Representation of data
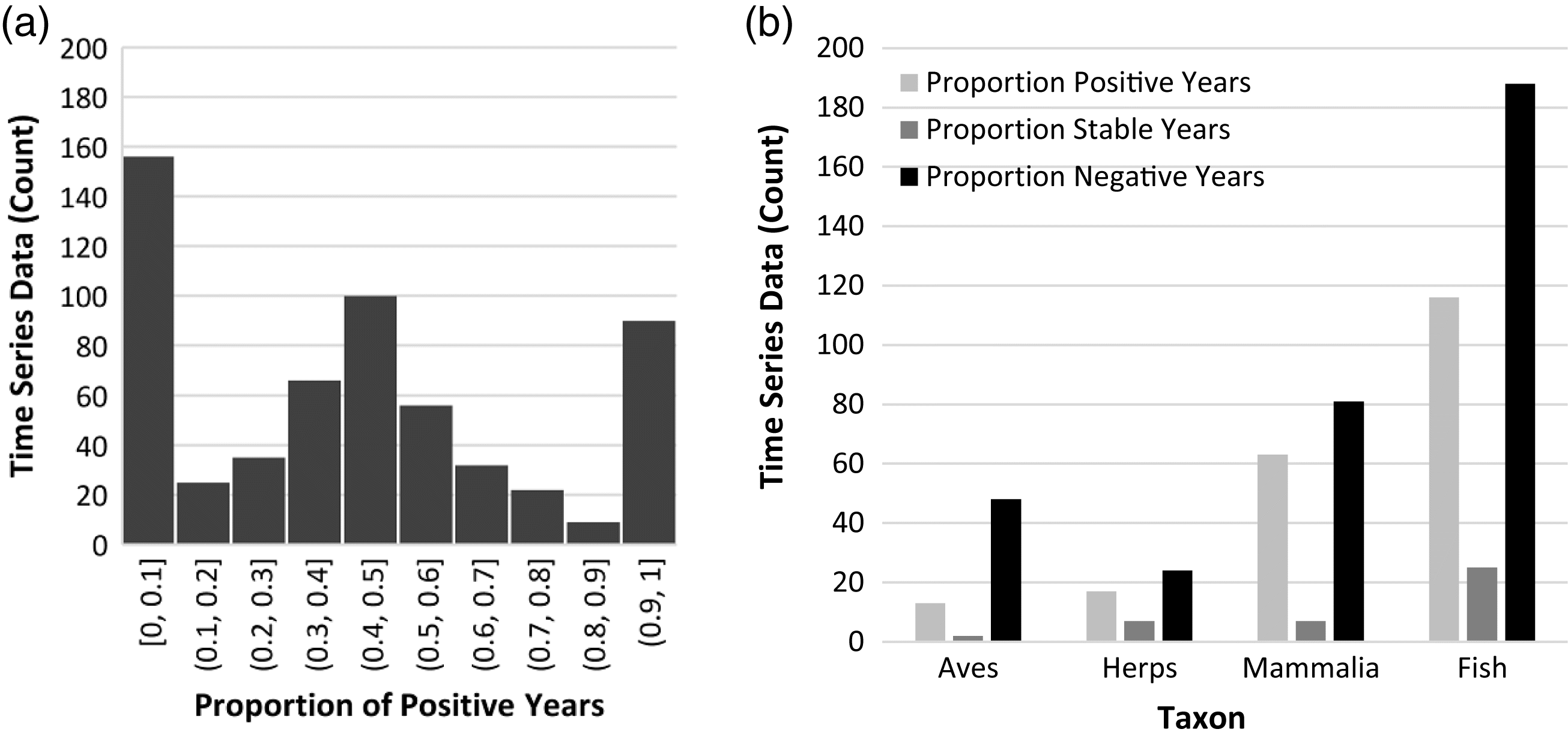
Birds were the best represented taxonomic group in our analysis, largely attributable to data from the Status of Birds in Canada (
ECCC 2015), which aggregates data from bird monitoring surveys across the country including the North American Breeding Bird Survey (
Pardieck et al. 2018) and the Christmas Bird Count (
NAS 2016). Fish, which represent 43.17% of Canadian at-risk species as designated by COSEWIC, were also well-represented in the analysis as a result of Research Vessel Trawl Survey data from Fisheries and Oceans Canada. These large-scale monitoring programs are valuable in determining large-scale trends, but as shown through the analysis of predictor variables and population trends, they may lack a level of geographic specificity to produce meaningful results.
