Macrofungal conservation in Canada and target species for assessment: a starting point
Abstract
Despite the ecological importance of fungi, we still know little about their diversity in Canada. One of the largest hurdles to implementing fungal conservation initiatives is the lack of fungal distribution data. As anthropogenic impacts accelerate the speed of environmental change, it is imperative that we fill this major information gap, critical for fungal protection. To gain insight on the conservation status of Canadian macrofungi, we took advantage of the large and growing body of fungal biodiversity data from government research (Wild Species 2020), citizen science, trained independent mycologists, university, and museum biodiversity research. The majority of macrofungi are data deficient; we do not know their geographic distribution or habitat requirements, occurrence, or abundance in Canada. For mushrooms that fruit only a few days of the year and are often difficult to positively identify, there is a lot of work to overcome the uncertainty of distinguishing under-sampling from rarity. Our work stresses the importance of building a strong network of professional and amateur mycologists to develop resources, disseminate information to make educated decisions, and advance conservation actions. We found that several fungi can be prioritized; we present a short list for consideration for formal conservation assessment.
Introduction
Fungi are critical components of food webs because of their roles in the cycling of energy and nutrients (Boer et al. 2005). They are the primary recyclers in most ecosystems, making the resources held within detritus available for other organisms. For example, white rot fungi are the only organisms that can fully utilize lignocellulose, the main structural component of wood (Ten Have and Teunissen 2001). Many fungi also form important mycorrhizal associations in which water and mineral nutrient resources from the soil are traded for photosynthetically fixed sugars from plants (Peay et al. 2016; Mommer et al. 2018). These are obligate symbiotic relationships without which neither the plant nor the fungal partners would survive. Fungi are also an important direct food source for a wide variety of organisms, from bacteria and soil invertebrates to mammals and birds. Preserving Canadian fungal diversity matters. Contrary to the hypothesis of functional redundancy, which posits that species performing a similar ecological function in an ecosystem are functionally interchangeable (Hubbell 2005), individual fungal species interact with one another and their environment in individual ways, with impacts on the outcome of the ecological function they perform, from decomposition to pathogenic or beneficial interactions with plants (Hazard et al. 2017; Wang et al. 2021). The 3F Initiative (Flora, Fauna, Funga, ffungi.org/eng/conservation/) is now being adopted by global educational and environmental institutions and organizations to refer to macroscopic life on Earth. 3F aims to acknowledge the existence and importance of fungi in governmental decisions and determine the probability of extinction of Fungi in every continent using International Union for Conservation of Nature (IUCN) Red List Criteria.
According to Buxton et al. (2021), enough data are available to make informed decisions regarding conservation in Canada, and it is necessary to shift from data collecting to implementation of conservation policies. Unfortunately, that is not true for fungi; fungal conservation is hampered by lack of data, even for “macrofungi” with individual or massed fruiting bodies >1 cm (Mueller et al. 2014), which are the focus of this document. Conservation plans often do not include fungi because there is insufficient data to demonstrate a population decline over time. This is evident from the few fungi present on the IUCN Red List. There are 60,000 plants assessed for the IUCN Red List; in contrast, as of September 2021, the Red List has only 545 fungi (427 Basidiomycota, 118 Ascomycota), most of which were assessed in the past decade. Dahlberg and Mueller (2011) translated IUCN criteria for fungi and reviewed past assessment criteria with a focus on Europe and the USA, which have led the way in fungal conservation (Dahlberg et al. 2010; Mueller et al. 2014; Heilmann-Clausen et al. 2015; Ainsworth et al. 2018; May et al. 2018). Very recently, articles advocating for an urgent need to include fungi more systematically in conservation actions (Cao et al. 2021; Gonçalves et al. 2021) have highlighted how fungi are different from other organisms: mycologists are still advocating for the consideration of fungi as a component of threatened biodiversity.
Aspects of fungal biology challenging classic conservation approaches
The ability to make informed decisions about the conservation status of an organism is premised on a clear understanding of its life history. Occasionally, we are able to observe patterns because the prevalence of one fungal species is so striking. For example, the Death Cap mushroom (Amanita phalloides) could be tracked in its invasive spread through North America because of its prolific fruiting and human interest (Pringle et al. 2009). However, most of the time, the presence of fungi is not evident even though fungi are key ecosystem players and are everywhere. The reason is that most fungal growth happens out of sight, by the mycelium embedded in its substrate (e.g., soil, wood). Seasonal, unpredictable, and ephemeral macrofungal fruiting and cryptic lifestyles make devising conservation management and monitoring systems difficult. Additionally, when mushrooms or other macrofungi fruit, mycologists cannot readily distinguish different individuals of the same species because different fruiting bodies may be arising from the same embedded network of microscopic filamentous cells. It is therefore difficult to answer basic questions key to conservation action plans such as: What is a fungal individual? How big is a fungal population?
Data-deficient species are more likely to be at risk (Bland et al. 2015). There are not nearly as many high-quality data points for fungi as for birds or plants, although their decline has been shown at least for some groups (Arnolds 1991). For most macrofungi, fruiting bodies are evanescent, and their morphology can be confusing. As a result, the vast majority of macrofungi in Canada are only known from a few collections and are incompletely sampled throughout their range, making them difficult to assess for rarity and threatened status. For example, it becomes challenging to tease apart how much of the recorded geographical distribution of a species of fungus is due to mycologists’ territories (where they live, collect, or go on holiday) rather than the geographical distributions of the fungi collected, leading to the question: Which fungi are rare and which are poorly sampled?
The lack of data is especially alarming in light of changing climatic conditions. One of the more insidious consequences of global climate change is that remote environments may be irreversibly disrupted before their baseline levels of diversity have been assessed. Without predisruption assessments of diversity, managing the recovery of these ecosystems becomes challenging or impossible, as there is no standard with which to assess the effectiveness of recovery efforts. The impacts of global climate change and other anthropogenic activities on the structure and function of fungal communities in Canada is not currently known, making it difficult to predict their effects on future landscapes. Fungi are especially sensitive to climate change (Körner 2003; Giauque and Hawkes 2013; Kivlin et al. 2013; Fernandez et al. 2017; Andrew et al. 2018a, 2018b) and atmospheric pollution, including nitrogen deposition (Treseder 2004; Lilleskov et al. 2011; Allen and Allen 2017; van Strien et al. 2018); changing or extreme weather patterns due to global warming may lead to fruiting body declines and shifts in phenology (Gange et al. 2007; Kauserud et al. 2012; Boddy et al. 2014; Gange et al. 2018).
