The role of science advice in recovery potential assessments in freshwater fish listing decisions under the Canadian Species at Risk Act
Abstract
Fishes assessed as Threatened or Endangered by the Committee on the Status of Endangered Wildlife in Canada are disproportionately less likely to be listed under the federal Species at Risk Act (SARA) compared to other taxa. We examined the extent to which the amount and type of science advice in a Recovery Potential Assessment (RPA) contributes to SARA-listing decisions for 34 wildlife species of freshwater fishes in Canada. We used a generalized linear mixed model to describe SARA listing status as a function of RPA completeness. Principal coordinates analyses were conducted to assess similarity in answers to RPA questions among listed and nonlisted species. The amount and type of science advice within an RPA were weakly related to SARA status. RPA completeness accounted for only 7.4% of model variation when family was included as a random effect, likely because nine species not listed under SARA (64%) belong to the sturgeon family. Our results suggest that, while potentially useful for informing recovery strategies, RPAs do not appear to be driving listing status for freshwater fishes in Canada. Factors beyond scientific advice likely contribute to nonlisted species and delays in listing decisions.
1. Introduction
Legal protection is vital in endangered species recovery (Restani and Marzluff 2002); however, the process of listing at-risk species under legal frameworks is often problematic (Favaro et al. 2014; Himes Boor 2014; McDevitt-Irwin et al. 2015). Challenges to the legal-listing process may arise due to socio-economic factors (Restani and Marzluff 2002; Findlay et al. 2009; Schultz et al. 2013) or taxonomic bias (Mooers et al. 2007; Walsh et al. 2012; Glass et al. 2017) and can be exacerbated by limited biological information available for species (COSEWIC 2019; Powles 2011). Not only can the paucity of accurate, species-specific information influence listing decisions but, if listed, may lead to deficiencies in species recovery planning (Rohlf 1991; Cooke 2008; Gehring and Ruffing 2008). Standardized guidelines, informed by a balance of socio-economic and scientific factors, are needed to ensure the assessment, legal protection, and recovery of at-risk species.
Legal protection in Canada is provided federally by the Species at Risk Act (SARA), which affords legal prohibitions against “harming, killing, harassing, capture or taking” of species listed under the Act (SARA, S.C. 2002, c. 29). To make an informed decision about listing species under SARA, the Canadian government uses the best available scientific advice recommended by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) and applies precaution when balancing threats, cost-effective measures, and scientific certainty (DFO 2013; Gregory and Long 2009). Within 90 days of receiving a COSEWIC assessment of an aquatic species, the Minister of Environment and Climate Change Canada (MoE) must publish a response statement on how they wish to proceed. Depending on the type of consultation undertaken, a final listing decision is made by the Governor in Council (GiC) within 24–36 months (Supplementary Material 1). Listing decisions are outlined in the Regulatory Impact Analysis Statement (RIAS) and while many factors contributing to the final decision are often not public (including detailed consultations and socio-economic analyses), biological risk assessments in the form of Recovery Potential Assessments (RPA) are publicly available.
In cases where the impact of listing a species is anticipated to be high, RPAs are conducted to inform RIAS of the listing decision and the subsequent legally required recovery actions (strategy, action plan, and identification of critical habitat) (DFO 2014). Information found in the COSEWIC assessment provides a baseline of biological information required to assess a species’ status. An RPA differs from a COSEWIC assessment by describing both the species status (Phase 1) and recovery potential (Phase 2 and 3). In 2007, Fisheries and Oceans Canada (DFO) developed their Revised Protocol for Conducting Recovery Potential Assessments, outlining a series of information requirements (termed tasks) that RPA documents should address based on the “best science advice possible” (DFO 2007). Although DFO adopts the language of “best science advice possible” in its guidelines, SARA acknowledges that COSEWIC must develop a status report based on the “best available information” and, thus, we used the latter terminology throughout this report (S.C. 2002, c. 29). The “best science advice possible” encompasses a species’ current status, recovery target, and potential mitigation and alternative options for managers (DFO 2007) to assess and prioritize both the threats to a species at population and species levels and their potential for recovery (DFO 2014). The RPA is peer reviewed during a Canadian Science Advisory Secretariat (CSAS) meeting and posted to the publicly accessible CSAS website, alongside the science advisory report proceedings and other associated documents (dfo-mpo.gc.ca/csas-sccs/index-eng.htm).
Despite a comprehensive mechanism by which DFO can communicate scientific advice in the SARA-listing process, species governed by DFO, in particular freshwater fishes, are disproportionately less likely to be listed under SARA compared with other taxa (Mooers et al. 2007; Favaro et al. 2014; Dorey and Walker 2018). Creighton and Bennett (2019) found that 57% of fish species assessed by COSEWIC between 2003 and 2017 were not listed under SARA. For freshwater fishes with an RPA written before a SARA-listing decision (n = 34), 41% have not been listed under SARA, each owing to delays in the SARA-listing process associated with extended consultations. Findlay et al. (2009) and McDevitt-Irwin et al. (2015) have both demonstrated the bias towards delayed listing for marine and freshwater fishes via extended consultation. Building on this work, we explore whether science advice in an RPA influences whether a species is listed under SARA or awaits a decision due to ongoing extended consultation.
