Introduction
Rabies, an acute disease of the nervous system, is caused by a Lyssavirus (
Fooks and Jackson 2020). Worldwide, rabies kills about 59 000 people a year, with 95% of human deaths occurring in Asia and Africa (
Hampson et al. 2015). Typically, the rabies virus is spread by dog bites; at the bite wound the victim is inoculated with infectious virus in the dog’s saliva. The tally of human deaths from rabies varies dramatically across countries. For example, in India from 2012 to 2014 there were 128 (42.7 per year;
Mani et al. 2016), in South Africa from 2008 to 2018 there were 104 (10.4 per year;
Dermaux-Msimang et al. 2019), and in Brazil from 2000 to 2017 there were 188 human rabies cases (27 per year;
Vargas et al. 2019). What about the association of bats and rabies?
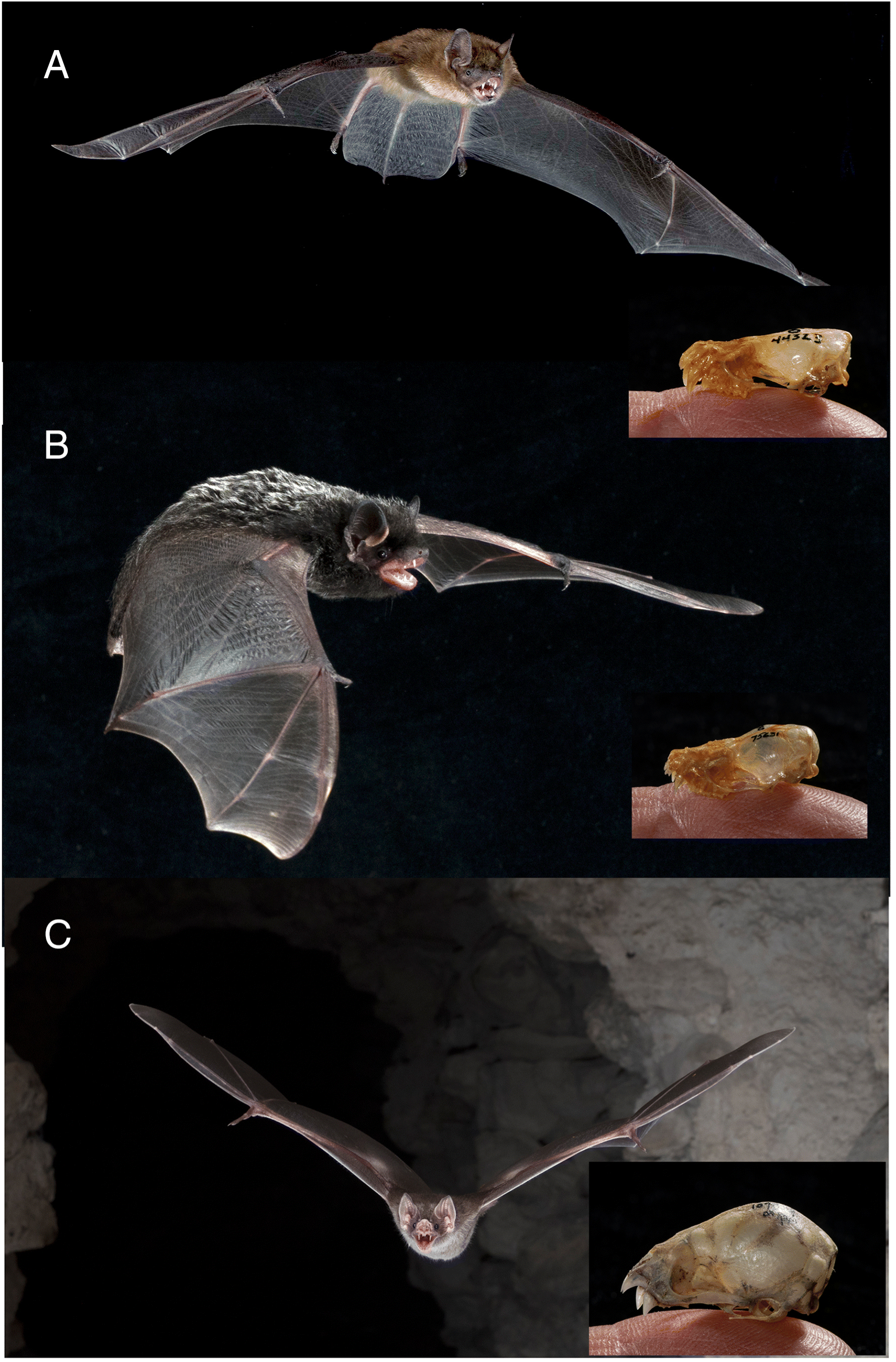
Rabies is also spread by bats (
Fig. 1). In Brazil, rabies from bats accounted for >40% of the 188 human cases and from dogs about another 40% (
Vargas et al. 2019). Bat-related rabies in Brazil is contracted from either blood-feeding vampire bats (
Fig. 1C) or insectivorous bats. Vampire bat bites on humans usually result from blood feeding, whereas bites from insectivorous bats (e.g., Brazilian free-tailed bat,
Tadarida brasiliensis) are normally delivered in self-defense but also can be from aggressive rabid bats.
Escobar et al. (2015) noted a considerable reduction in rabies in dogs in Brazil, while the numbers of cases of bat rabies has remained steady for about 10 years. They noted that bats appeared to be the principal reservoir for rabies in the Neotropics.
Rabies in humans is much less common in the USA and Canada where, from 1950 to 2009, there were 60 cases of rabies acquired domestically (
Fenton et al. In press). Bat rabies variants caused a number of these fatalities, but 33% involved no history of exposure to bats, and in 28% there was no report of direct contact with a bat (
De Serres et al. 2008;
Jackson 2011a). In Canada, seven of the nine most recent human rabies fatalities were caused by bat virus variants (
Table 1). Notably, none of these involved rabies virus variants associated with big brown bats (