One of the recent triumphs towards fungal conservation in Canada was the production of Wild Species 2020, in which the known status of ∼7,000 species of macrofungi was assessed and listed (wildspecies.ca/reports, available 2022). Much of the data used in the assessment came from fungaria with digital records and local mushroom club surveys, highlighting the crucial importance of these institutions and organizations in fungal conservation. A second major advance in our knowledge was the recent release of over 200,000 digitized fungal specimens from Agriculture and Agri-Food Canada’s Canadian National Mycological Herbarium, Canada’s largest collection of nonlichenized fungi, now searchable in Mycoportal.org. In the light of these major advances, as well as updates in the Canadian Species at Risk Act (Mooers et al. 2010), the meaningful integration of Indigenous knowledge (Turner 2020; Turcotte et al. 2021), and the recent interest in global fungal conservation, we present an update of the state of fungal conservation in Canada.
Contribution of Canadian mushroom clubs and amateur mycologists
Driven by passion and enthusiasm, members of mycological organizations have long made crucial contributions to advancing knowledge of fungi (Watling 1998; Perry 2008; Lemelin and Fine 2013). Mushroom club members and amateur mycologists have greatly improved our collective knowledge of fungal diversity and occurrence through many activities and projects. The role of the nonprofessional mycological community initiatives has been multifold, with the overarching goal to encourage enthusiasm for fungi. Such organizations have increased awareness of the need to document fungal diversity and have prioritized the training of community members to create high-quality, vouchered observations.
Gathering field data has been part of the regular activities of mushroom clubs and organizations through organizing forays and surveys (e.g., Foray Newfoundland and Labrador, Fédération québécoise des groupes de mycologues, Mycoquébec, Pacific NorthWest Key Council). These typically annual events have resulted in the development of robust data sets on fungal biodiversity for target regions in Canada that have been used for developing valuable reference material for fungal taxonomy and biogeography (e.g., Mycoquébec.org, including the app “La Fonge du Québec”, Mushroom Expert, Bolete Filter, MatchMaker/MycoMatch, Pictorial Key to Mushrooms of the Pacific Northwest). Reference books and magazines have also been an invaluable product of community activities in Canada, for example, the exceptionally thorough “Répertoire des cortinaires du Québec” (Landry et al. 2021) or the Foray Newfoundland and Labrador journal Omphalina. Many nonprofessionals have enthusiastically embraced the use of DNA sequence data paired with morphological investigation to aid in specimen identification, especially in North America, where many species await formal taxonomic description, for example, Mycoquébec, which has contributed ∼3000 sequences obtained from public or private Quebec fungaria or through surveys of undersampled areas since 2017, or the Puget Sound Mycological Society’s Danny’s DNA discoveries (alpental.com/psms/ddd/). Through these activities nonprofessionals—often in collaboration with professional mycologists—have described and published new species in official outlets (e.g., Xerocomellus diffractus N. Siegel, C.F. Schwarz & J.L. Frank, in Frank et al. 2020; Hygrophorus canadensis Lebeuf & P.-A. Moreau, in Bellanger et al. 2021; Cortinarius amabilis Bojantchev, Ammirati & Pastorino, in Bojantchev 2015). Seattle-based architect Benjamin Woo (1923–2008) is an example of an independent mycologist who made a large contribution to our understanding of a taxonomic group. Over 30 years (1974–2007), Woo developed an expertise in the genus Russula and his collection was used to describe several new species (Hyde et al. 2017), understand the local diversity of Russula (Bazzicalupo et al. 2017), develop keys for identification of Pacific Northwest Russula species (alpental.com/psms/PNWMushrooms/PictorialKey/Russula.htm, zoology.ubc.ca/∼biodiv/mushroom/) and inform a regional book, “Mushrooms of British Columbia” (MacKinnon and Luther 2021).
Recently, iNaturalist and MushroomObserver have emerged as tools for citizen scientists to upload geotagged biodiversity observations, allowing researchers to study fungal diversity and biogeography with an unprecedented level of resolution. In Canada alone, approximately 40,000 contributors have uploaded over half a million fungal observations. There are currently ongoing projects that leverage iNaturalist data to improve the knowledge of patterns of biodiversity for select taxa such as chanterelles (Cantharellus spp., Craterellus spp.) and hedgehog mushrooms (Hydnum spp.) in New Brunswick (inaturalist.org/projects/chanterelles-and-hedgehog-mushrooms-in-new-brunswick) and in Newfoundland (inaturalist.org/projects/foray-nl-hydnum-project). These genera have been widely foraged as edible for decades, and identified as similarly looking European species, but the actual species present in Canada have only been described in the past 5 years (Thorn et al. 2017; Niskanen et al. 2018; Swenie et al. 2018), and additional undescribed taxa very likely remain to be found. Similar conservation-focused efforts are underway, such as the Rare Fungi Challenge for Northeastern North America (inaturalist.org/projects/fundis-rare-fungi-challenge-northeast), which aims to generate new observations for 20 potentially rare species of macrofungi.
Amateur mycologists have contributed to our collective knowledge of fungal species and their efforts have been used in many forms from identification keys, to fungarium collections to species assessments for Wild Species 2020 (wildspecies.ca/reports, available 2022). Community science mycological organizations will be key in the service and advancement of fungal conservation.
Species and habitats: conservation priorities
The most complete assessment of fungal species in Canada is the Wild Species 2020 report, which includes ∼7,000 species of macrofungi. This milestone report was produced by compiling national data from across Canada through collaboration with every provincial and territorial government in an effort to assess the status of species at both the national and subnational level. To calculate a national rank for each species, provincial and local species occurrence data were used. The species were assessed based on a rank calculator that included range extent and occurrence. Species categories are based on a system used by NatureServe, a US-based conservation organization that provides conservation tools. Their categories are already used by the regional Conservation Data Centres in Canada, and these categories are similar (but not identical) to those used by the IUCN. In the past 5 years there was a sharp uptick in the number of species assessed from 87 in the Wild Species 2015 report (CESCC 2016) to ∼7,000 in the 2020 report, a hopeful and exciting result.