Our study examines the extent to which the best available scientific advice provided in an RPA contributes to SARA-listing decisions for freshwater fishes at risk in Canada. Specifically, we evaluate two questions: (i) Does the completeness of the RPA influence SARA-listing decisions? (ii) Does the type of scientific information within an RPA influence the SARA listing decisions? By evaluating the role of an RPA in the SARA-listing process, our study examines whether not listing a species is related to the quality, quantity, and availability of science advice above the threshold provided in a COSEWIC assessment. Our study identifies knowledge gaps in the listing-decision process and provides recommendations to improve the RPA for freshwater fishes in Canada. Understanding the role of science advice and RPAs in conservation legislation is integral to ensure meaningful science contributes to effective public policy and to improve the overall protection for freshwater fishes at risk in Canada.
2. Methods
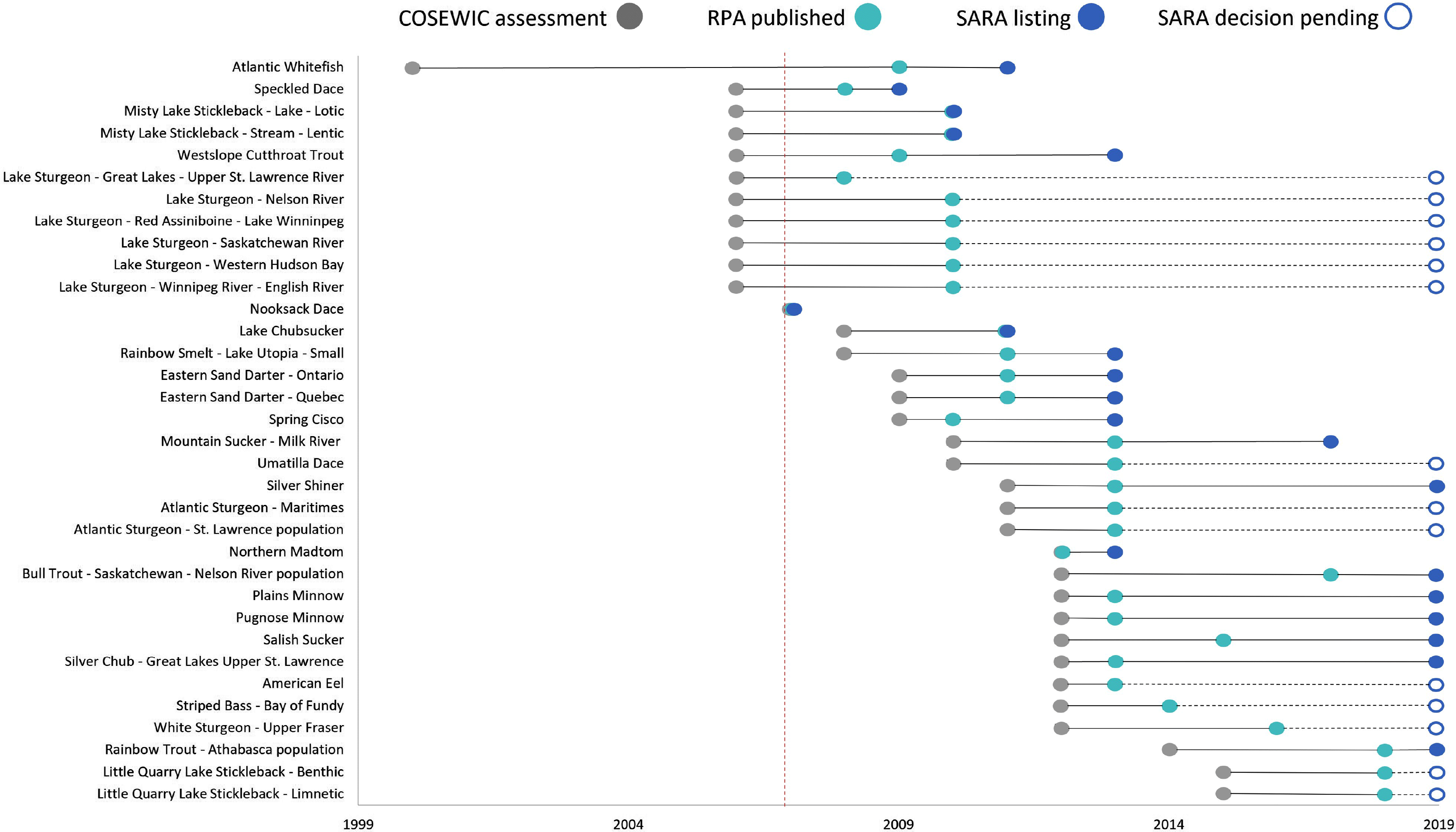
We compiled a list of 34 species at risk (SAR), or designatable units (DU), in Canada based on the following criteria: (i) the species or DU is a freshwater fish or diadromous fish that spends a substantial portion of its life cycle in freshwater habitat; (ii) the species or DU was (re)-assessed by COSEWIC as Threatened or Endangered after 2007, the year in which standardized guidelines to completing an RPA were published (DFO 2007); and (iii) there is a written RPA for the species or DU published after the COSEWIC assessment and before the SARA-listing decision (Fig. 1; Supplementary Material 2). SARA-listing decisions were classified as Threatened, Endangered, or no status. Species with a SARA-listing decision of no status are currently not listed under Schedule 1 for one of the following reasons: (i) the MoE or GiC decided not to list the species under Schedule 1, (ii) the MoE response outlined that further consultation is needed prior to a decision, or (iii) the MoE recommendation was to refer the species back to COSEWIC for further additional scientific information required to make the decision.