Fig. 1A), which is perhaps the most common house bat across southern Canada. Details of two recent human deaths from bat rabies illustrate the complexity of the situation.
In Montreal in 2000, a 9-year-old boy died of rabies caused by the silver-haired bat (
L. noctivagans,
Fig. 1B) virus variant. This variant also is known from the tricoloured bat (
Perimyotis subflavus) (
Turgeon et al. 2001;
Despond et al. 2002;
Elmgren et al. 2002). This case illustrates four issues presented by bats and rabies. First, the two bat species associated with the silver-haired rabies virus variant rarely roost in buildings and thus are expected to have infrequent human contact. Second, the boy and his family had stayed in a rented cottage in a park in Quebec. Two bats, identified by the parents as little brown myotis (
Myotis lucifugus), were captured in the cottage by the parents and released outside. Third, the victim had a slight abrasion on his upper arm, which in retrospect may have been a bat bite, but apart from this scrape there was no report or clear evidence of a bite. Two of us (MBF and ACJ) visited the cottage in December 2000, and careful searching revealed no physical evidence (e.g., droppings) that bats had roosted in the cottage. But another cottage within 1 km of the site was used as a roost by bats, based on the accumulation of droppings under specific roosting sites. Fourth, did the bat exposure happen in the cabin, and if so, how could bats have entered the cottage occupied by the boy and his family? Could bats possibly have come into the house on firewood or flown down the metal chimney into the wood stove? Unfortunately, the lack of details precludes drawing definitive conclusions about how the boy’s exposure to a rabid bat had occurred.
In 2019 in British Columbia, a 21-year-old man died of rabies caused by the silver-haired bat virus variant. This was the first human death in Canada from indigenously acquired rabies since 2007 (
Table 1). The victim had been exposed to a bat on Vancouver Island around the middle of May and showed clinical symptoms of rabies 6–8 weeks afterwards. The exposure route is unknown, possibly from a scratch rather than a bite. The time lag between exposure and appearance of symptoms is typical.