For conservation lists to be effective, baseline data (taxonomic, ecological, biogeographic) need to be generated and evaluated for fungal species occurring in Canada. Taxonomic expertise, in particular, is badly needed to be able to accurately reflect which species we should prioritize for fungal conservation. A representative example is the mushroom genus Pluteus, which has been the subject of much taxonomic work in the past 10 years (e.g., Justo et al. 2014). In the current version of the Canada Wild Species 2020, three species of Pluteus are included, but none of them are confirmed to occur in North America based on current taxonomic work. On the other hand, five other species of Pluteus present in Canada (P. elaphinus, P. eos, P. leucoborealis, P. oreibatus, and P. rangifer) should be included in the Natureserve list and highly prioritized in conservation actions because of their relative rarity and endemicity to Eastern North America or the circumboreal area. Conservation efforts for macrofungi have been hampered by the lack of basic taxonomic knowledge; research funds dedicated to taxonomic research are hard to obtain. Since 2010 the Natural Sciences and Engineering Research Council of Canada has awarded only 8 small grants (less than CAN$27,000) directly related to the taxonomy or diversity of macrofungi, and none were Canada-wide in scope (nserc-crsng.gc.ca/ase-oro/index_eng.asp).
Although species of fungi occur across governmental borders, governments have different laws and priorities for their protection. Two main approaches are used for conservation implementation. Firstly, assessments can target specific habitats (e.g., Carolinian forest in Ontario and the Okanagan valley in British Columbia). Some species are likely limited to threatened habitats, for example sand dunes or old growth forests (Ruokolainen et al. 2018; Yang et al. 2021), but data are limited for threatened habitats and the species inhabiting them. One drawback of this specific approach in Canada is that it is often restricted to species that are at the edge of their northern range, making their habitat very limited. As another approach, assessment can target species endemic only to Canada or species having a large proportion of their global geographic range in Canada. It is difficult to designate a species as endemic to Canada because detailed (or even approximate) distribution data are lacking for most macrofungal species in North America. The conservation directive is that Canada becomes responsible to maintain those species in the world. The practical consequences of the “conservation directive” depends on the case that can be presented to government agencies to act upon the conservation issue. By identifying priority targets we can make the case for the species that need more urgent action. While identifying targets does not imply that other conservation measures will happen, without first identifying targets nothing will happen because there is no case to be presented.
With the recent momentum of the Canadian Institute of Ecology and Evolution’s Virtual Working Group in Fungal Conservation as well as the Canadian Fungal Research Network (CanFunNet, fungalresearch.ca, Horianopoulos et al. 2020), we aim to unite those working with macrofungi in Canada towards establishing conservation goals. In Table 1, we provide a preliminary list of fungi to be considered for formal conservation assessment, with justification. As formal frameworks can aid actionable conservation recommendations, in Fig. 1 we outline a process for assessment of Canadian macrofungi for conservation. Representative fungi from our list of potential candidate species for assessment are illustrated in Fig. 2. While by no means exhaustive, our species list is a starting point to invite a broader conversation in the Canadian mycological community to determine macrofungal conservation priorities. For example, Phaeocollybia species found in western Canada also deserve our attention as they occur in threatened old growth forests (Redhead and Norvell 1993; Kroeger and Berch 2017).
Table 1.
| Species | Biogeographical pattern | Background | Justification | Mycoportal Canadian records |
|---|---|---|---|---|
| Arrhenia chlorocyaneaa | European, west coast | This species is reported throughout northern Europe, where it is sometimes common in highly disturbed sandy soils with regenerating vegetation (Boomsluiter 2006). | Restricted to coastal sand dunes on the west coast of North America and uncommon in coastal BC (Redhead 1989, as Omphalina viridis; map) | 15 collections from BC in DAOM (6), UBC (4), Mushroom Observer (5) |
| Battarrea phalloides | Global | This species, or species complex (Martín et al. 2013), has a global distribution and is currently under assessment by the IUCN (Gargano et al. 2020). It is predominantly restricted to sandy soils but not uncommonly occurring in disturbed habitats in some parts of the world. | Mainly found in the US southwest, but rarely seen in BC (Schalkwijk-Barendsen 1991; Kroeger and Berch 2017), SK and YT | 11 collections from YT (2), BC (8), SK (1) in DAOM (1), UBC (6), UC (1), NYBG (1), Mushroom Observer (2) |
| Cortinarius kroegeria | Western cordilleran endemic | This taxon was recently segregated from the morphologically similar C. limonius, based on material from BC and Washington State (US) (Liimatainen 2016). Collections of C. limonius from eastern Canada and Costa Rica appear conspecific with European C. limonius (Landry et al. 2021) | Even if regionally common or widespread in BC, it would have a very restricted global range. | 14 collections from BC, in UBC (10) and Mushroom Observer (4) |
| Crepidotus cinnabarinusa | Circumboreal: hardwood forest | Conspicuous member of the genus found uncommonly on rotting hardwoods, especially aspen, throughout the Northeast and westward to Alberta (Luther and Redhead 1981; Redhead 1989, map) | 25 records from AB (7), MB (12), ON (2) and QC (4) in DAOM (19), CMMF (2), UBC (2), and HRLb (1), iNaturalist (1) | |