Fig. 1.
2.1. Analysis of RPAs
The RPAs were coded according to the Revised Protocol for Conducting Recovery Potential Assessments, developed in 2007 (DFO 2007). This document lists 17 tasks in total, divided into three phases. Phase 1 addresses questions related to the current species status, Phase 2 addresses issues related to recovery, and Phase 3 identifies potential mitigation and alternatives. The 17 tasks were subdivided into 42 questions that were each coded to assess the best available scientific information for each species (Supplementary Material 3). Tasks were subdivided into multiple questions when they contained multiple types of information. For example, task two, “evaluate recent species trajectory for abundance, range, and number of populations”, was subdivided into three questions, each answered separately, to examine whether recent species trajectory was assessed for (i) abundance, (ii) range, and (iii) number of populations. Sixteen questions were coded as either containing qualitative (1), quantitative (2), or no data (0), and 21 questions were coded as either not applicable (NA), no data (0), or data. For these 21 questions, “data” answers varied to reflect the type of answers often found in the RPAs (e.g., whether data were presented for a surrogate species or the species of interest). Five of these questions were coded as the count of unknown, low, medium, and high threats and the total number of threats. For the complete list of questions and detailed information on coding criteria see Supplementary Material 3 – Coding Criteria.
In addition to the RPA, Recovery Potential Modelling (RPM) and other supplemental documents in support of the RPA were used as supplemental information, as they were considered part of the scientific advice that contributes to the final listing decision. Referenced documents considered in the coding process for each species can be found in Supplementary Material 2.
Coding was performed by seven researchers in the Mandrak Biodiversity and Conservation of Freshwater Fishes Lab at the University of Toronto Scarborough. Each researcher independently conducted a quantitative assessment of each RPA. Afterward, a consensus among the seven researchers was reached using the small-group Delphi paradigm, which allows for flexibility while minimizing bias (Mitchell 1991; Lofaro 2015).
2.2. Does the RPA completeness influence SARA-listing decisions?
In total, 42 questions were coded to quantify the best available scientific advice provided in an RPA and supplemented with RPM and other supporting documents. We measured the RPA completeness as the percentage of questions answered in total and within each phase. Kruskal-Wallis tests, followed by Dunn’s post-hoc tests, were used to examine significant differences in phase completeness, across taxonomic families.
Phase 1 completeness reflects the amount of available science advice on the species’ status, similar to what is found in a COSEWIC assessment, whereas Phases 2 and 3 completeness reflects the amount of available science advice on species recovery and management potential, beyond the baseline of a COSEWIC assessment. We used a generalized linear model (GLM) to assess whether the completeness of science advice in each phase of the RPA influenced SARA-listing decisions. First, we asked: “do the number of questions answered by any or all of the three phases of the RPA questionnaire explain the variability in whether the GIC chooses to list a species or not under SARA?” If Phases 2 and 3 explain more variation in the SARA-listing decision, then the addition of qualitative and quantitative information found within an RPA plays an important role in the SARA-listing process. We fit a binary response of SARA-listing decision (0 = no status; 1 = listed on Schedule 1) via a logit link with Phase 1, 2, 3 completeness, using the function “glm” in package lme4 in R (R 3.5.3; R Core Team 2019). The full model was compared to the partial models, using Akaike’s information criterion (AICc) corrected for a small sample size (n = 34).
The recommended COSEWIC status for fishes within the same family may be more similar due to heritable attributes and information provided in their RPA may overlap; therefore, we assume species within the same family may not be independent with respect to SARA-listing status. We extended these linear models to examine the effect of within-group clusters at the family level. We then asked: “does the taxonomic family affect our findings with respect to questionnaire completeness?” Adding a family group as a random effect allowed each group (taxonomic family, n = 10 groups) to have its own mean value with respect to listing status by using a random-intercepts generalized linear mixed model (GLMM) (Gelman and Hill 2006; Schielzeth and Nakagawa 2013). All other model variables were kept the same as in the linear models presented in Table S4b, with Phases 1, 2, and 3 all included as fixed effects. We tested for the significance of random effects by using an ANOVA to compare the full model (family as random effects) with a reduced model (individual as random effects and random effects set to 0). The full model received significantly more support (χ2 = 18.32, df = 2, p = 0.0002), supporting the use of family as random effects. Variance explained was measured using pseudo-R2 with the function r.squaredGLMM in the MuMIN package in R (R Core Team 2019; Bartoñ 2020). The contribution of the three fixed‐effect variables was compared to the full model by stepwise removal of nonsignificant fixed effects (p > 0.05) using likelihood ratio tests.