Recognizing a bat exposure
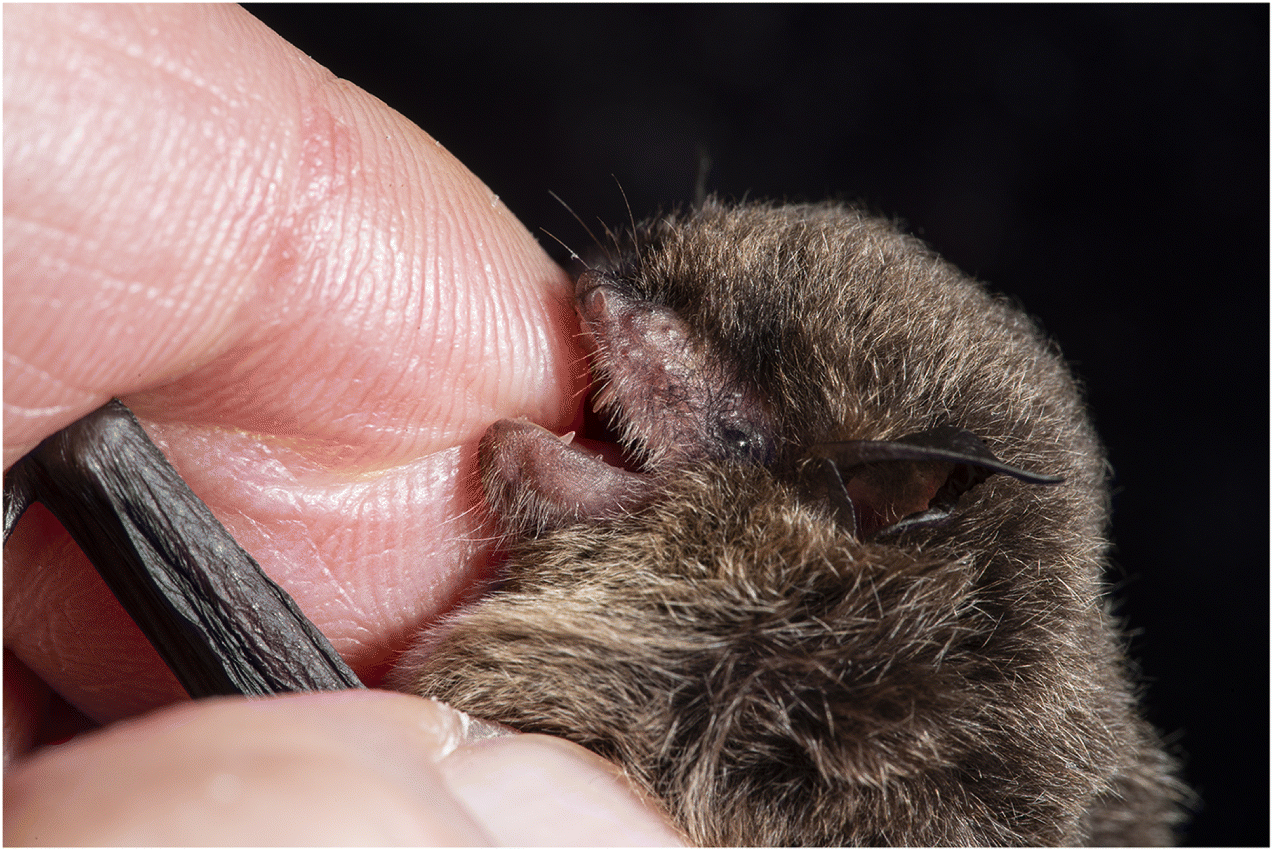
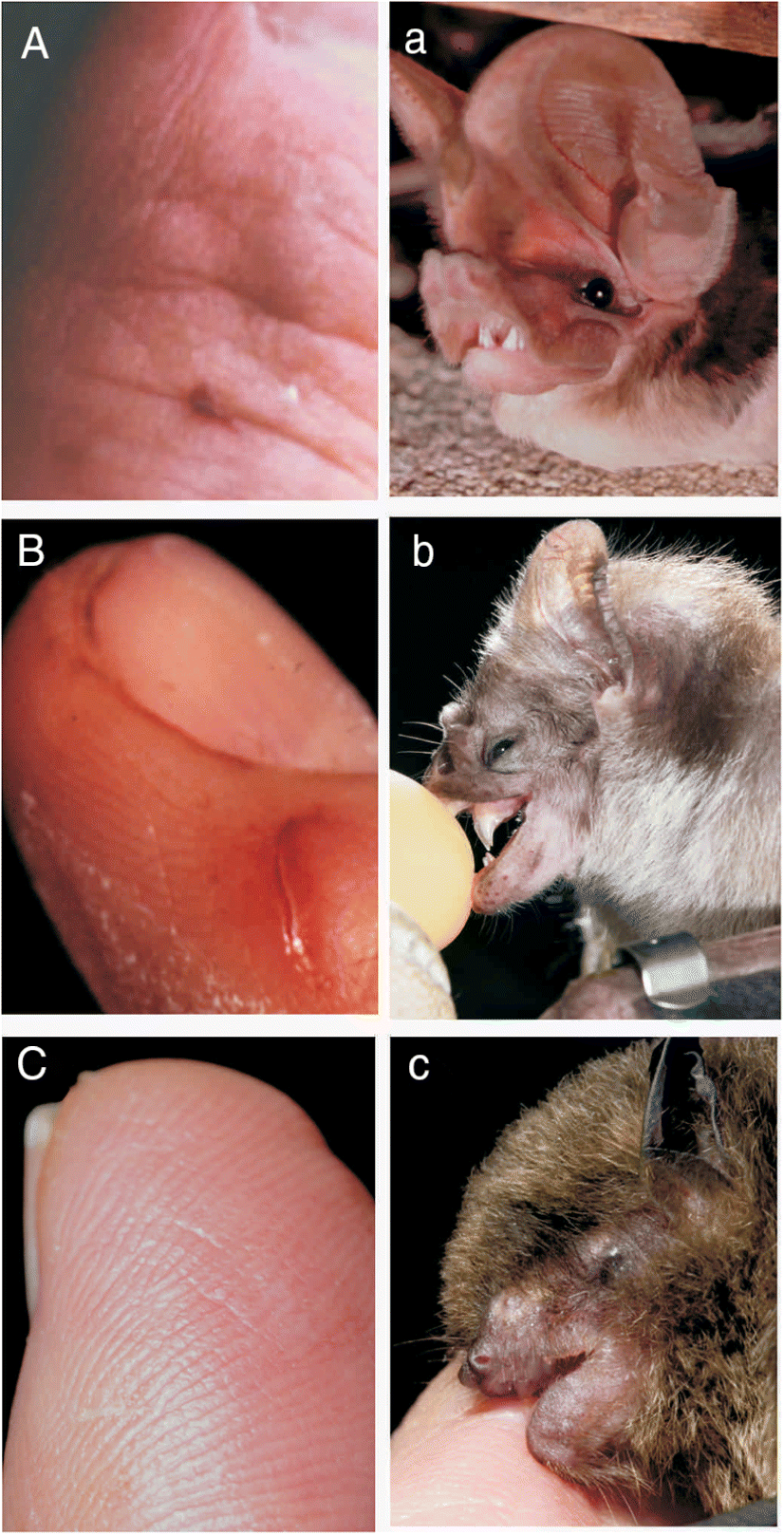
Normally, a bite from a wild mammal indicates possible exposure to a rabid animal. But bat bites can be hard to recognize (
Fig. 2). When do bats bite? Normally bats, like most mammals, bite only in self-defense. Even the three species of vampire bats that bite to eat (drink) blood also bite in self-defense. Bats expressing clinical signs of furious rabies may bite indiscriminately and without provocation (see below). These bites and defensive bites usually leave puncture wounds, which may be small and difficult to recognize when inflicted by an insectivorous bat (e.g.,
Figs. 2A,
2C). The blade-like upper incisor and canine teeth of vampire bats slice rather than puncture tissue and leave obvious, bleeding wounds (
Fig. 2B).