The low representation of amphibians and reptiles in the data set is consistent with their overall representation in the global LPI database (
McRae et al. 2017;
Saha et al. 2018). Although the natural history, biology, and physiology of most amphibian and reptile species are generally documented, there is a comparatively poorer understanding of biological population size and distribution—partially attributed to a lack of baseline data and the difficulty in monitoring species of solitary and cryptic behavior (
CESCC 2011).
Threat profiles
Given that most species face multiple threats, a determination of (
i) which threats are the most prevalent and (
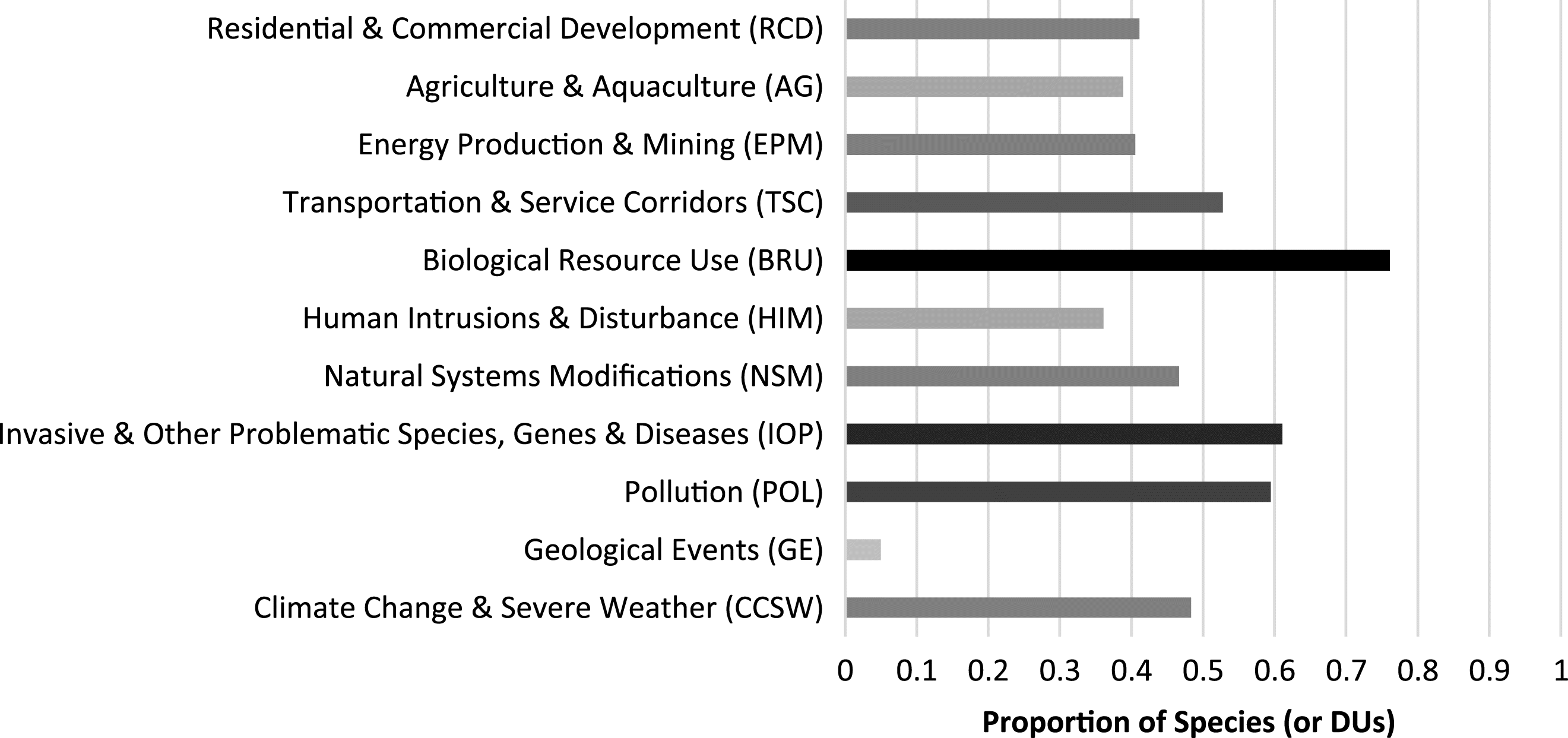
ii) which threats act synergistically on population abundance, would seem crucial for the recovery of many species. BRU was the most frequently cited threat to at-risk vertebrate species in our sample, consistent with previous studies, which found BRU as the most frequently cited threat of scientifically assessed at-risk flora and fauna in Canada (