2.3. Does the type of scientific information within an RPA influence SARA-listing decision?
To visualize how SARA listing (Threatened, Endangered, no status) differs based on the scientific information provided in an RPA, a Principal Coordinates Analysis (PCoA) was constructed. Before analysis, the species (n = 34) by question (n = 41) matrix was converted to a distance matrix using Gower distance, which allows for missing data and mixed data types (Gower 1971; Podani 1999). Question 16b regarding population viability analysis and mitigation uncertainty was excluded as the question was scored NA for all species. An analysis of similarities (ANOSIM) was completed on the Gower distance matrix using the vegan package in R (R 3.5.3, R Core Team 2019; Oksanen et al. 2019) to test for significant differences in SARA-listing decision based on the best available scientific advice provided in an RPA (and supplementary documents). Significant differences were examined further using post-hoc pairwise ANOSIM comparisons between all possible status pairs.
3. Results
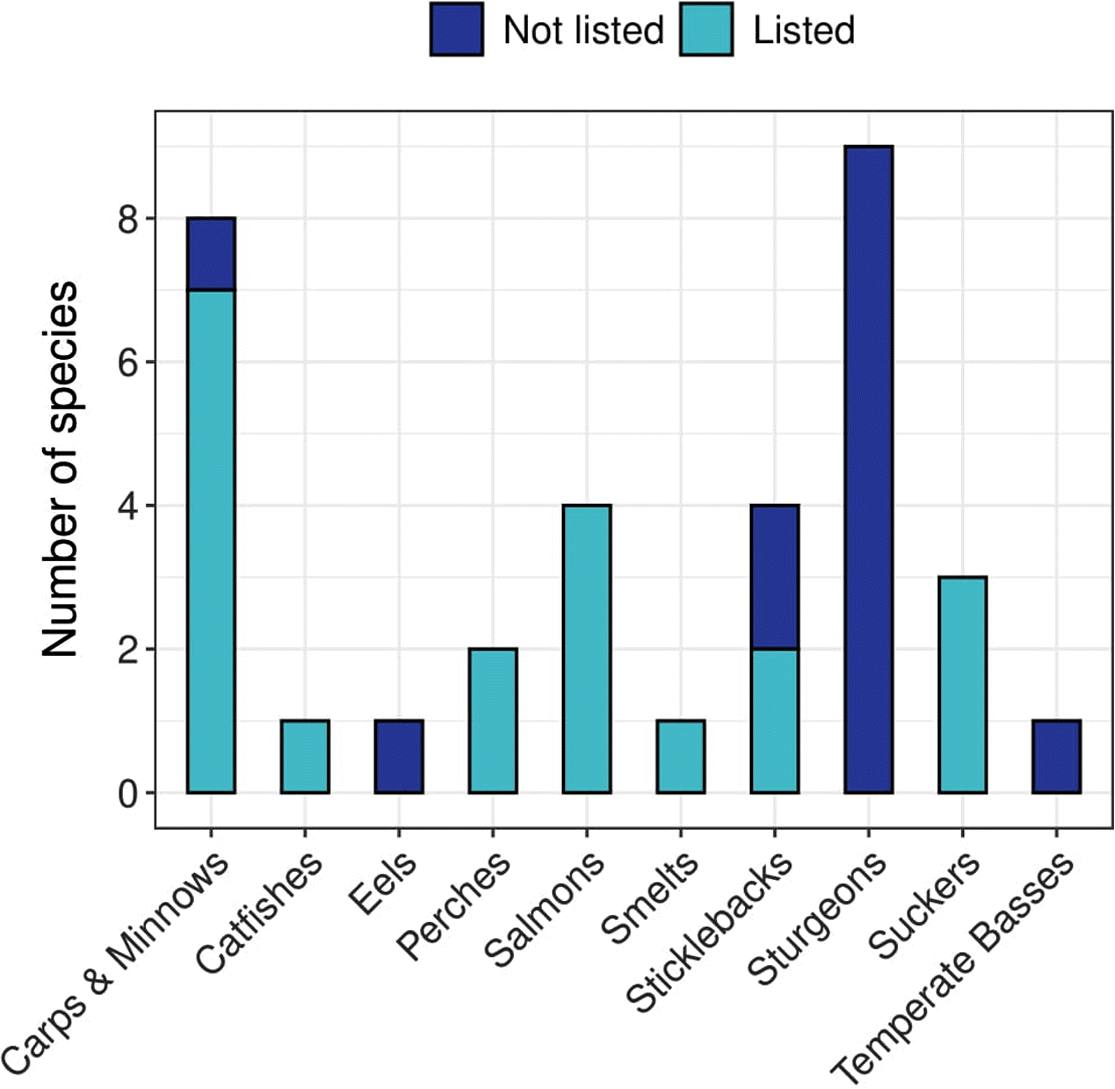
Of 34 species, 20 (59%) had the same COSEWIC assessment and SARA listing status (10 Threatened, 10 Endangered). Species with matching statuses belong to carps and minnows, catfishes, perches, salmons, smelts, sticklebacks, and suckers (Fig. 2). For the fourteen no status species (7 Endangered, 7 Threatened), a listing decision was still pending in every case owing to consultation periods that extended beyond the standard 24-month process (GOC 2018; Supplementary Material 1). Sixty-four percent of these species belong to the sturgeon family, and the remaining 36% are temperate basses, eels, sticklebacks, and carps and minnows (Fig. 2).
