Introduction
Large-scale population monitoring is fundamental to wildlife management and provides the basis for conserving or controlling animal populations. In addition to guiding decision-making around when and where to deploy management efforts, these data are also central to ecological studies seeking to reveal patterns and processes underlying spatiotemporal population dynamics (
Liebhold and Kamata 2000;
Johns et al. 2016). In recent decades, large-scale population data sets have been bolstered by contributions from volunteer scientists, leading to a substantial increase in the scale and resolution of data for many key species (also known as “community science” or “citizen science”; e.g.,
Howard and Davis 2009;
Ingwell and Preisser 2011;
Gardiner et al. 2012;
Pocock and Evans 2014). As an added benefit, engaging volunteers in scientific endeavors also enhances public interest and knowledge on important environmental issues (
Dickinson et al. 2010). However, the design and implementation of these programs comes with several challenges. For a program to be successful it generally requires: (
i) a project design within the capabilities of volunteers, (
ii) effective recruitment and retention approaches, (
iii) procedures to validate collected data, and (
iv) well-defined scientific questions that can be answered with the data (
Dickinson et al. 2010;
Worthington et al. 2012). These features underlie the conundrum researchers often face when designing community science programs—how does one satisfy the data needs of landscape-scale scientific questions while also ensuring high data quality and sustained public participation?
It was with this conundrum in mind that we conceived the Budworm Tracker Program (BTP), a contributory community science project to monitor a major native forest defoliator, the spruce budworm (
Choristoneura fumiferana Clemens, hereafter budworm) (Lepidoptera: Tortricidae). Although BTP was designed for budworm, the approach is broadly applicable to insect monitoring for pest management and ecological research across a range of forestry, urban forestry, and agricultural ecosystems. Budworm outbreaks are typically wide-spread and severe—the previous outbreak between 1963 and 1992 left in its wake nearly 52 million hectares of moderate to severe defoliation in northeastern North American forests, including Ontario, Quebec, New Brunswick, Nova Scotia, and Newfoundland in Canada, and Maine in the United States (
National Forestry Database 2018). The most recent budworm outbreak emerged in Quebec around 2006 and grew to over ∼7.2 million hectares during the next decade with no sign of slowing (
Ministère des Forêts, de la Faune et des Parcs 2017). One particularly striking feature of budworm outbreaks occurs in mid-July to early August when newly emerged budworm moths begin to fly in search of mates and spruce and fir trees on which to lay eggs. After dusk, budworm moths will often ascend and hover above the tree canopy where they can be picked up by wind and dispersed locally or hundreds of kilometers to distant stands (
Greenbank et al. 1980;
Dobesberger et al. 1983). Under the right conditions, so-called mass-exodus flights can disperse millions of moths throughout a region, concentrated in extensive plumes that can be detected and tracked with weather radar (
Boulanger et al. 2017). The arrival of these moths in new areas is often conspicuous (
Fig. 1) and draws considerable public interest and media attention (e.g.,
Kitts-Goguen 2016). Nearly a half century of research has been dedicated to understanding the role of budworm dispersal in budworm outbreak ecology, though many questions remain unresolved (
Pureswaran et al. 2016).
Regional budworm moth monitoring provides much needed data for numerous scientific applications. For example, survey data and samples are needed to help answer long-standing questions around moth dispersal and its influence on outbreak dynamics (
James et al. 2015) as well as questions related to population genetics (
Lumley and Sperling 2011) and dispersal ecology (
Dickinson et al. 1983;
Dobesberger et al. 1983). Most moth monitoring is carried out using pheromone traps baited with a synthetic blend of the sex pheromone emitted by female moths to attract males for mating (
Silk et al. 1980;
Silk and Kuenen 1986). Historically, it has been the role of governmental agencies to distribute pheromone traps throughout their regions. This entails driving to selected sites to set up traps in early summer then returning in late fall, after the flight period, to collect the traps and captured moths. Although the traps themselves are relatively inexpensive, labor and travel expenses to set-up and collect traps can be quite high. For example, in 2016 provincial staff in the province of New Brunswick deployed 300 traps at 100 sites (i.e., 3 traps per site) throughout the province (an area of ∼ 73 000 km
2 at a cost of ∼$27 700 CAD; unpublished data).