Prugh et al. 2010) and worldwide (
Maxwell et al. 2016). Importantly, BRU was not merely an effect of the greater number of fish species included in our analysis, given that BRU was among the most frequently cited threats for multiple taxa. In Canada, despite no comparable assessment for 8 years, the similarities of analyses are prevalent: BRU was the most frequently cited threat, followed by invasive and other problematic species, genes and diseases—while catastrophic geological events was last (
Prugh et al. 2010).
Conversely, in an analysis of threats extracted from recovery strategies for SARA-listed flora and fauna in Canada, human intrusion and disturbance (recreational, military, and other activities) was the most frequently cited threat (
McCune et al. 2013). However, species considered threatened by BRU according to COSEWIC were (
i) less likely to be listed under SARA, (
ii) infrequently has BRU as a listed threat, and (or) (
iii) lacked a final recovery strategy for data extraction (
McCune et al. 2013). Consequently, as an example only six marine fish were included in the analysis by
McCune et al. (2013) compared to the 41 included in this analysis—all of which had BRU as a listed threat. This bias against listing harvested fish and northern mammals under SARA has been well-documented in the literature (
Mooers et al. 2007;
Findlay et al. 2009;
Creighton and Bennett 2019) and likely contributes to the discrepancies between our analysis and that of
McCune et al. (2013).
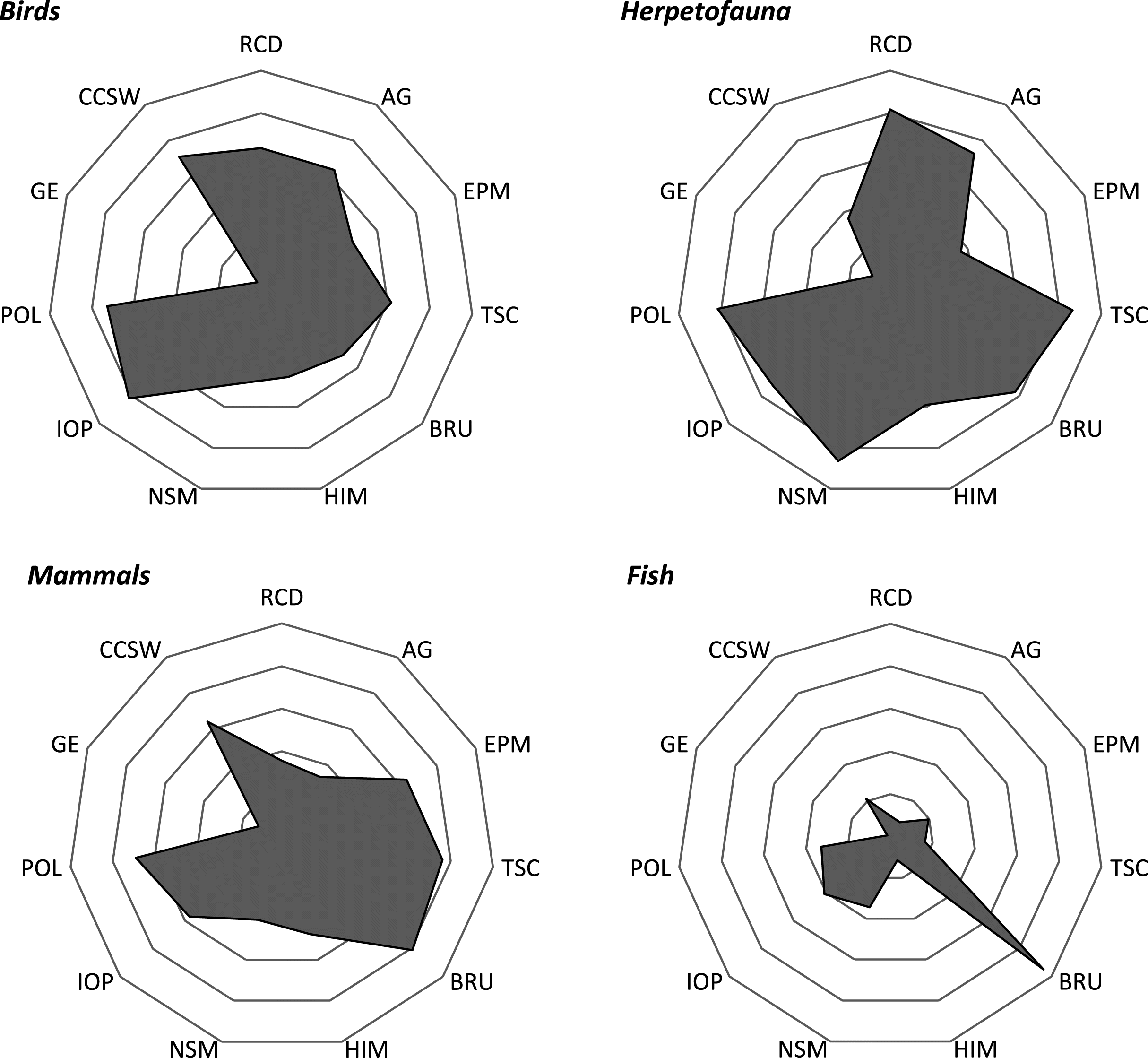
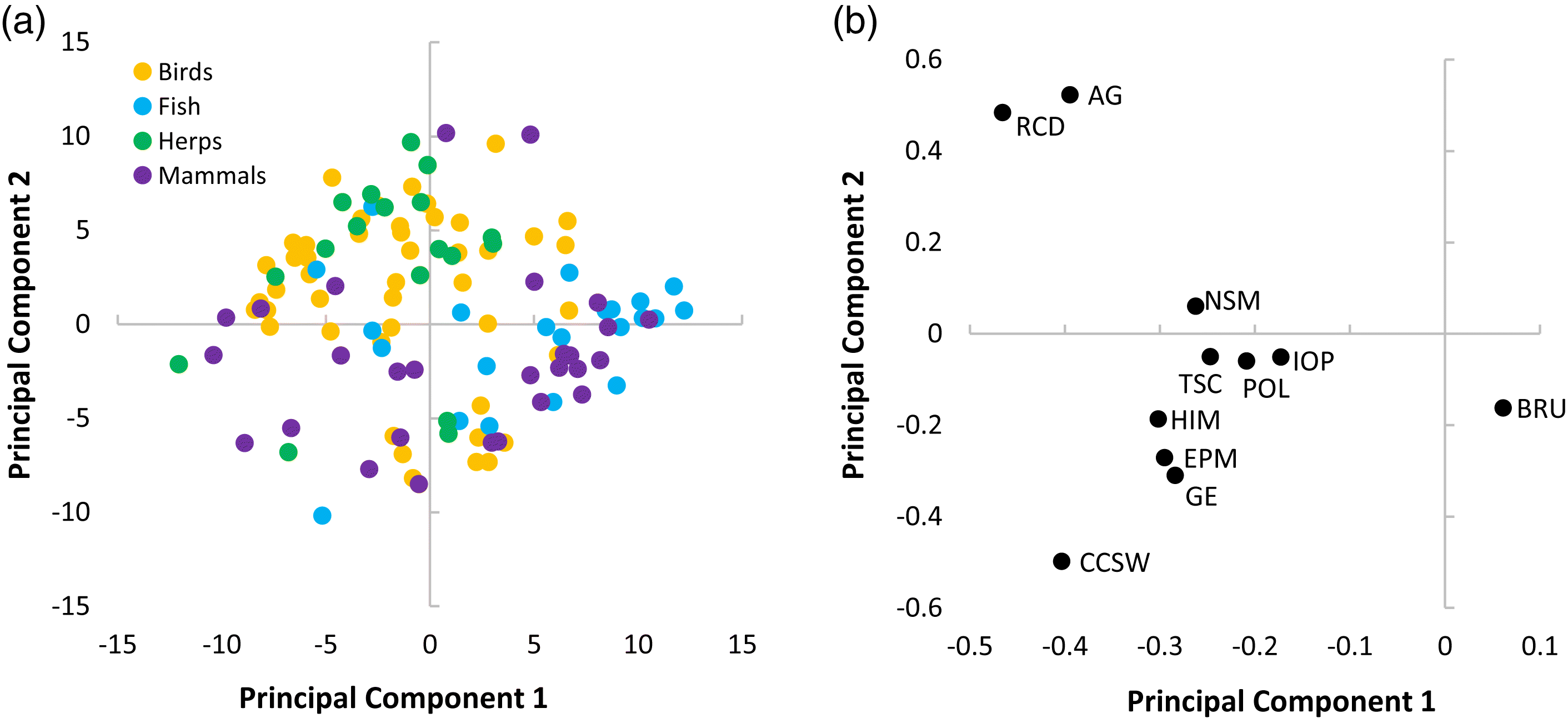
Within the analysis, threats were rarely listed in isolation. Rather, species were generally affected by multiple, compounding pressures. There were some patterns (e.g., taxonomic groups) where signals could be detected among the distribution of threats. For instance, PCA scores demonstrated an isolated cluster of RCD and AG, likely due to their prevalence in similar geographic space—southern Canada. Given that species richness gradients are strongly correlated to climate, species are concentrated within the southern regions of Canada (
Coristine and Kerr 2011) where many at-risk species reach their northern range limits (
Gibson et al. 2009). Hotspots of at-risk vertebrates are particularly prevalent in southern Ontario and Quebec, the prairies, and the Okanagan Valley of British Columbia (
Coristine and Kerr 2011;
WWF Canada 2019)—areas characterized by intensive land-use by agriculture and development (
Coristine and Kerr 2011;
Coristine et al. 2018). This is particularly true for birds (
eBird Canada 2018) and herpetofauna inhabiting the northern periphery of their distribution (
Lesbarrères et al. 2014)—taxa that occupy similar PCA space as RCD and AG.