Bats are among the smallest mammals that are rabies vectors (
Fenton and Simmons 2015). This has important public health implications because their small size can make bat bites hard to notice. For example, it is easy to overlook the bite inflicted by an adult silver-haired bat with a mass of about 9.5 g (
Jackson and Fenton 2001), which could place the victim in jeopardy (
Table 1). About 20 bat species have been reported in Canada (
Van Zyll de Jong 1985), with hoary bats (
Lasiurus cinereus; ∼30 g) being the largest and eastern small-footed bats (
Myotis leibii; ∼4 g) among the smallest. Hoary bat bites are obvious and painful, while those from eastern small-footed bats may not break the skin (e.g.,
Fig. 2C).
Any mammal, including bats, will bite while in the grip of furious rabies. Any unprovoked bat bite should be treated as a potential exposure to rabies. The best way to confirm the diagnosis of rabies is by submitting the head of the animal (in the case of bats, the whole body) to a certified laboratory for detection of rabies virus antigen in the brain. This also facilitates accurate identification of the species involved. But if the animal that delivered the bite is not available, how is it possible to confirm exposure especially if there is no evidence of a bite?
Diagnostic laboratory tests for rabies in humans or animals include detection of rabies virus antigen in post-mortem brain tissue. In humans, antemortem diagnostic tests include detection of rabies virus antigen in skin biopsies or rabies virus RNA using the reverse transcription polymerase chain reaction in saliva, skin biopsies obtained from the nape of the neck, and cerebrospinal fluid (
Jackson 2020a). The presence of serum neutralizing anti-rabies virus antibodies is also of diagnostic value in previously unvaccinated individuals (also in cerebrospinal fluid whether previously vaccinated or not).
Humans directly exposed to a known or suspected rabid animal should receive rabies post-exposure prophylaxis (PEP) treatment. Exposures to bat rabies could occur through bites, scratches, or having infected saliva contact abrasions or cuts in the skin (
NACI 1998). Since the mid-1990s, PEP has also been administered to persons sleeping unattended in a room with a bat when the individual was unable to exclude the possibility of a bat “exposure” and the bat in question was unavailable for testing. Over time, this recommendation had been expanded to include additional situations where persons are unable to report possible bat exposures, such as children or intoxicated/cognitively impaired individuals in the same room as a bat (
NACI 2002).
The cost and questionable benefits of widespread administration of rabies PEP (
Huot et al. 2008;
De Serres et al. 2009) led the National Advisory Committee on Immunization to recommend PEP only when there has been direct human contact with a bat that is unavailable for testing or when a possible bat exposure (e.g., bite; scratch; saliva from a bat contacting a wound or skin abrasion; or unattended sleeping person, child or intoxicated/cognitively impaired individual in the same room as a bat) cannot be excluded (
NACI 2009;
Warshawsky and Desai 2010).
Could these measures have saved either victim? We think not. Even though neither person reported directly handling or intentionally touching a bat, both victims (or their families) later recalled the victim having some type of bat incident or possible exposure. Almost all humans who have died from rabies in Canada and elsewhere did not receive PEP before the onset of clinical symptoms of rabies.
Rabies kills bats
To the best of our knowledge, all bats are susceptible to rabies and once a bat shows clinical signs of rabies, the illness is fatal (
Rupprecht et al. 2002).
Nadin-Davis et al. (2001) molecularly characterized rabies virus variants from bat species native to Canada and found four principal phylogenetic groups. Group I included colonial species such as mouse-eared bats (
Myotis spp.) and the big brown bat. Group II included the so-called migratory bats: the eastern red bat (
Lasiurus borealis), hoary bat, and silver-haired bat. Group III variants circulate in big brown bats and likely emerged more recently in this host. Group IV includes some insectivorous and hematophagous bats of Latin America.