In this article, we outline the BTP, including the project design, volunteer recruitment and retention approaches, data validation procedures, as well as some of the core scientific questions it was designed to address. The BTP involves outsourcing pheromone trap data collection to volunteers living throughout the eastern outbreak range of budworm. Volunteers receive in the mail pheromone-baited traps, collection accessories, and detailed instructions on how to use the traps to capture and store moths. Aside from providing an inexpensive means of deploying traps, the BTP provides unique data on the relative timing of moth flight throughout the region, which can reveal deeper ecological insights into the range, destinations, phenology, and intensity of moth dispersal throughout the region (
Pureswaran et al. 2017). Since its inception in 2015, the program has expanded to all six eastern Canadian provinces (from Ontario east) and Maine, United States. We also evaluate the efficacy of the BTP using results from the first three years (2015–2017) and briefly discuss its contributions to pest management and ecological research using the 2016 trapping season as an example.
Methods
Spruce budworm life cycle
Spruce budworm in eastern Canada undergoes one generation per year. In early spring, young larvae emerge from diapause to mine year-old needles on spruce (
Picea sp.) or balsam fir (
Abies balsamea (L.) Mill). As the young shoots begin to shed their bud cap, larvae enter the shoots and spin a protective tunnel in the midst of the expanding needles to protect them as they feed. Larvae continue to feed on young foliage until pupation, which occurs usually around mid-summer (late June to mid-July in eastern Canada). About 10–12 d after pupation, adult moths emerge and proceed to mate, lay eggs, and in some instances disperse to new stands. As soon as moths emerge from cocoons, the females begin to emit a multi-component sex pheromone to attract males in search of mates (
Silk et al. 1980). Eggs hatch after 10–12 d and the newly emerged larvae seek out crevices on the tree branches or trunk in which to spin a hibernaculum for protection during overwintering.