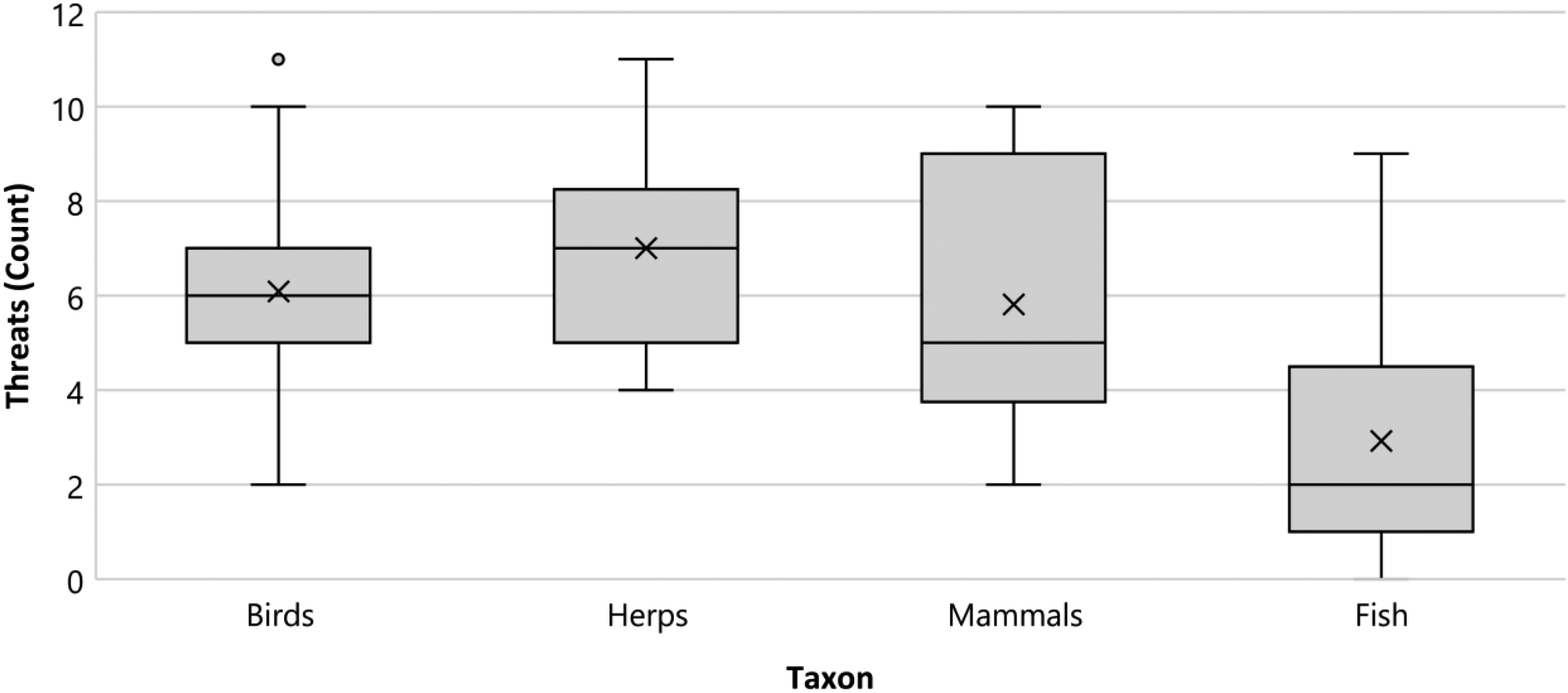
Amphibians and reptiles were considered the most threatened by compounded pressures, with a comparatively greater proportion of threats cited within their respective COSEWIC reports. According to the IUCN Red List, amphibians are the most threatened taxa (
Baillie et al. 2010) and often epitomize the current biodiversity crisis (
Sodhi et al. 2008). Of the species or DUs assessed by COSEWIC, 65.12% of amphibians and 90.57% of reptiles are designated as at-risk or Extirpated (
sararegistry.gc.ca). To put this into perspective, of all flora and fauna assessed by COSEWIC with active designations, 77.33% are designated at-risk or Extirpated, and 19.76% are classified as Not at Risk, underscoring the vulnerability of herpetofauna within national borders. More than any other taxa, herpetofauna are restricted to the southern latitudes of Canada at the northern periphery of their distribution (
Lesbarrères et al. 2014), where human footprint is highest (
Venter et al. 2016)—which translates into numerous threats for these species.