| Cystoderma granosuma | Eastern deciduous forest endemic | Distinctive wood rotter, but infrequently observed (Smith and Singer 1945; Thorn 1986). | 29 records from ON (15) and QC (14), in DAOM (12), NYBG (2), TRTC (3), CMMF (5), ARIZ (1), MICH (4), iNaturalist (1), Mushroom Observer (1) | |
| Dendrocollybia racemosaa | European, west coast | Unmistakable species uncommonly encountered along the west coast of North America (Machnicki et al. 2006) but very rarely observed in the east | 12 records from BC (11) and ON (1), TRTC (1), UBC (7), MICH (1)c, iNaturalist (1), Mushroom Observer (4) | |
| Gyromitra sphaerosporaa | Eastern deciduous forest endemic | Distinctive member of the genus, but rarely observed since its description from New York in 1875 (Seaver 1942; Thorn 2006, as Pseudorhizina sphaerospora) | 12 records from BC (1), MB (4), ON (2), QC (3) and Canada (2), in DAOM (6), FH (1), CMMF (2), BPI (2), Mushroom Observer (1) | |
| Hapalopilus croceusa | Uncertain | Listed by IUCN as globally vulnerable, in North America it is restricted to the northeast.d | 8 records from ON (4) and QC (3), in DAOM (1), NYBG (3), CMMF (3), and UC (1)c; collections from ON are from 1890–1918 | |
| Laricifomes officinalis | Circumboreal | Widespread throughout the northern hemisphere where it is restricted to old growth conifers. In Europe, this species is becoming increasingly rare due to anthropogenic stressors such as deforestation (Mukhin et al. 2005); Endangered on IUCN Red List | Associated with old growth conifers; no recent records from eastern Canada (Thorn 2006) | 52 records from BC (37), AB (2), ON (11), and QC (2), in DAOM (19), CUP (1), FH (8), NYBG (4), OSU (2), ARIZ (1), GB (2), MICH (2), TENN-F (1), CFMR (2), and BPI (5)c; Mushroom Observer (5), most recent collections from ON and QC are from 1923 |
| Myriostoma coliforme | Mediterranean-Continental | Included on the Red Lists of 18 European countries (Evans et al. 2006; Sousa et al. 2017; Sousa et al. 2019). Under assessment by the IUCN. | Known in Canada from just three localities in southern Ontario, one cited in Coker and Couch (1928) | 13 records from ON, in DAOM (3), BPI (2), NYBG (2), TRTC (2), UBC (1), and MICH (3)c |
| Naiadolina flavomerulina | Boreal endemic | A monotypic genus of small, brightly coloured mushrooms fruiting on dead stems of wetland monocots | The only agaric genus known only from Canada (Redhead 1981; Redhead 2013) | 11 records from QC, in DAOM (10) and HRLb (1) (one photographic sighting from Nova Scotia (Redhead et al. 2013)) |
| Resupinatus dealbatus | Eastern deciduous forest endemic | Very rarely observed since its initial description from Ohio in 1847, but recently rediscovered in Canada after 125 years. | weirdandwonderfulwildmushrooms.blogspot.com/2018/03/a-missing-mushroom-reappears-after-125.html | 2 records from ON (1) and QC (1), in DAOM (1) and NYS (1) |
| Sarcosoma globosuma | Circumboreal | Red-listed in 10 European countries (Dahlberg 2015), Near Threatened globally: iucnredlist.org/species/58515314/58515381; the prime cause for decline is changing land management, above all the practice of clear cutting old-growth forests. | Associated with old-growth boreal forests | 22 records from BC (1), ON (17), QC (3) and NB (1) in FH (3), NBM (2), TRTC (1), S (1), CMMF (2), UC (1), WIS (1), RMS (2), BPI (3), and HRLb (1)c, iNaturalist (1), Mushroom Observer (3) |
| Sarcosphaera coronariaa | Circumboreal | Distinctive species from a monotypic genus, presumed ectomycorrhizal with deciduous or coniferous hosts. This species is already red-listed throughout several European countries (Lizoň and Zelený 2006) | Ontario records are from just two areas in Carleton and Norfolk Counties. | 45 records from YT (1), BC (21), AB (3), MB (1), ON (17), QC (2), in DAOM (11), CMMF (3), ARIZ (2), UBC (5), BPI (1), and HRLb (1), Mushroom Observer (22) |
| Stereopsis humphreyi | Asian (?), Pacific coastal | Known from the Pacific coast of North America, with an uncertain record from Tibet (Redhead and Reid 1983; Redhead 1989, map); Near Threatened globally (IUCN) | 25 records from BC, in DAOM (5), UBC (12), MICH (1), and WTU (1)c, Mushroom Observer (6) | |
| Typhula [Macrotyphula] fistulosa ssp. fistulosaa | Amphi-Atlantic: European, east coast | Apparently common in Europe, however in North America it almost exclusively occurs in Southern Ontario, where it is only rarely found (Petersen 1972). Macrotyphula fistulosa var. contorta, found on dead hardwood branches in winter in Newfoundland, represents a separate species, Typhula contorta (Voitk 2012, Olariaga and Salcedo 2013). | Records of Clavariadelphus fistulosus or M. fistulosa include T. contorta, leaving T. fistulosa s.str. as quite uncommon | 19 records (not counting those that were identified as T. contorta or could be recognized as that taxon by occurrence on woody branches) from ON (13) QC (4) and NS (2) in DAOM (10), ACAD (1), CMMF (1), UC (2), MICH (1), and HRLb (1)c, iNaturalist (1), Mushroom Observer (2) |
| Underwoodia columnaris | Eastern deciduous forest endemic | Large, distinctive species from monotypic genus. Rarely observed in Canada and Northeastern US (Seaver 1928; Barron 1999) | 8 records from MB (7) and ON (1), in DAOM (7) and BPI (1); 3 ON records in Mushroom Observer appear to be misidentified |
a
Denotes species pictured in Fig. 2.
b
HRL represents the private fungarium of author Renée Lebeuf.
c
Individual record(s) duplicated in different fungaria.
d
This species is a good example of the need for taxonomic evaluation of species that must occur in parallel with conservation assessments. The DNA data currently available in GenBank suggest that what has been called Hapalopilus croceus globally is a complex of 3 distinct species, one each in Europe, Asia and North America, and it is likely that all of them have conservation concerns (see Supplementary Material 1).
