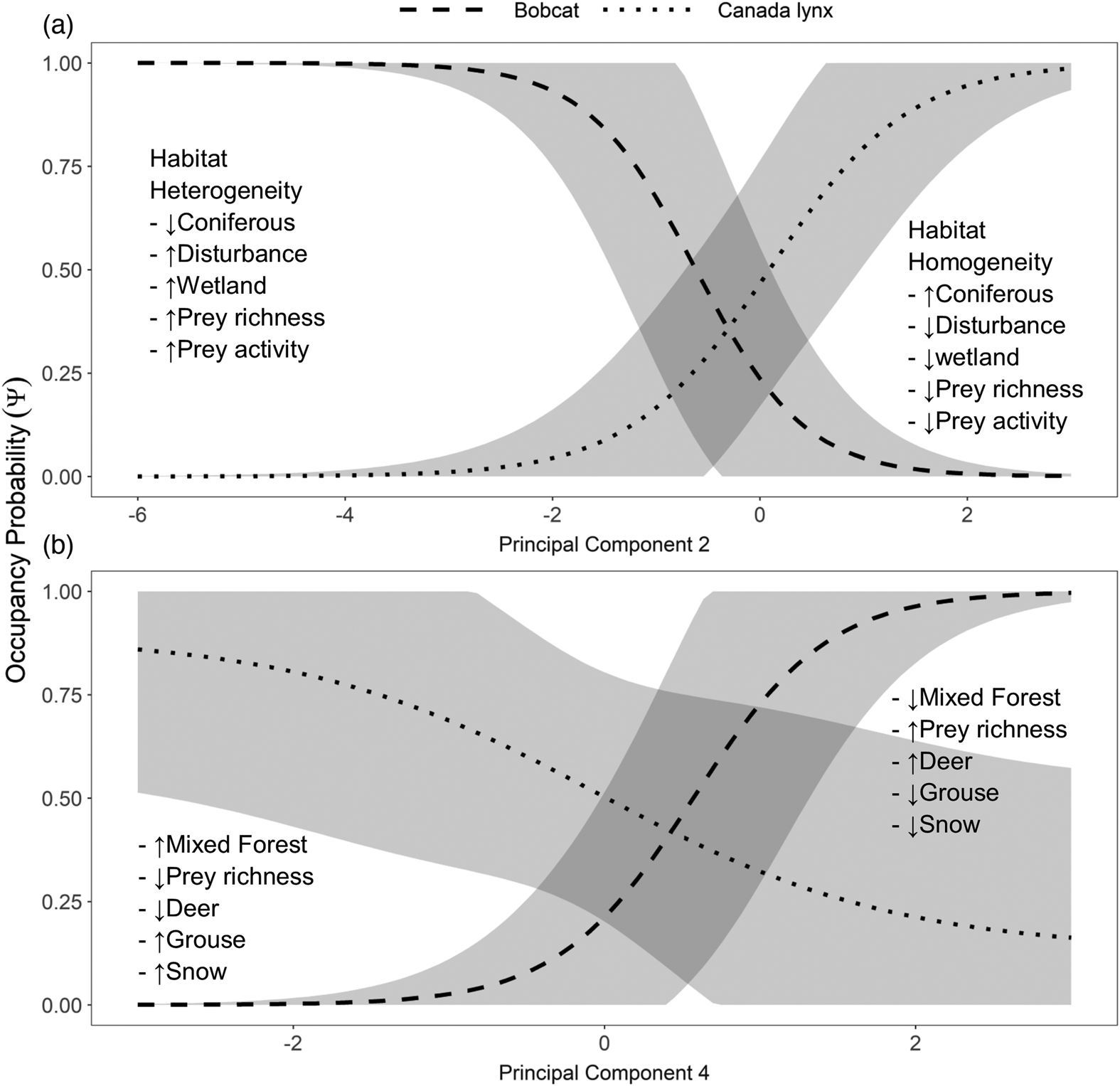
Principal component 2 represented a gradient of snowshoe hare, grouse, squirrel, prey richness, anthropogenic disturbances, and coniferous forest (
Table 1). The habitat on one end of the gradient was heterogeneous landscape with several options of prey and high prey activity, and the other end of the gradient was habitat with a homogenous landscape with a low number of prey options and low prey activity. The occupancy probability for bobcats and for lynx increased at opposing ends of this gradient. Bobcat occupancy increased with heterogeneity and lynx occupancy increased with homogeneity (
Fig. 3a). More specifically, human-disturbed areas with high activity of snowshoe hare, grouse, and squirrel; high prey richness; and a low proportion of coniferous forest were areas where bobcat probability of occupancy was high and lynx probability of occupancy was low. Conversely, homogenous areas less disturbed by humans with low activity of snowshoe hare, grouse, and squirrel; low prey richness; and a high proportion of coniferous forest were areas where bobcat probability of occupancy was low and lynx probability of occupancy was high.
Principal component 4 represented a gradient of grouse, deer, prey richness, snow depth, and proportion of mixed forest. On this gradient, deer activity increased with prey richness but decreased with increasing grouse activity, snow depth, and mixed forest. Bobcat probability of occupancy increased towards higher values of this predictor, but the effect of this gradient seemed negligible for lynx (
Fig. 3b). The probability of occupancy of bobcats was higher in areas with high deer activity, high prey richness, low grouse activity, shallow snow, and a low proportion of mixed forest.