Nadin-Davis et al. (2001) found remarkable levels of spillover of rabies virus variants into mouse-eared bats (genus
Myotis) from bats of other genera. The silver-haired bat variant showed the greatest level of interspecies spillover (6/19 or 32%) (
Nadin-Davis et al. 2001). Spillover of bat rabies virus variants into other mammal species are less common. In Canada, mammals such as cats (
Felis catus), foxes (
Vulpes fulva), raccoons (
Procyon lotor), and skunks (
Mephitis mephitis) sometimes catch and eat bats, may be bitten in the process, and may contract rabies if the bat was rabid. In Brazil there are records of cats becoming rabid from interactions with bats (
saude.gov.br/o-ministro/961-saude-de-a-a-z/raiva/41858-situacao-epidemiologica).
We lack comprehensive data about the natural incidence of rabies in bats in Canada. Most data on rabies surveillance is passive. The most accurate (and exceptional) data to date come from bats killed at wind turbines that later tested positive for rabies;
Klug et al. (2011) found a low incidence of rabies in silver-haired (1%,
n = 96) and hoary bats (0%,
n = 121). The most commonly reported data on the incidence of rabies comes from bats submitted for rabies testing, which is arguably not a random or representative sample from natural bat populations. We suspect that the proportion of bats naturally infected with rabies virus is quite low and probably has changed little over time even though rabies surveillance and the absolute number of animals submitted for testing has increased (
Constantine 1967;
Baer and Adams 1970;
Bradley 1979;
Rosatte 1987).
Signs of rabies
The rabies virus infects the central nervous system, causing mild inflammation of the brain (encephalitis) and spinal cord (myelitis) without prominent degeneration affecting neuronal cell bodies (
Rossiter and Jackson 2020). Rabies causes a marked degeneration of neuronal processes, including axons and dendrites due to oxidative stress caused by mitochondrial dysfunction (
Jackson 2020b). The onset of rabies in bats is characterized by a short (1–3 d) prodromal phase of nonspecific signs, which then progresses to severe neurologic disease before the onset of coma, respiratory failure, and death (
Courter 1954). Rabies is manifested in two distinct clinical forms—furious (encephalitic) rabies and dumb (paralytic) rabies—and the underlying pathogenesis responsible for each remains poorly understood (
Jackson 2020a). There are case reports of furiously rabid bats attacking and biting humans (e.g.,
Constantine 1967;
Brass 1994), and at least one of us (PAF) has seen big brown bats with furious rabies bite aggressively and indiscriminately.
A bat expressing furious rabies is easier for lay persons to recognize because it is likely to be hyperactive, aggressive, irritable, or restless and fly erratically. It may make unproved attacks on other animals, including humans or inanimate objects (e.g., its cage). Furiously rabid bats are agitated and very sensitive to sensory stimuli. They are prone to excessive biting, including self-mutilation and hang-on biting, which likely increases the probability of the bite wound being inoculated with infectious saliva and thus successful disease (virus) transmission. Furious rabies occurs in solitary and colonial bats, although it may be more common in solitary or tree-roosting species (
Bell 1980;
Schowalter 1980;
Kuzmin and Rupprecht 2007;
Constantine 2009). Furious rabies in bats is more often observed after intracranial laboratory inoculations of rabies virus compared to bats infected intramuscularly, intradermally, or naturally from other infected bats (
Baer and Bales 1967;
Constantine 1967).
Bats expressing dumb (paralytic) rabies often show moderate to severe paralysis, losing motor coordination and becoming ataxic with tremors and convulsions. These signs could reflect a greater burden of involvement in the peripheral motor nerves, nerve roots, and spinal cord in paralytic rabies than in encephalitic rabies (
Jackson 2020a). Bats with dumb rabies may appear abnormally tame, friendly, inquisitive, and (or) placid. These behavioural changes can cause the sick bat to approach conspecifics or other animals (including humans) without fear (and vice versa), thereby placing the rabid bat closer to another animals, which facilitates biting and disease transmission. Paralytic rabies may be more common in highly social colonial species (
Constantine et al. 1968;
Kuzmin and Rupprecht 2007). Arguably dumb rabies is more dangerous to people than furious rabies: it is easy for a sympathetic and unsuspecting human to recognize an animal that is in distress, paralyzed and (or) otherwise helpless, and try to come to its aid and get bitten in the process.