Project design
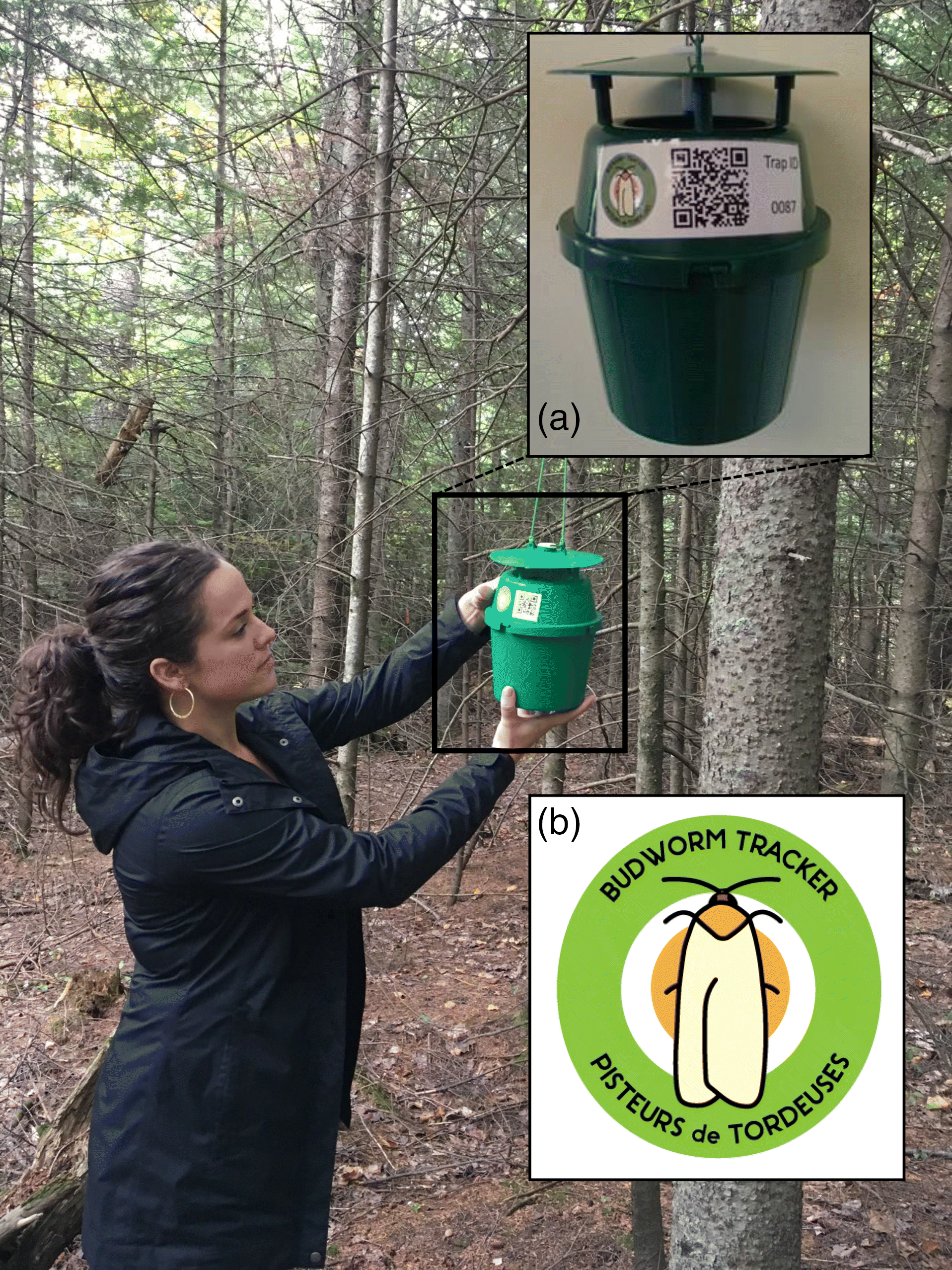
We provided BTP start-up kits free-of-charge to volunteers. Kits consisted of the following components: supply checklist and set-up instructions, pheromone trap (Unitrap, Contech Enterprises Inc., pre-assembled with a unique identity number;
Fig. 2a), a budworm pheromone lure, an insecticidal strip, prelabeled paper bags (40), a freezer bag, one pair of gloves, a wooden stick for sweeping moths into bags, a pencil or pen, a data collection sheet, a contact information sheet, and a prepaid and addressed return envelope (Fig.
S1). In 2018, the insecticidal strip was switched for a solution of soapy water, which provided a simpler and cleaner way to kill moths in traps. Each year, volunteers who wished to continue with the program were encouraged to keep and store their trap, and they were shipped a small refill kit with the same components except the pheromone trap, which was sent at a reduced shipping cost. Kits were sent in a box large enough to enclose all its components (for our full kit (L × W × H): 20 cm × 20 cm × 25 cm box; for refill kits (no trap): 30 cm × 22 cm × 5 cm bubble envelope). We purchased, packaged, and stored all kit components between February and April in preparation for the upcoming season, starting in mid-June. To preserve their quality, sex pheromone lures were kept frozen and were not placed in the delivery box until just before their distribution. BTP protocols were provided in English and French (Fig.
S1). We branded all kits and outreach material with our easily recognizable logo (e.g., on trap in
Fig. 2b). In 2015, we shipped or hand-delivered BTP kits to local organizations to distribute and collect kits, data, and samples at the end of the season. In all subsequent years, we used mail service (prepaid) almost exclusively to deliver traps and have the data returned.
Following delivery of kits, we guided assembly using a combination of the component checklist, and photographic and video assembly guides (Figs.