Note:
Biogeographical patterns are derived from Redhead (1989). BC, British Columbia, UBC, University of British Columbia Herbarium; DAOM, Canadian National Mycological Herbarium; IUCN, International Union for Conservation of Nature; US, United States; SK, Saskatchewan; YT, Yukon Territory; UC, Herbarium of the University of California, Berkeley; NYBG, New York Botanical Gardens; AB, Alberta; MB, Manitoba; ON, Ontario; QC, Quebec; CMMF, Cercle des mycologues de Montréal Fungarium; TRTC, The Royal Ontario Museum Fungarium; ARIZ, University of Arizona Herbarium; MICH, University of Michigan Herbarium; FH, Farlow Herbarium of Harvard University; BPI, U.S. National Fungus Collections; CUP, Cornell Plant Pathology Herbarium; OSU, Oregon State University Herbarium; GB, University of Gothenburg Herbarium; TENN-F, University of Tennessee Fungal Herbarium; CMFR, Center for Forest Mycology Research Herbarium; NYS, New York State Museum Herbarium; NBM, Herbarium, New Brunswick Museum; S, Herbarium, Swedish Museum of Natural History; WIS, Wisconsin State Herbarium; RMS, W. G. Solheim Mycological Herbarium, University of Wyoming; WTU, University of Washington Herbarium; NS, Nova Scotia; ACAD, E.C. Smith Herbarium, Acadia University.
Fig. 1.
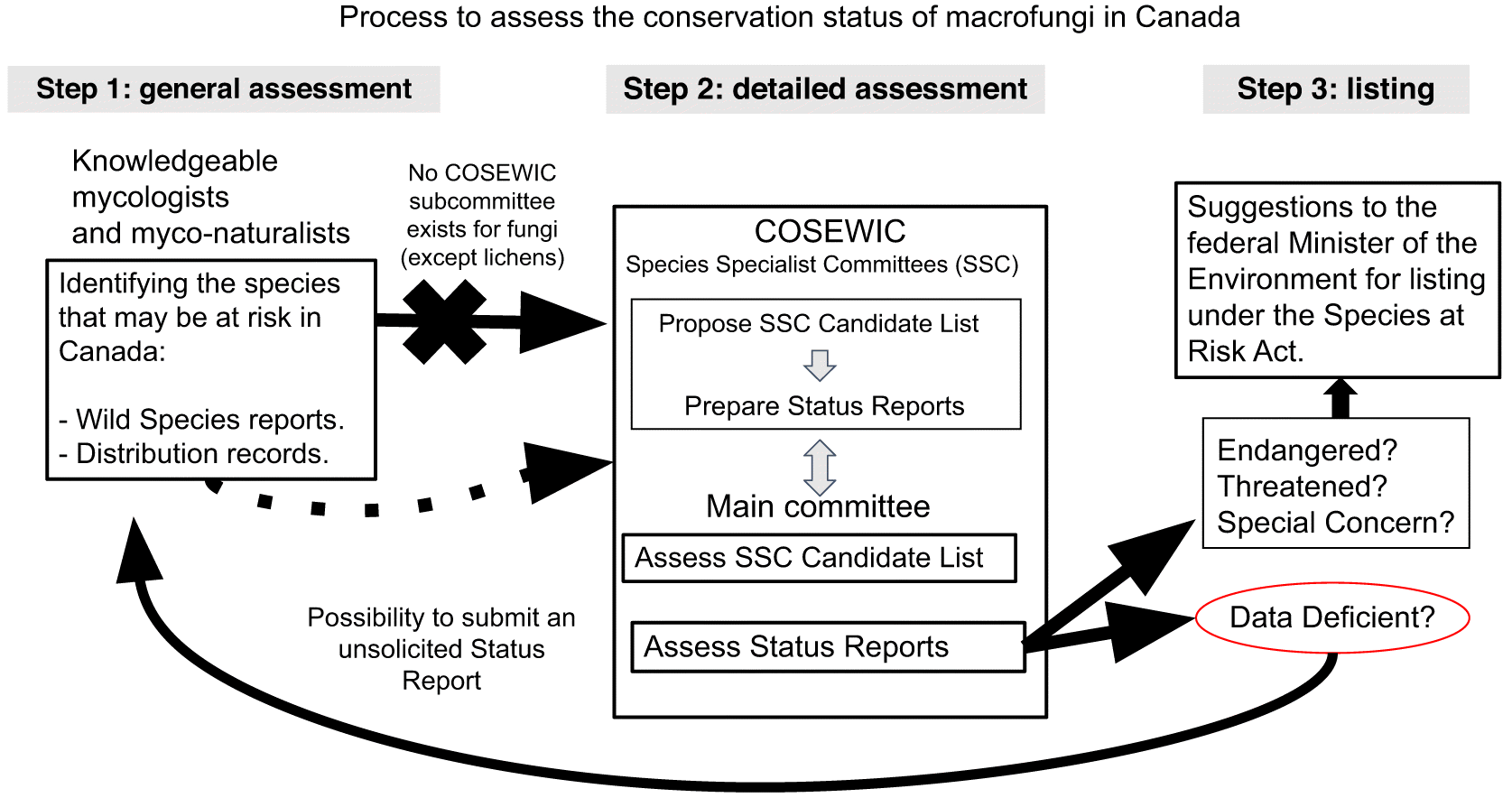
Fig. 2.

Conclusion
Challenges to assessment of fungal species remain. Important habitats across Canada are under-surveyed as species records are generally clustered near large urban areas (see example for Canadian records of the easily recognizable genus Amanita in Supplementary Material 2). Old growth and native prairies are threatened ecosystems and may host rare fungal species; they remain under-sampled for fungi. In this paper we have summarized the state of fungal conservation in Canada and provided a short preliminary list of fungal species to be prioritized for conservation status assessment in Canada. The use of historical and digital records and the collaboration of academic institutions, governmental agencies, citizen scientists, independent mycologists, museum biodiversity researchers, and Indigenous Peoples will be key in the implementation of conservation actions for fungi in Canada.
Acknowledgements
The authors gratefully acknowledge the Canadian Institute of Ecology and Evolution for Virtual Working Group funding. AKW also acknowledges NSERC Discovery Grant funding (No. NSERC—2017-04325). We thank reviewers including Dr. Scott Redhead (Agriculture and Agri-Food Canada’s Canadian National Mycological Herbarium) for valuable comments on an earlier draft of this manuscript.