Rabies and bats
Not all bats exposed to rabies virus develop disease because bats can have lethal or nonlethal infections. After an exposure to rabies virus, bats may not have clinical disease because they develop neutralizing serum anti-rabies virus antibodies that confer protective immunity and result in an abortive infection (
Franka et al. 2008). Maternally derived serum-neutralizing anti-rabies virus antibodies cross the placenta and presumably confer temporary protective immunity to newborn young (
Constantine et al. 1968).
The incubation period for rabies virus in insectivorous bats is highly variable. Longer incubation periods may facilitate higher viral titres in the salivary glands of infected animals and thus disease transmission (
Baer and Bales 1967). Some bats shed virus in their saliva for 12–15 d before showing clinical signs (
Constantine 1971). Bats inoculated experimentally in the laboratory with “fixed” rabies (laboratory-adapted) virus strains tend to exhibit shorter incubation times than bats infected with “street virus” (wild type) from natural sources. This could reflect use of higher than natural viral doses in the lab as well as rapid uptake and direct spread in peripheral nerves. Experimentally inoculated bats can have incubation times from 5 to 14 d and even 6 months (
Baer and Bales 1967). Incubation periods in bats captured from the wild can be longer (e.g., 28–209 d—
Moore and Raymond 1970;
Shankar et al. 2004). Laboratory infection studies reveal that viral replication slows and incubation times are longer in cooler temperatures (
Sadler and Enright 1959;
Sulkin et al. 1960), suggesting that the onset of disease can be prolonged by hibernation. This could produce seasonal variation in the cycle of infectivity which, in turn, may facilitate long term maintenance of rabies virus in some bat populations (
Constantine 2009;
George et al. 2011).
Recent studies using modern methods for detecting rabies virus infection indicate that bats with clinical signs of rabies do not recover from disease. This contradicts earlier suggestions that bats were asymptomatic carriers of rabies because they could transmit infectious virus but show no clinical signs of illness and not succumb to the disease. Inoculated bats that remain healthy and have survived infection are not carriers of rabies (
Moreno and Baer 1980;
Franka et al. 2008). Moreover, bats and other mammals can shed infectious virus in their saliva several days (or even weeks) before they begin to show signs of disease onset. But once neurological signs of rabies are evident, the outcome is an inevitable progression to death.
How do bats get rabies? In most cases, probably from bites by other bats (e.g.,
Bell 1980). The tendency of many bat species to roost together and form social colonies may make them especially vulnerable to rabies transmission. In summer, bats that roost with others (usually conspecifics) often do so to keep warm (
Willis and Brigham 2007). Big brown bats and other colonial species live in fission–fusion societies in which individuals roost with an extended group of roost-mates. On any given day, the individuals comprising a “social group” within a roost may differ from the composition of the day before or the group composition one week later. Individuals have a repertoire of roosts and roost mates, potentially facilitating transfer of rabies virus among individual conspecifics and, possibly, heterospecifics (
Nadin-Davis et al. 2010). Recent work with proximity tags reveals longer term associations between individuals (e.g., mother and young;
Ripperger et al. 2019), perhaps altering the host social structure available to rabies virus. We know that the social systems of vampire bats can be used to advantage by humans for spreading fatal anticoagulants (e.g., diphenadiane) or vaccines in the wild (
Bakker et al. 2019).