S1 and
S2). Once traps were assembled, we asked that volunteers choose trap locations that were convenient to visit every few days and that had an appropriate host tree (i.e., spruce or balsam fir) with an accessible lower branch from which to hang the trap at about eye level (
Fig. 2). Traps were often placed near homes, offices, field stations, parks, or in nearby forests. Following trap set up, volunteers were encouraged to begin checking the trap immediately. We encouraged volunteers to check traps ideally 2–3 d per week; however, trap check frequency varied among volunteers depending on their schedule. If multiple checks per week were not possible, we asked for a minimum of one weekly trap check, ideally on the same day of the week. Spruce budworm moth flight occurs after dusk (
Greenbank et al. 1980), so we recommended checking traps during daylight hours. When checking traps, volunteers placed captured moths into one of the provided paper bags and filled in the label with trap number and collection date. Bags with moths were to be placed in the provided freezer bag and then into a freezer to prevent mold. We encouraged volunteers to count collected moths, though there was no requirement to do so because we always recounted moths once they were returned. We asked that volunteers use the provided paper data sheet to record each time the trap was checked and the number of moths that were caught. We told volunteers that even when there was nothing captured they should still report their observations—from our perspective noting the absence of moths on a given day was just as important as noting their presence. To assist BTP volunteers to report data on their moth counts we prepared a webpage and mobile phone applications (Android or Apple iOS). Each trap was given a unique QR code applied as a sticker on each trap (
Fig. 2b). This QR code directed users of the mobile phone application to their personal profile on the BTP webpage where they could report the number of moths in their trap. These tools were to be used in addition to the paper records written on the provided data sheets.
We asked that volunteers cease collecting and return moths no sooner than the end of August, which was weeks after most moth flight would have ended in the region. After an initial reminder to return collected moth samples and data, we sent occasional follow-up reminders through social media, post cards, and e-mail. If samples were not received by mid-October, we followed up with another e-mail or phone call.
Volunteer recruitment, retention, and outreach
To recruit volunteers we used many of the same outreach tools that have been used in other community science programs (
Mulder et al. 2010;
Graham et al. 2011;
Council and Horvath 2016). Our first step was to ask local organizations to solicit their members or employees to sign up as volunteers (e.g., forestry companies, government agencies and departments, woodlot associations, Co-ops, park groups, outfitters). Next, we engaged in several periods of concentrated outreach and recruitment of the public via social media and traditional media (i.e., newspaper ads, radio and TV interviews).
When contacted by groups or individuals asking to join the program, we requested a contact name, mailing address, and contact information (phone and e-mail). We also requested the likely location of the trap (i.e., latitude and longitude, determined by GPS or site information provided by volunteer). As the volunteer network developed and the spatial pattern of trap locations began to emerge, we sought to fill in any gaps on the map by targeting communities in the nonsampled area via outreach to local media outlets and community groups.
Providing feedback to volunteers regarding their contribution to the program is a key part of retaining volunteers and ensuring that the outreach and educational benefits of volunteer science are achieved. Thus, at the end of each year, we prepared an annual report for volunteers summarizing the monitoring season, any interesting insights, and adjustments to the protocol for the upcoming year (e.g., Fig.
S2). We provided periodic updates and additional information on budworm during the off-season in the form of written and video blogs to keep volunteers engaged in the program and to prepare them for the upcoming season.
Data validation procedures
All moths returned to the program were verified as spruce budworm based on morphological features and counted. Moth counts were compared to those provided by the volunteers and, where there was a discrepancy, we used our own counts for subsequent analyses. All samples were placed in a freezer (−18 °C) for later analyses in related ecological studies (e.g., morphological, isotopic, and genomic). All other information provided by the volunteers on the data sheets was digitized (e.g., when traps were set up and taken down, weather, sampling frequency, etc.). We used these to assess compliance rates of volunteers with the program’s sampling protocol (e.g., were set up and take down of traps around the recommended dates, were samples collected at least weekly, etc.).