Fig. 2.
3.1. Does RPA completeness influence SARA-listing decisions?
Across all 34 species examined, there were no cases where all 17 tasks (and 42 questions) outlined in the RPA guidelines were completed. On average, RPAs were 64% complete, ranging from 43% to 81%. Phase completeness differed significantly (χ2 = 49.9, df = 3, p < 0.001) across the 34 species (Fig. 3a). Post-hoc Dunn’s pairwise tests determined that Phase 1 and Phase 2 did not significantly differ from each other (χ2 = 0.8, p = 0.21) and that both were significantly more complete than Phase 3 (χ2 = 6.47, p < 0.001; χ2 = 5.68, p < 0.001) (Fig. 3a). Specifically, Phase 1 (q. 1–7) was generally the most complete, ranging from 50% to 90% complete. Phase 2 (q. 8–11) contained the greatest range in completeness, ranging from 38% to 92% complete. Lastly, Phase 3 (q. 12–17) was consistently the least completed, ranging from 22% to 55% complete, except for the RPA for Lake Sturgeon (Great Lakes–St. Lawrence population) which was 66% complete (Fig. 3).
Fig. 3.
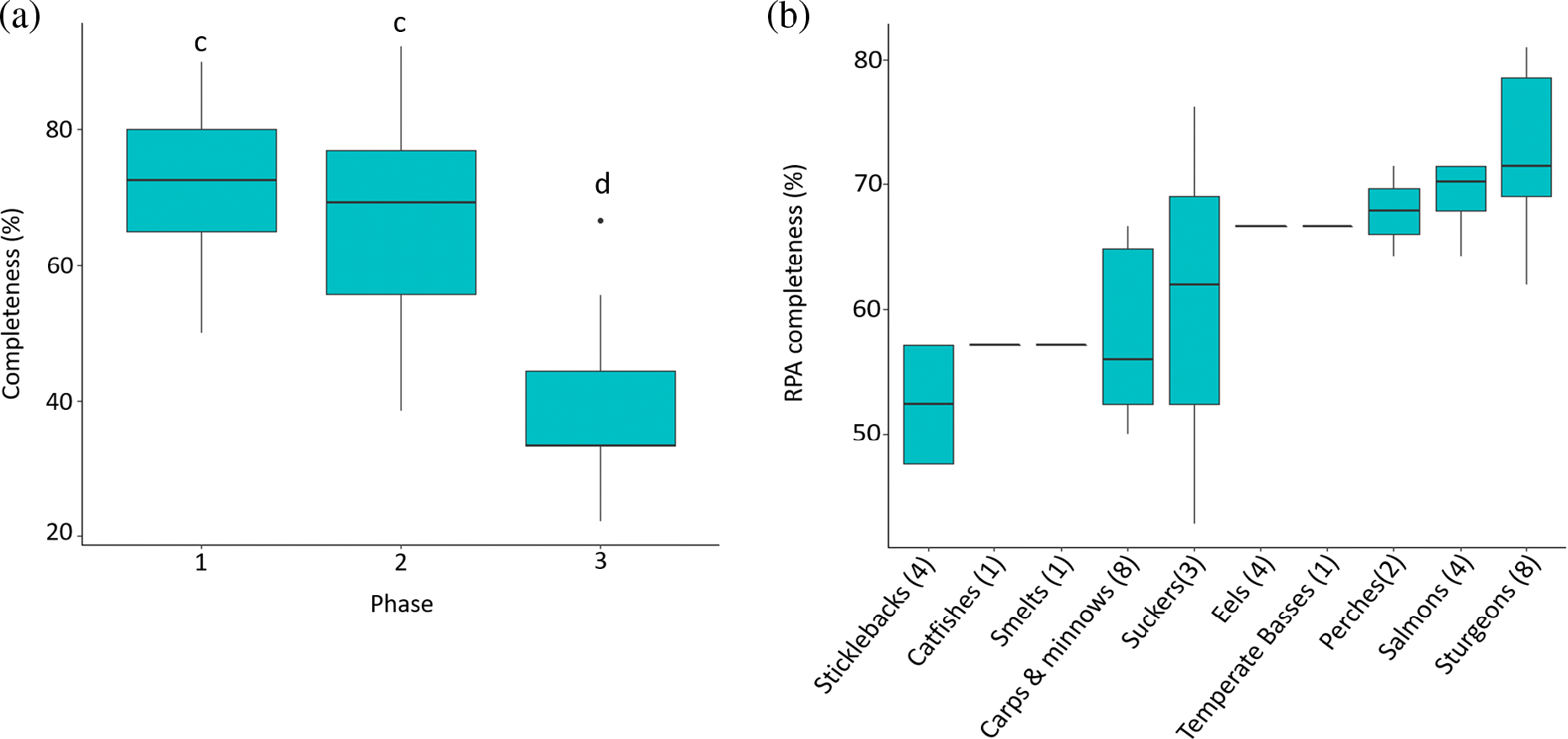
The total number of questions answered varied by family, ranging from 52% to 73% complete. The RPAs for perches, salmons, trout, whitefish, eels, sturgeons, and gars were among the most complete, whereas those for sticklebacks, sculpins, smelts, lampreys, and catfishes were among the least complete (Fig. 3b).