References
Ainsworth A, Canteiro C, Dahlberg A, Douglas B, Furci G, Minter D, et al. 2018. Conservation of fungi. In State of the world’s fungi. Edited by KJ Willis. Royal Botanic Gardens, Kew, UK. pp. 70–77.
Allen M, and Allen E. 2017. Mycorrhizal mediation of soil fertility amidst nitrogen eutrophication and climate change. In Mycorrhizal mediation of soil. Edited by NC Johnson, C Gehring, and J Jansa Elsevier, Amsterdam. pp. 213–231.
Andrew C, Halvorsen R, Heegaard E, Kuyper TW, Heilmann-Clausen J, Krisai-Greilhuber I, et al. 2018a. Continental-scale macrofungal assemblage patterns correlate with climate, soil carbon and nitrogen deposition. Journal of Biogeography, 45(8): 1942–1953.
Andrew C, Heegaard E, Høiland K, Senn-Irlet B, Kuyper TW, Krisai-Greilhuber I, et al. 2018b. Explaining European fungal fruiting phenology with climate variability. Ecology, 99(6): 1306–1315.
Arnolds E. 1991. Decline of ectomycorrhizal fungi in Europe. Agriculture, Ecosystems & Environment, 35(2–3): 209–244.
Barron G. 1999. Mushrooms of Ontario and eastern Canada. Lone Pine Publishing, Edmonton, CA. 336 p.
Bazzicalupo AL, Buyck B, Saar I, Vauras J, Carmean D, and Berbee ML. 2017. Troubles with mycorrhizal mushroom identification where morphological differentiation lags behind barcode sequence divergence. Taxon, 66: (4): 791–810.
Bellanger JM, Lebeuf R, Sesli E, Loizides M, Schwarz C, and Moreau PA, et al. 2021. Hygrophorus sect. Olivaceoumbrini: new boundaries, extended biogeography and unexpected diversity unravelled by transatlantic studies. Persoonia, 46: 272–312.
Bland LM, Collen B, Orme CDL, and Bielby J. 2015. Predicting the conservation status of data-deficient species. Conservation Biology, 29: (1): 250–259.
Boddy L, Büntgen U, Egli S, Gange AC, Heegaard E, Kirk PM, et al. 2014. Climate variation effects on fungal fruiting. Fungal Ecology, 10: 20–33.
Boer WD, Folman LB, Summerbell RC, and Boddy L. 2005. Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiology Reviews, 29(4):795–811.
Bojantchev D. 2015. Nomenclatural novelties. Index Fungorum, 247: 1–2.
Boomsluiter MW. 2006. Some ecological notes on Omphalina chlorocyanea. Field Mycology, 7(2): 52–53.
Buxton RT, Bennett JR, Reid AJ, Shulman C, Cooke SJ, Francis CM, et al. 2021. Key information needs to move from knowledge to action for biodiversity conservation in Canada. Biological Conservation, 256: 108983. [online]: Available from sciencedirect.com/science/article/pii/S0006320721000355.
Cao Y, Wu G, and Yu D. 2021. Include macrofungi in biodiversity targets. Science, 372(6547): 1160–1160.
Coker WC, and Couch JN. 1928. The Gasteromycetes of the eastern United States and Canada. The University of North Carolina Press, Chapel Hill. 446 p.
CESCC. 2016. Wild species 2015: the general status of species in Canada. [online]: Available from wildlife-species.canada.ca/species-risk-registry/virtual_sara/files/reports/Wild%20Species%202015.pdf.
Dahlberg A. 2015. Sarcosoma globosum. The IUCN red list of threatened species 2015. [online]: Available from https://doi.org/10.2305/IUCN.UK.2015-4.RLTS.T58515314A58515381.en.
Dahlberg A, Genney DR, and Heilmann-Clausen J. 2010. Developing a comprehensive strategy for fungal conservation in Europe: current status and future needs. Fungal Ecology, 3(2): 50–64.
Dahlberg A, and Mueller GM. 2011. Applying IUCN red-listing criteria for assessing and reporting on the conservation status of fungal species. Fungal Ecology, 4(2): 147–162.
Evans S, Henrici A, and Ing B. 2006. Red data list of threatened British fungi. Report by the British Mycological Society (BMS), Working With the Joint Nature Conservation Committee. [online]: Available from britmycolsoc.org.uk/field_mycology/conservation/red-data-list.
Fernandez CW, Nguyen NH, Stefanski A, Han Y, Hobbie SE, Montgomery RA, et al. 2017. Ectomycorrhizal fungal response to warming is linked to poor host performance at the boreal-temperate ecotone. Global Change Biology, 23(4): 1598–1609.
Frank JL, Siegel N, Schwarz CF, Araki B, and Vellinga EC. 2020. Xerocomellus (Boletaceae) in western North America. Fungal System Evolution, 6: 265–288.
Gange A, Gange E, Sparks T, and Boddy L. 2007. Rapid and recent changes in fungal fruiting patterns. Science, 316(5821): 71–71.
Gange AC, Heegaard E, Boddy L, Andrew C, Kirk P, Halvorsen R, et al. 2018. Trait-dependent distributional shifts in fruiting of common British fungi. Ecography, 41(1): 51–61.
Gargano ML, Venturella G, and Ferraro V. 2020. Is Battarrea phalloides really an endangered species? Plant Biosystems, 155: 759–762.
Giauque H, and Hawkes CV. 2013. Climate affects symbiotic fungal endophyte diversity and performance. American Journal of Botany, 100(7): 1435–1444.
Gonçalves SC, Haelewaters D, Furci G, and Mueller GM. 2021. Include all fungi in biodiversity goals. Science, 373(6553): 403–403.
Hazard C, Kruitbos L, Davidson H, Taylor AF, and Johnson D. 2017. Contrasting effects of intra-and interspecific identity and richness of ectomycorrhizal fungi on host plants, nutrient retention and multifunctionality. New Phytologist, 213(2): 852–863.
Heilmann-Clausen J, Barron ES, Boddy L, Dahlberg A, Griffith GW, Nordén J, et al. 2015. A fungal perspective on conservation biology. Conservation Biology, 29(1): 61–68.