Scientific applications
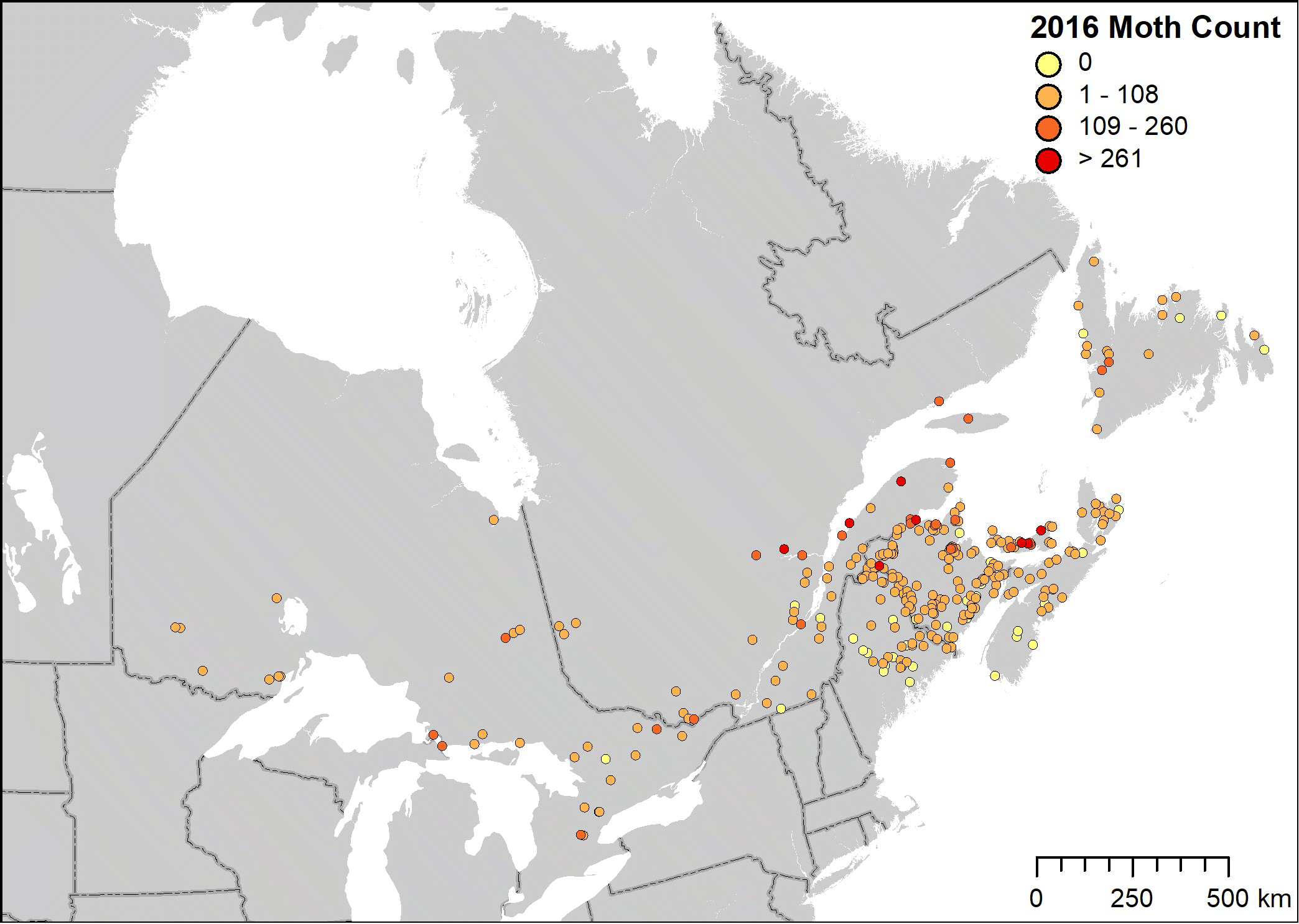
Data from the BTP provided two types of data for use in managing and studying spruce budworm outbreaks. First, budworm moth monitoring is an important part of gauging year to year variations in the distribution and abundance of budworm throughout the region. To this end, the overall moth density data collected by BTP complements that collected by provincial and state jurisdictions. In New Brunswick for example, data from BTP essentially doubled the total number of trapping sites where pheromone traps were set up. These data can help to map regional trends of budworm moth abundance and are could be used to identify areas of potential interest for more intensive sampling of overwintering larvae to inform decisions to manage populations (
Johns et al. 2019).