Bats may be labelled by their choice of roosts. In Canada, house bats may roost in buildings (e.g.,
Myotis spp. and big brown bats), whereas tree-roosting bats do not (e.g., eastern red and hoary bats). As previously mentioned, silver-haired bats rarely enter or roost in buildings. Arguably, humans are more likely to encounter bats that roost in buildings. This leaves us with a conundrum: three of the last four cases of human rabies acquired in Canada from a bat involved the silver-haired bat rabies virus variant (
Table 1). The transmission of this variant among bat species may help to explain this situation (
Nadin-Davis et al. 2001). In other words, a little brown myotis could be infected by the silver-haired bat rabies virus variant.
Recommendations
The combination of realities we have outlined makes it imperative for people to avoid being bitten by bats. Biologists and others that regularly contact and handle bats should receive a rabies pre-exposure immunization series and have their protective antibody titre measured regularly, every 6 to 24 months (depending on the profession or activities), and obtain booster doses of vaccine as needed. Wherever possible, only biologists or other trained wildlife professionals should handle bats. Humans bitten by a bat should immediately wash and thoroughly flush and clean the wound with soap and water, and then immediately report the incident to a local Medical Officer of Health or a Canadian Food Inspection Agency (CFIA) veterinarian who can help to arrange appropriate PEP. This recommendation also applies to bites from other wild or feral mammals. In the case of bat bites, the bat should be contained so a trained professional can euthanize it and submit the body for rabies testing and species identification.
Two other situations are grim reminders of possible hazards involving humans and bat variants of the rabies virus. First, transmission to humans has occurred through accidents in the laboratory associated with the aerosolization of concentrated virus (
Winkler et al. 1973;
Tillotson et al. 1977). Second, and more problematic, are reports of people being exposed to rabies virus through the air in caves harbouring millions of bats (
Constantine 1962). Either situation makes it impossible to categorically deny the possibility of aerosol transmission even though it remains doubtful that this occurs commonly (i.e., it remains a possibility in very rare circumstances). Perhaps fortunately, the very high densities of bats observed in some places, like the ∼20 million Brazilian free-tailed bats that roost in Bracken Cave, Texas, USA, do not occur in Canada.
Some reflections
Associating bats with frightening zoonotic diseases adversely affects both the public image and conservation of bats (
Tuttle 2017). When it comes to public health, a negative image associated with the ∼1400 species of bats reflects a mixture of truth and misinformation. Bats are long-term reservoirs for rabies virus and we have explored this situation. Some other assertions about bats and diseases are not based on strong evidence. For example,
Yang et al. (2019) identified an Ebola-like virus associated with bats. The virus was isolated from a liver sample from one fruit bat in China, and the bat was not identified beyond the level of genus (
Rousettus). What does this prove about bats or Ebola? Sample sizes of
n = 1 are worrisome. Some researchers may have been utterly mislead in tying bats to Middle East respiratory syndrome (MERS) based on data from one bat (
Memish et al. 2013). Careful research about viruses in bats is one thing, but assertions that bats are long-term reservoir hosts for feared diseases such as Ebola (e.g.,
Calisher et al. 2006) or severe acute respiratory syndrome (SARS) (
Racey et al. 2018) are another. Too often assertions are perpetuated as truths with little regard for actual evidence and (or) context (e.g., “bat flu”—
Karakus et al. 2019).
As noted above, we typically lack important details about situations where humans are exposed to rabid bats. Take Brazil, for example. There
Schneider et al. (1996) interviewed 129 of 160 people from one remote Amazonian village to assess the incidence of vampire bats feeding on humans. In this and other villages, vampire bats feed on humans and the incidence of bites can vary widely from 23% through 41%–88% of residents (
Fenton et al. 2020). It is difficult to put these numbers in a broader perspective because according to the World Health Organization there were three human deaths from rabies in Brazil in 2016 and all involved bats (
paho.org/salud-en-las-americas-2017/?tag=rabies). For Latin America,
Escobar et al. (2015) reported 0.00–0.10 human deaths from rabies per 100 000 in the population. In the final analysis, the chances of any one person contracting rabies from a vampire bat are exceedingly low (
Fenton et al. 2020). In short, people should neither dismiss the possibility of rabies associated with a bite by a wild bat nor use fear of rabies to label all bats as a looming threat to humans.