Horianopoulos LC, Gluck-Thaler E, Benoit Gelber I, Cowen LE, Geddes-McAlister J, Landry CR, et al. 2020. The Canadian fungal research network: current challenges and future opportunities. Canadian Journal of Microbiology, 67: (1): 13–22.
Hubbell SP. 2005. Neutral theory in community ecology and the hypothesis of functional equivalence. Functional Ecology, 19: 166–172.
Hyde KD, Norphanphoun C, Abreu VP, Bazzicalupo A, Thilini Chethana KW, Clericuzio M, et al. 2017. Fungal diversity notes 603–708: taxonomic and phylogenetic notes on genera and species. Fungal Diversity, 87(1): 1–235.
Justo A, Malysheva E, Bulyonkova T, Vellinga EC, Cobian G, Nguyen N, et al. 2014. Molecular phylogeny and phylogeography of Holarctic species of Pluteus section Pluteus (Agaricales: Pluteaceae), with description of twelve new species. Phytotaxa, 180(1): 1–85.
Katoh K, and Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30(4): 772–780.
Kauserud H, Heegaard E, Büntgen U, Halvorsen R, Egli S, Senn-Irlet B, et al. 2012. Warming-induced shift in European mushroom fruiting phenology. Proceedings of the National Academy of Sciences, 109(36): 14488–14493.
Kivlin SN, Emery SM, and Rudgers JA. 2013. Fungal symbionts alter plant responses to global change. American Journal of Botany, 100(7): 1445–1457.
Körner C. 2003. Ecological impacts of atmospheric CO2 enrichment on terrestrial ecosystems. Philosophical Transactions of the Royal Society of London. Series A: Mathematical, Physical Engineering Sciences, 361(1810): 2023–2041.
Kroeger P, and Berch S. 2017. Macrofungus species of British Columbia. Province of BC, Victoria, BC. Technical Report 108, 88 p. [online]: Available from for.gov.bc.ca/hfd/pubs/Docs/Tr/TR108.htm.
Landry J, Lamoureux Y, Lebeuf R, Paul A, Lambert H, and Labbé R. 2021. Répertoire des cortinaires du Québec. Québec, Mycoquébec. 252 p.
Larsson A. 2014. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics, 30: 3276–3278.
Lemelin RH, and Fine GA. 2013. Leisure on the recreational fringe: naturework and the place of amateur mycology and entomology. PAN: Philosophy Activism Nature, 10: 77–86.
Liimatainen K. 2016. Nomenclatural novelties: Cortinarius kroegeri. Index Fungorum, 294: 1.
Lilleskov E, Hobbie EA, and Horton T. 2011. Conservation of ectomycorrhizal fungi: exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecology, 4: 174–183.
Lizoň P, and Zelený, L. 2006. Sarcosphaera coronaria in Slovakia. Catathelasma, 8: 5–9.
Luther BS, and Redhead, SA. 1981. Crepidotus cinnabarinus in North America. Mycotaxon, 12(2): 417–430.
Machnicki N, Wright, LL, Allen A, Robertson CP, Meyer C, Birkebak JM, et al. 2006. Russula crassotunicata identified as host for Dendrocollybia racemosa. Pacific Northwest Fungi, 1(9): 1–7.
MacKinnon A, and Luther K. 2021. Mushrooms of British Columbia. Royal BC Museum, Victoria, BC.
Martín, MP, Rusevska K, Dueñas M, and Karadelev M. 2013. Battarrea phalloides in Macedonia: genetic variability, distribution and ecology. Acta Mycologica, 48(1): 113–122.
May TW, Cooper JA, Dahlberg A, Furci G, Minter DW, Mueller GM, et al. 2018. Recognition of the discipline of conservation mycology. Conservation Biology, 33: 733–736.
Miller MA, Pfeiffer W, and Schwartz T. (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop, New Orleans, LA. pp. 1–8. [online]: Available from computer.org/csdl/proceedings/gce/2010/12OmNy7h3cn.
Mommer L, Cotton TA, Raaijmakers JM, Termorshuizen AJ, van Ruijven J, Hendriks M, et al. 2018. Lost in diversity: the interactions between soil-borne fungi, biodiversity and plant productivity. New Phytologist, 218: 542–553.
Mooers AO, Doak DF, Scott Findlay C, Green DM, Grouios C, Manne LL, et al. 2010. Science, policy, and species at risk in Canada. Bioscience, 60(10): 843–849.
Mueller GM, Dahlberg A, and Krikorev M. 2014. Bringing fungi into the conservation conversation: the global fungal red list initiative. Fungal Conservation, 4: 12–16.
Mukhin VA, Kotiranta H, Knudsen H, Ushakova NV, Votintseva AA, Corfixen P, et al. 2005. Distribution, frequency and biology of Laricifomes officinalis in the Asian part of Russia. Mikologiya i Fitopatologiya, 39(5): 34–42.
Niskanen T, Liimatainen K, Nuytinck J, Kirk P, Ibarguren IO, Garibay-Orijel R, et al. 2018. Identifying and naming the currently known diversity of the genus Hydnum, with an emphasis on European and North American taxa. Mycologia, 110(5): 890–918.
Olariaga I, and Salcedo IJM. 2013. New combinations and notes in clavarioid fungi. Mycotaxon, 121(1): 37–44.
Peay KG, Kennedy PG, and Talbot JM. 2016. Dimensions of biodiversity in the Earth mycobiome. Nature Reviews Microbiology, 14: 434–447: Available from
Perry B. 2008. Citizen Science. Mycena News. [online]: Available from mykoweb.com/articles/CitizenScience.html.
Petersen RH. 1972. Notes on Clavarioid Fungi. XII. Miscellaneous notes on Clavariadelphus, and a new segregate genus. Mycologia, 64(1): 137–152: Available from
Pringle A, Adams RI, Cross HB, and Bruns TD. 2009. The ectomycorrhizal fungus Amanita phalloides was introduced and is expanding its range on the west coast of North America. Molecular Ecology, 18: (5): 817–833.