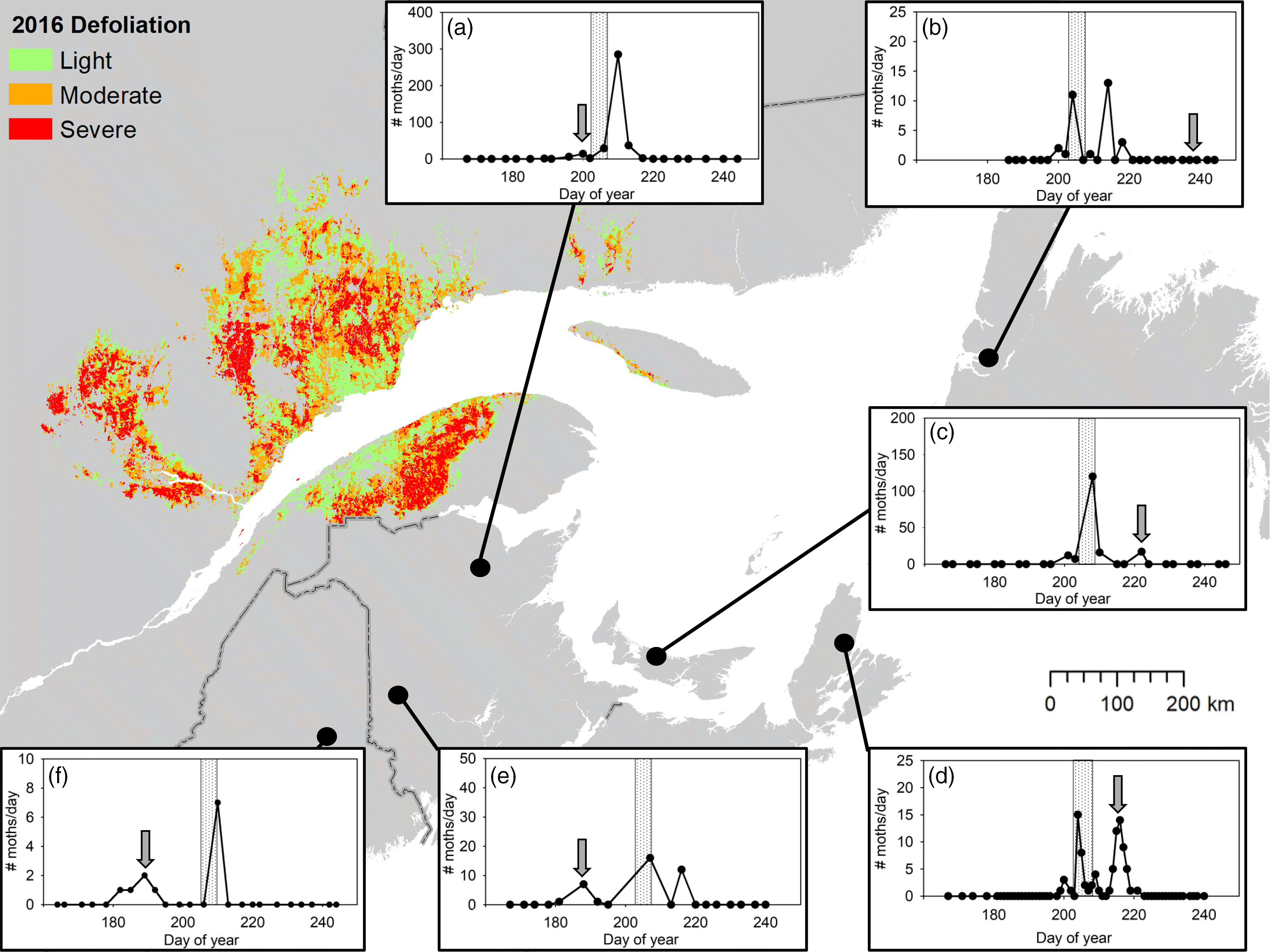
Second, the more frequent estimates of budworm moth density provided by volunteers (compared with conventional trapping) can offer additional insight on the potential origin of captured moths. Because of the large geographic range of the spruce budworm, different populations experience different temperature regimes. This results in correlated, spatial variations in the pacing of larval development across the range of the insect and associated regional variations in the timing of moth flight. Fortunately, the timing (i.e., phenology) of moth flight can be reliably forecast using a temperature-based model (i.e., BIOSIM:
Régnière et al. 2013). However, since typical survey programs only sample traps once during a season these patterns of flight can be obscured since discrete flight “events” are all combined in a single trap. By using a combination of information on the date and location of moth capture from the BTP and projected phenologies from temperature-based models, we can distinguish between flights of local, phenologically synchronous, spruce budworm populations and influxes of migrant, phenologically asynchronous, moths. By collecting moths weekly, the BTP volunteers provide high-frequency temporal data on moth flight phenology that can be used to determine the extent of moth flight, for instance during local and mass dispersal events (e.g.,
Dobesberger et al. 1983;
James et al. 2015;
Pureswaran et al. 2017). We illustrate this approach in the Results section using a subset of our trapping sites from the 2016 season. The 2016 trapping season was punctuated by a mass-exodus flight event that occurred roughly between 20 and 22 July (i.e., day of year 202–204). This event was first detected by weather radar imagery and indicated that moths were swept southward from the main outbreak defoliation range on the north and south shores of Quebec through Atlantic Canada.