Occupancy and overlap
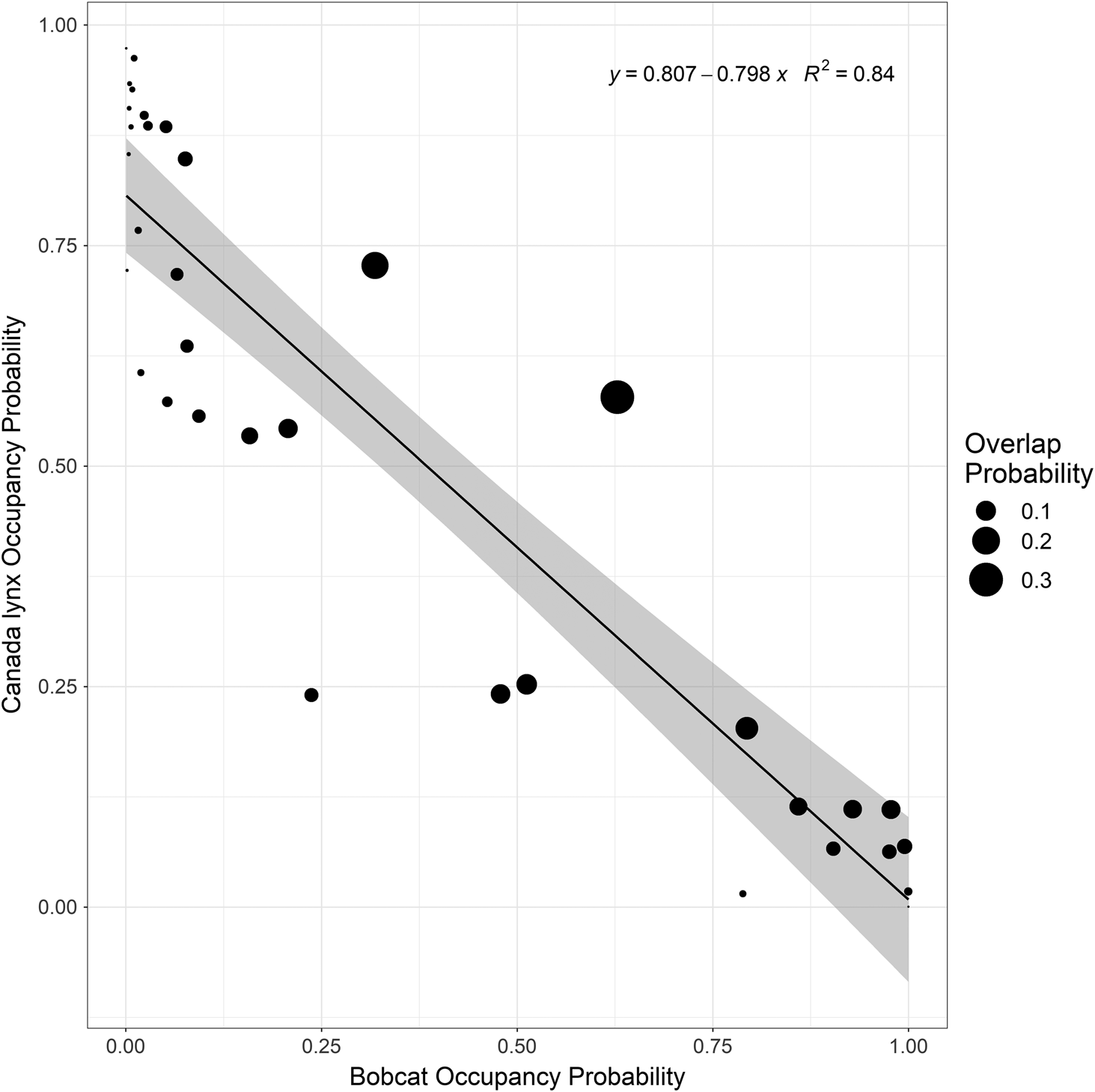
We predicted the probability of occupancy of both species and found that bobcats occupied areas closer to the shore of Lake Huron whereas lynx occupied areas away from the shores (
Fig. 2). There was a discrepancy in this pattern near the shore just to the east of the middle of the study area, where bobcat occupancy was low, and lynx was much higher. However, bobcat occupancy was higher on the shore eastward. Finally, we found that as bobcat occupancy increased, lynx occupancy decreased in our study area (
R2 = 0.84;
Fig. 4).
Acknowledgements
This work was supported through funding from NSERC (CREATE and Discovery Grants to JB and CGS to RRM), WCS Canada (W. Garfield Weston Fellowship to SJM), Trent University and OMNRF. We thank Dr. Marco Festa-Bianchet, Dr. Erin Koen, and two anonymous reviewers for all their helpful and insightful comments. We also thank A Walpole, B MacDonald, B Tang, C Sadowski, D Hamlin, J Greenhorn, J Trottier, M Browning, M Purvis, M Turcott, Natalie Pulham, and S Konieczka for all their help during the field season. Finally, we thank the many hunters, trappers, nature enthusiasts, cottagers, cottage associations, propriety owners, Ontario Parks, and the Ontario Federation of Snowmobile Clubs for allowing us to use their trails to do our surveys.