Of the 7 possible models involving Phase 1–3, the model with the lowest AICc score described SARA-listing decision (no status vs listed) as a function of the number of questions answered in Phase (ph) 1 (beta = -0.78 +/− 0.3) and Phase 2 (beta = +0.52 +/− 0.3) of the RPA (AICc (null) =48.19; Log likelihood (null) = −23.03, null deviance = 46.1; AICc (ph1, ph2) = 42.1, k = 3, Log likelihood (ph1, ph2) = −17.8, residual deviance = 35.3) (Supplementary Material 4). When family was included as a random effect, SARA-listing decision was only minimally related to phase completeness. After controlling for the effect of family, phase completeness explained 7.4% of the variation in SARA listing. However, the marginal R2 values are very small and Phases 1−3 of the RPA explain very little variation in SARA-listing decision.
3.2. Does the type of scientific information in an RPA influence the SARA-listing decision?
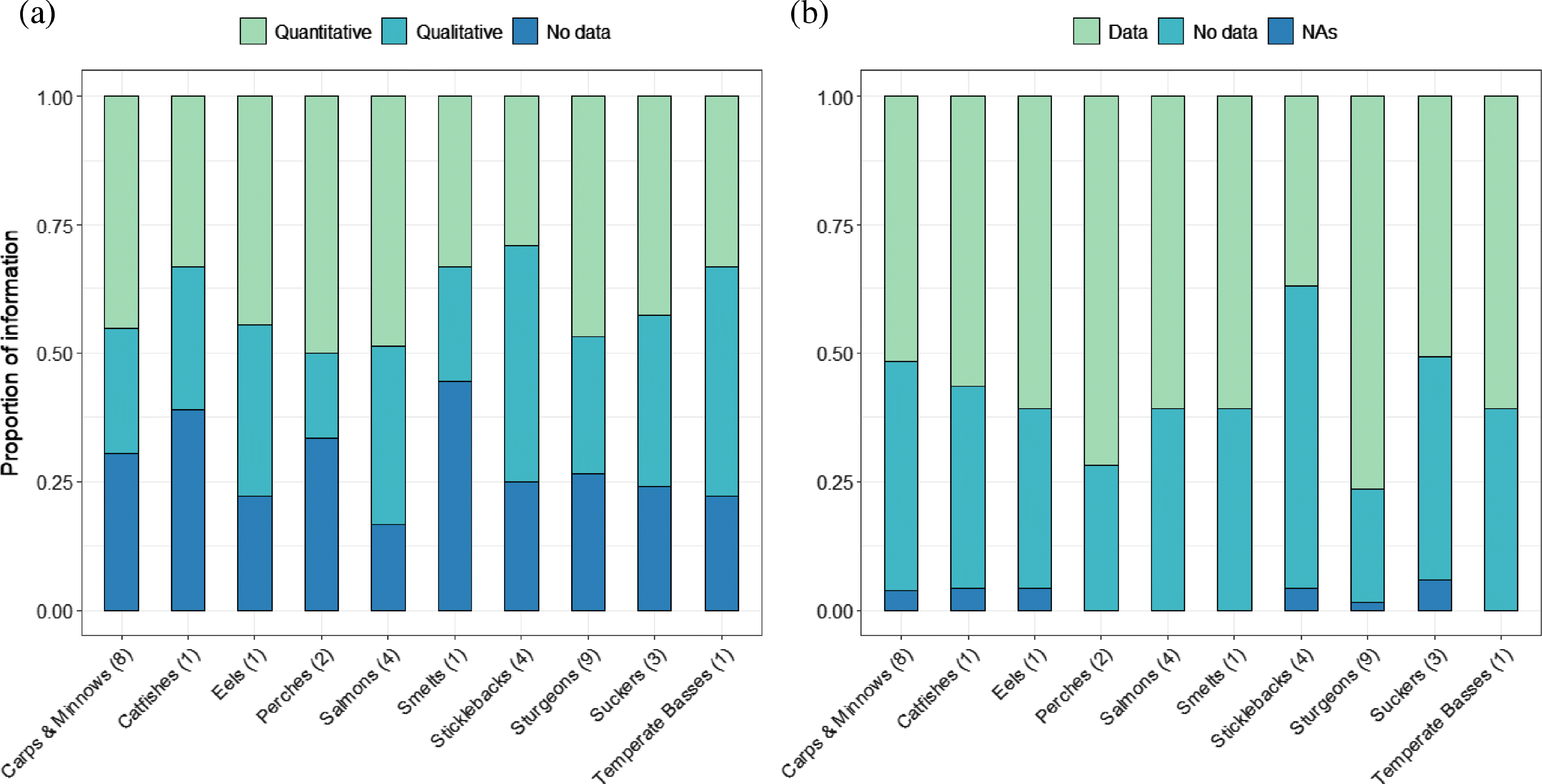
The type of information provided in an RPA varied across families (Fig. 4). Carps and minnows, perches, salmons, trouts and whitefishes, and sturgeons contained larger proportions of quantitative answers for 17 quantitative questions, whereas answers were largely qualitative or not provided for catfishes, smelts, sticklebacks, and temperate basses (Fig. 4a). Over 50% of 21 qualitative questions were answered for nine of 10 families, except for sticklebacks for which only 37.5% of those questions were answered (Fig. 4b).