Discussion
Community science is being used increasingly for monitoring insects throughout the world and for a variety of purposes, including for invasive species detection (
Maistrello et al. 2016), assessment of native species richness, abundance, and diversity (
Lye et al. 2012;
Gardiner et al. 2012;
Prudic et al. 2017), as well as science education of students and the public (
Braschler 2009;
Oberhauser and Le Bruhn 2012). Often these programs are inexpensive to deploy but require some baseline level of expertise or training for volunteers to identify or characterize their target groups. This, in turn, leaves room for volunteer error and necessitates validation processes for the data they submit (
Wiggins et al. 2011). Our BTP offers an alternative approach that requires some initial investment to set up the program, but the specific attraction of the pheromone essentially eliminates issues of misidentification. The simplicity of the approach and the high probability of having moths captured in traps (especially during outbreaks) may at least partially explain why return rates on data were >68% during our first three years. It is worth noting that we began the program in 2015 with a high proportion of industry and provincial foresters deploying our traps, whereas 2016 and 2017 had a much higher proportion of volunteers from the general public. This may in part explain the apparent decline in volunteer return rate. The most common error was for volunteers to sample too infrequently, though these data were still useful for providing overall density estimates for that trapping location. This approach has specific applications in pest management in forestry, agriculture, and horticulture and broad applications were it to be adapted for insects for which pheromones or other semiochemical attractants can be synthesized and a simple trapping method is available.
Although pheromone trap monitoring is quite common in pest management, engaging volunteers to assist with trapping may provide several benefits not offered through the more centralized-deployment approach used by many jurisdictions. First and foremost, the BTP is simple and cost-effective to deploy across large areas. This in large part owing to the significant reduction in transportation costs required for trap set up and collection. Second, traps can be checked and samples collected more frequently throughout the flight season, which can provide higher-resolution data (e.g.,
Fig. 4). This approach to volunteer-based monitoring is easily scaled with only a small total increase in cost per sampling point, especially where kits can be distributed using postal or delivery services. Finally, adoption of a similar, centrally coordinated community science programs at a regional scale could help to harmonize data collection methods and reduce some of the inconsistencies that arise when different jurisdictions develop or deploy monitoring programs independently.
Aside from providing important data, community science programs can also make important contributions to public education and engagement (
Dickinson and Bonney 2012). Public engagement in scientific research can draw much needed attention to critical environmental issues and, in the case of outbreak-prone insects such as budworm that cause economic losses, offers an entry point to discussions around how and when (and even if) we should manage populations. The BTP coordinators are frequently approached by volunteers seeking information not only about budworm, but also about other insects. Some volunteers made sampling a family activity and used the program as a means of educating their children about science and entomology; several reported that it was often their children who reminded them to check traps. Some BTP traps were deployed in parks, and in some of these parks staff reported using the BTP as an educational activity for visitors. Social media and media engagement occurred mainly during the trapping season but were especially intense following a mass dispersal event in 2016 that centered on Campbellton, New Brunswick. Having the social media infrastructure in place around the program provided a source for the public and media when questions arose around the event—people from the BTP team had ∼98 media hits in the month following the mass dispersal event with a potential reach of 3 million people based on outlet readership/viewership. This engagement has been especially important in recent years given the recent adoption of an area-wide management approach for controlling budworm at a regional scale in Atlantic Canada (i.e., Early Intervention Strategy;
Pureswaran et al. 2016;
Johns et al. 2019). Based on our experience, we expect that many pest management programs could benefit appreciably from implementing some level of community science, from both the unique data they receive and from the rapport it can help cultivate with the general public.