Analyzing drivers of trends
In general, advancing the understanding of factors—threat profile, TX, GL, and PAs—that influence trends in abundance, may help to prioritize biological attributes, physical elements, and conservation actions to maximize species recovery. Our analysis shows that the biotic factors selected (TX and GL) were considered stronger determinants of population trends in comparison to threat profiles and protected areas. In particular, our results suggest an association between TX and population trends, echoing results of
Leung et al. (2017). In the literature, however, there remains mixed evidence of GL as a predictor of population trends or extinction risk (
Purvis et al. 2000;
Collen et al. 2011). By comparison, PA was not a good predictor of population trends, though a lack of variance may have attributed to the absence of a PA effect—where only 7.95% of population time series had data collection within a PA. Finally, despite the association between taxon and population trends and its apparent correlation with threats as per the PCA results, we found no evidence of the predictive power of threat profiles on the proportion of annual increases for population time series of COSEWIC-assessed at-risk species.
Given that multiple threats are often acting in synergy (
Brook et al. 2008), the use of threat profiles was favored over the examination of individual threats, but the approach had limited predictive power and was therefore not as powerful as initially anticipated. Below, we provide a series of justifications as to why, and some insights regarding how these issues could be remedied to strengthen future iterations of this work.
Lack of precise spatial data
PA itself may not be a robust predictor of decline but may improve when paired with regional considerations. For instance,
Craigie et al. (2010) found that when considering the individual effect of PA of mammal population trends in Africa, western populations exhibited large declines, eastern populations experienced moderate declines, and southern populations revealed stable or increasing trends. Our analysis did not account for geographical differences due to inadequate spatial data associated with LPI trends. Specifically, population trends were often associated with large regions (e.g., provincial scale), rather than unique or refined geographic locations, limiting our ability to appropriately harness spatial data. Consequently, the absence of an effect of PA may be attributed to variation of population trends within PAs in the absence of regional differences. Nevertheless, PA did not emerge as a statistically significant fixed effect in similar studies (e.g.,
Spooner et al. 2018) despite the inclusion of spatial data.
Biases and outdated classification of threats
Importantly, the subjective nature of listing threats to species has been well documented for the IUCN Red List (
Hayward 2009). The potential bias in listing and further interpretation of these threats can therefore limit quantitative analyses aimed at comparing threat profiles and trends. For example, though nearly half of species in our analysis had Climate Change and Severe Weather (CCSW) as a listed threat, an increased incidence of CCSW categorization was revealed in the analyzed COSEWIC Status Reports. Of the assessed species 62.68% of COSEWIC assessments in the last 5 years had CCSW as a listed threat, compared with 40.62% in the 5 years prior.
In Canada, COSEWIC defines threats as “activities or processes that directly negatively affect the Canadian population” (
COSEWIC 2015). In November 2015, COSEWIC approved a threat classification and assessment calculator that applies the IUCN threat classification system. COSEWIC mandated the systematic application of threat classification for all threat categories in future COSEWIC status reports. Under this system, threats are also characterized with respect to “scope”, “severity”, and “timing”, which can be used to generate the relative impact of a given threat. Despite implementation of this detailed and rigorous approach to listing threats, COSEWIC reports conducted prior to 2015, which contributes 90.56% of species in our analysis, were likely prone to greater biases in listing of threats, and interpretation and correlation of listed threats to IUCN categories.
Poor sample size and variation among predictive factors
In our analysis, we were unable to partition out individual taxa owing to poor sample size. Consequently, we struggled to include appropriate cross-taxa measures such as body size, where length is typically recorded for fish and herpetofauna (
Froese and Pauly 2016;
Santini et al. 2018) and mass is generally used for birds and mammals (
Myhrvold et al. 2015).
Collen et al. (2011), and we also suggest that a lack of significant predictors could also be attributed to small sample sizes or a lack of variance across given predictors. In our data set, only 7.95% of population time series were within PAs, suggesting that a lack of variance may have attributed to the absence of a PA effect. We were also unable to look at phylogenetically induced effects as fitting genus and species in a nested structure resulted in convergence errors and did not improve model fit.