Fig. 4.
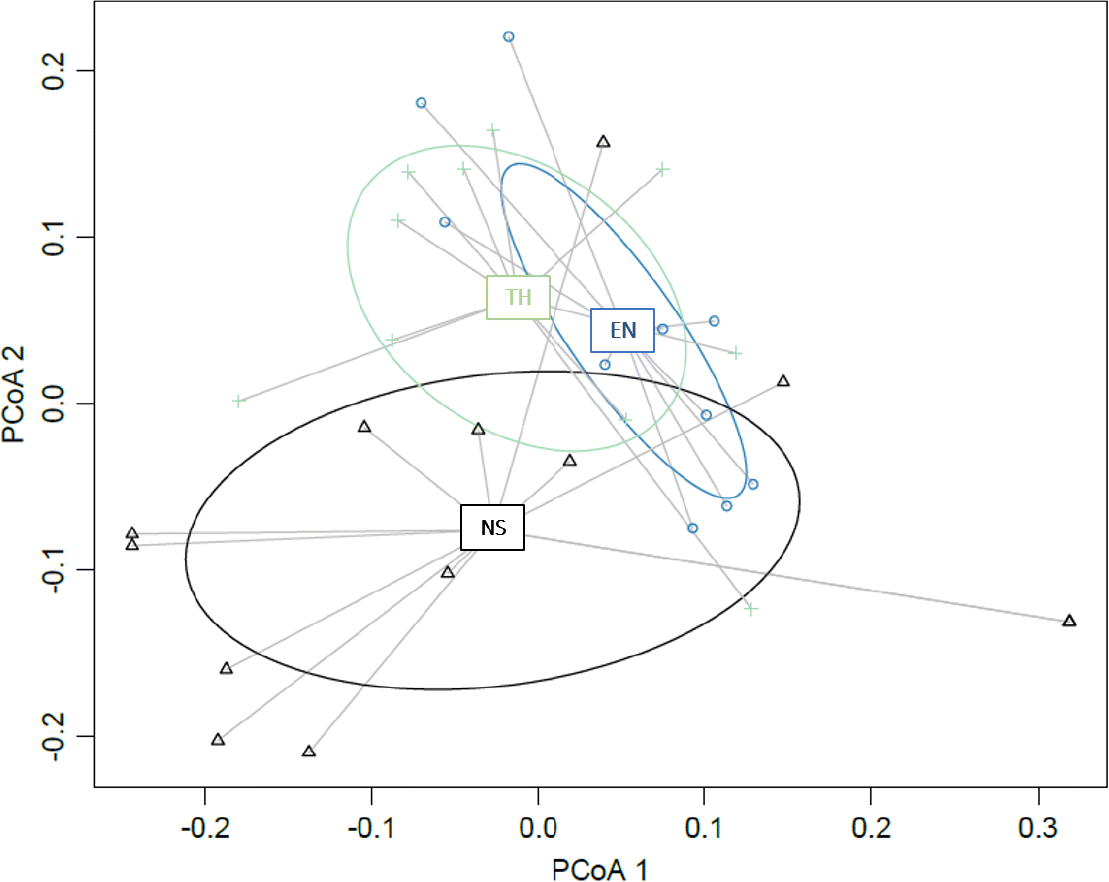
Science advice communicated within RPA, RPM, and supplementary documents does not differ between fishes listed as Threatened, Endangered, or no status based on the PCoA (Fig. 5). Based on the ANOSIM, the Gower distance matrix based on 41 questions coded for each RPA did not differ among species with different SARA listing statuses. There were no significant differences in post-hoc comparisons between all possible pairs of Endangered, Threatened, and no status listings based on Holm–Bonferroni-corrected p values despite significant differences found when comparing all groups (ANOSIM R statistic = 0.18; p = 0.003).
Fig. 5.
4. Discussion
The amount and type of science advice provided in an RPA does not appear to be a major driving factor in the SARA-listing process for freshwater fishes more than expected by chance. RPA completeness was only weakly associated with SARA listing and the variation in the information content of RPAs does not influence whether a species is listed under SARA or awaiting a decision. It is noteworthy that several freshwater fishes, whose RPAs were written after a SARA listing decision was made, were still given the same listing status as recommended by COSEWIC (Supplementary Material 2), which further supports that RPAs may not, and should not, delay the SARA-listing process. RPAs, while potentially useful for recovery strategies (DFO 2007), do not appear to be essential for listing freshwater fishes under SARA.
4.1. RPA Completeness
Although DFO has standardized guidelines for providing detailed scientific advice to the MoE and GIC, many species ultimately lack the information to complete an RPA, and this does not appear to influence the likelihood of receiving legal protection under SARA. Notably, the sturgeon family had the highest overall RPA completeness (73%), yet each DU is currently awaiting a decision under SARA due to extended consultation (Figs. 2 and 3; Supplementary Material 1). We found limited evidence of an effect of RPA completeness or content on listing. The results suggest that the best available science advice in an RPA above the baseline of science advice identified in COSEWIC assessments does not influence listing decisions for freshwater fishes.