Research programs that are considering something like our BTP approach should also keep in mind its weaknesses. For example, the BTP required some initial capital investment (e.g., for traps, outreach, online infrastructure, etc.) as well as personnel and administrative costs (e.g., managing outreach, planning, media relations, and sample processing). However, once the trapping network was established, the yearly capital costs declined substantially, mostly because the majority of returning volunteers kept their traps (e.g.,
Table 1). Our experience with the relatively low usage of the apps also suggests that the investment in this technology may not always be necessary to keep volunteers engaged. As discussed previously, the use of a species-specific pheromone has benefits in limiting identification errors and inadvertent collection of nontarget species; however, this specificity also limits some of the questions that can be asked. Most sex pheromones only attract male moths, whereas it is the egg-carrying female moths that may be more strongly associated with population abundance or other processes influencing outbreaks.
We share the conviction of other community science advocates that a key part of developing a sustainable program is that it must be built from and guided by clearly formulated scientific questions (
Dickinson and Bonney 2012). Our scientific questions guiding the BTP were focused on fundamental issues around moth dispersal ecology, which remains a key point of debate in the quest to explain the dynamics of budworm outbreaks (
Pureswaran et al. 2016). Results to date suggest that budworm disperse long distances during outbreaks, though on-the-ground demographic data do not suggest that dispersers are sparking population rise well beyond the leading outbreak edge (
Johns et al. 2019). Moreover, moths collected from sites indicating immigration can be subjected to genetic (e.g.,
James et al. 2015;
Larroque et al. 2019), morphological (e.g.,
Delisle 2015), and isotopic (e.g.,
Hood-Nowotny and Knols 2007) analyses to better pinpoint their origin. These data and samples have other applications as well, ranging from helping to validate weather-transport modelling seeking to predict mass dispersal movements, to population modelling seeking to understand if and how long-distance moth dispersal might contribute to landscape genetics and population dynamics of budworm outbreaks.
Community science offers an attractive solution to the issue of collecting detailed data for insects that span broad geographical areas, with the potential to provide inexpensive population estimates for use in both pest management and ecological research. The BTP offers a versatile approach to gather population data through engaging volunteers, which can be complementary to the conventional trap-deployment approach used by most governmental agencies. The BTP approach satisfies the core features needed for a successful community science program, including having a simple project design, effective recruitment and retention strategies, data validation procedures, and well-defined scientific questions to address with the collected data. Furthermore, this method is easily adaptable to any insect for which a species-specific pheromone, or for which a chemical attractant is known, making it useful for standardized collection of data related to conservation, management, or general ecology of species.
Acknowledgements
This program would not be possible without the support of a great many collaborators. First and foremost, we thank our many Budworm Trackers who are too numerous to list by name—they are the foundation of this program and we are grateful for their continued participation and enthusiasm. Several groups were instrumental in getting the program off the ground, including the New Brunswick and Nova Scotia Departments of Natural Resources, Healthy Forest Partnership, New Brunswick and Nova Scotia Marketing Boards, Acadian Timber, SERG International, University of Maine, AV Group, Twin Rivers Paper Company, JD Irving Ltd., Fornebu Lumber, State of Maine, Cooperative Forestry Research Unit, Fundy Model Forest, as well as other provincial and state governmental organizations—we thank their employees for organizing the set-up of monitoring traps in remote areas. We also thank our own staff’s considerable efforts in processing, digitizing, cleaning, and organizing the data, including K. Moore, I. Abel, M. Robichaud, E. Papagiannis, and T. Somers. We also need to acknowledge the various people who have helped in outreach and communication efforts for the program, including S. Blatt, C. Boone, B. Daigle, C. Donahue, N. Hay, B. Roth, M. Wright, D. Lavigne, S. Pegler, G. Penny, B. Richard, and J. Smith as well as the provincial and National Parks who participated. Several people, including C. Simpson and M. Stastny, offered comments and corrections on an earlier version of the manuscript. Several news organizations, including the CBC and Radio Canada, Campbellton Tribune, and Maine Forestry, were also instrumental in promoting the program through their coverage in articles and radio. Finally, we thank Gillian Goldie of “This is Duke” for the design of the Budworm Tracker logo. Financial support was provided by Natural Resources Canada, Atlantic Innovation Fund, and SERG International.