Redhead SA. 1981. Agaricales on wetland Monocotyledoneae in Canada. Canadian Journal of Botany, 59(5): 574–589.
Redhead SA. 1989. A biogeographical overview of the Canadian mushroom flora. Canadian Journal of Botany, 67: 3003–3062.
Redhead SA. 2013. Nomenclatural novelties. Index Fungorum, 15: 1–2.
Redhead SA, Malloch DW, and Ginns J. 2013. Naiadolina flavomerulina. Omphalina, 4: 18–20.
Redhead S, and Norvell L. 1993. Phaeocollybia in western Canada. Mycotaxon, 46: 343–358.
Redhead SA, and Reid DA. 1983. Craterellus humphreyi, an unusual Stereopsis from western North America. Canadian Journal of Botany, 61(12): 3088–3090.
Ruokolainen A, Shorohova E, Penttilä R, Kotkova V, and Kushnevskaya H. 2018. A continuum of dead wood with various habitat elements maintains the diversity of wood-inhabiting fungi in an old-growth boreal forest. European Journal of Forest Research, 137:(5): 707–718.
Schalkwijk-Barendsen HME. 1991. Mushrooms of Western Canada. Lone Pine, Edmonton. 414 p.
Seaver FJ. 1928. The North American cup fungi (operculates) (reprint 1978). Lubrecht and Cramer, Monticello, NY. 377 p., 74 p.
Smith AH, and Singer R. 1945. A monograph on the genus Cystoderma. Papers of the Michigan Academy of Sciences, Arts and Letters, 30: 71–124.
Sousa JO, Baseia IG, and Martín MP. 2019. Strengthening Myriostoma (Geastraceae, Basidiomycota) diversity: Myriostoma australianum sp. nov. Mycoscience, 60: 25–30.
Sousa JO, Suz LM, García MA, Alfredo DS, Conrado LM, Marinho P, et al. 2017. More than one fungus in the pepper pot: Integrative taxonomy unmasks hidden species within Myriostoma coliforme (Geastraceae, Basidiomycota). PLoS ONE, 12: e0177873.
Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30(9): 1312–1313.
Swenie RA, Baroni TJ, and Matheny PB. 2018. Six new species and reports of Hydnum (Cantharellales) from eastern North America. MycoKeys, 42: 35–72.
Ten Have R, and Teunissen PJ. 2001. Oxidative mechanisms involved in lignin degradation by white-rot fungi. Chemical Reviews, 101: 3397–3414: Available from
Thorn RG. 1986. Mushrooms of Algonquin Provincial Park. Friends of Algonquin Park, Whitney, ON. 32 p.
Thorn RG. 2006. Checklist of the conspicuous fungi of Algonquin Provincial Park. Algonquin Park Tech. Bulletin, Friends of Algonquin Park, Whitney, ON. 25 p.
Thorn RG, Kim JI, Lebeuf R, and Voitk A. 2017. The golden chanterelles of Newfoundland and Labrador: a new species, a new record for North America, and a lost species rediscovered. Botany, 95: 547–560.
Treseder KK. 2004. A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytologist, 164: 347–355.
Turcotte A, Kermany N, Foster S, Proctor CA, Gilmour SM, Doria M, et al. 2021. Fixing the Canadian species at risk act: identifying major issues and recommendations for increasing accountability and efficiency. FACETS, 6(1): 1474–1494.
Turner NJ (ed). 2020. Plants, People, and Places. McGill-Queen’s University Press, Montreal. 554 p.
van Strien AJ, Boomsluiter M, Noordeloos ME, Verweij RJ, and Kuyper TW. 2018. Woodland ectomycorrhizal fungi benefit from large-scale reduction in nitrogen deposition in the Netherlands. Journal of Applied Ecology, 55(1): 290–298.
Voitk A. 2012. An epiphany about Macrotyphula contorta. Omphalina, 3(1): 3–7.
Wang P, Xu J, Wu G, Liu T, and Yang ZL. 2021. Genomic and experimental investigations of Auriscalpium and Strobilurus fungi reveal new insights into pinecone decomposition. Journal of Fungi, 2021: 679.
Watling R. 1998. The role of the amateur in mycology–what would we do without them! Mycoscience, 39: 513–522.
Yang S, Limpens J, Sterck FJ, Sass-Klaassen U, Cornelissen JH, Hefting M, et al. 2021. Dead wood diversity promotes fungal diversity. Oikos, 130: 15.
Supplementary materials
Supplementary Material 1
- Download
- 220.29 KB
Supplementary Material 2
- Download
- 711.25 KB
Information & Authors
Information
Published In

FACETS
Volume 7 • Number 1 • January 2022
Pages: 448 - 463
Editor: Emile Gluck-Thaler
History
Received: 20 November 2021
Accepted: 30 January 2022
Version of record online: 24 March 2022
Notes
This paper is part of a collection titled “The Canadian Fungal Network 2021 Annual Meeting – Connecting fungal researchers across Canada”.
Copyright
© 2022 Bazzicalupo et al. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Data Availability Statement
All relevant data are within the paper and in the Supplementary Material.
Key Words
Sections
Subjects
Plain Language Summary
Towards Mushroom Conservation in Canada
Authors
Author Contributions
All conceived and designed the study.
AB, RH, AJ, GK, RL, BM, RGT, and AKW performed the experiments/collected the data.
All analyzed and interpreted the data.
AJ, GK, RGT, and AKW contributed resources.
AB, SCG, RH, AJ, GK, RL, BM, RGT, and AKW drafted or revised the manuscript.
Competing Interests
The authors have declared that no competing interests exist.
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Anna Bazzicalupo, Susana C. Gonçalves, Rémi Hébert, Sigrid Jakob, Alfredo Justo, Gavin Kernaghan, Renée Lebeuf, Bruce Malloch, R. Greg Thorn, and Allison K. Walker. 2022. Macrofungal conservation in Canada and target species for assessment: a starting point. FACETS.
7(): 448-463. https://doi.org/10.1139/facets-2021-0180
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
Cited by
1. Reviewing the contributions of macrofungi to forest ecosystem processes and services
2. Editorial: Biodiversity and conservation of fungi and fungus-like organisms