Species most often lacked information to complete Phase 3 of the RPA, and this was mostly the case for sticklebacks, sculpins, smelts, lampreys, and catfishes (Fig. 3). Phase 3 is arguably the most data-intensive phase of the RPA, focused on population projections and proposed mitigation and alternative scenarios, requiring detailed analysis built on information from Phases 1 and 2 (Supplementary Material 3; Table S3). While often answered using population viability analysis (PVA), PVA itself may be too data-intensive to be useful for many species (Wolf et al. 2015). It may be unrealistic to expect that the data and analyses required to answer the complex questions found in Phase 3 of the RPA are collected and completed within the 12 months allowed for consultation and analysis (GOC 2018; Table S1a; Table S1b).
Allocating limited resources towards completing RPAs in their entirety may not be a strategic conservation investment towards species listing. Simplified, updated protocols would ensure questions in the RPA better reflect which facets of science advice are required for listing decisions versus the development of recovery strategies and for defining critical habitat. Streamlined questions may optimize the allocation of resources available and better inform listing decisions within the legislated 270-day window. Although this point has been noted in some papers (Lundquist et al. 2002; Prugh et al. 2010; Ng et al. 2014), the majority of studies consider the economic costs of recovery after a species has been listed. This seems likely due to the inherent difficulties of estimating the financial cost of a lengthy assessment process. Evaluation of economic costs associated with the development of socio-economic analyses, RPAs, and RIAS may provide valuable insight into the economic-ecological tradeoffs associated with these steps in the listing process.
4.2. RPA Information Content
The type of information provided in an RPA (e.g., qualitative, quantitative, or no data) is similar among species with different listing statuses, given our small sample size and limited statistical power. Listing decisions were only weakly associated with the type of data available for each question, including those related to population recovery, critical habitat, and alternative scenarios to reduce mortality and productivity (Supplementary Material 4). This suggests that variation in the information content of RPAs (above the COSEWIC threshold) does not influence whether a species is listed versus awaiting a decision.
4.3. Data Limitations
Our results are mainly limited by the small numbers of freshwater fishes for which both COSEWIC assessments and RPAs were available before listing decisions. Given our small sample size, sturgeon DUs dominate a large portion of the “not listed” species group (n = 9; 64% of “no status” species), making it difficult to tease out whether taxonomic bias at the family level plays a large role in SARA listing or whether this is a product of our data set. These limitations speak to the difficulties of statistical modelling to better understand factors associated with listing decisions when working with small group sizes, such as freshwater fishes at risk in Canada.
Another limitation to our study is that detail, such as the specific context of qualitative and quantitative answers, was not captured in our coding technique (e.g., a reported population trajectory of 5 vs 50 years to recovery). Although we find limited evidence that RPA information content influences SARA-listing decisions, it is also possible that differences in RPAs are either: (i) not captured in the coding or (ii) driven by non-scientific factors. However, the no status species in our data set lack detailed reports of what information (whether from an RPA or not) warrants extended consultation, and subsequent lack of decision, so we cannot effectively test these alternative hypotheses. While many have addressed concerns over the delays attributed to extended consultations and/or loopholes in the SARA process (e.g., Mooers 2004; Hutchings and Festa-Bianchet 2009; McCune et al. 2013; Waples et al. 2013; Otto et al. 2016), the underlying factors influencing the decision for extended consultation and the direct role that information in an RPA plays, remains difficult to study due to barriers associated with access to information (e.g., Access to Information Act s. 20(1)(b)).
5. Conclusions
Despite our study limitations, it appears that the nature (e.g., qualitative/quantitative) or extent (e.g., completeness) of information in an RPA does not greatly influence a species’ listing status. The best available science advice in an RPA that is beyond the baseline identified in COSEWIC assessments is only weakly associated with listing freshwater fishes under SARA. Given that all no status species in this study are awaiting decisions, and the science advice in an RPA does not strongly contribute to the species’ listing decision, it is apparent that factors beyond science have led freshwater fishes to face long-extended delays for listing decisions. However, when species are awaiting listing decisions due to “extended consultation”, reasons supporting this decision are not publicly available in the Response Statement or RIAS to better understand what scientific information may be missing to prevent timely decision-making. Failure to include detailed reasoning for extended consultation makes it difficult for scientists and managers to effectively address gaps in the process of providing science advice. Although input from the consultation is important in guiding legal decisions, it comes at a cost of delaying decisions for species, by definition, at risk of extinction and in need of immediate protection.
If the data are available, future research should evaluate the socioeconomic or sociocultural costs incurred by stakeholders, Indigenous Peoples, or the federal government associated with listing a species, whether it goes to extended consultation and how long it spends there. Evaluation of additional realized/imagined costs to listing will help to better understand how, and to what degree, nonscientific factors pose as barriers to freshwater fishes receiving prompt legal protection under SARA.
An updated evaluation of DFOs 2007 Revised Protocol for Conducting Recovery Potential Assessments could streamline which data are imperative for species listing decisions as opposed to subsequent legally required recovery actions (strategy, action plan, identification of critical habitat). Streamlined RPAs could maximize resources towards species recovery, lead to more effective use of science in the SARA-listing process, and improve how freshwater species at risk are protected in Canada.
Acknowledgments
Funding for this project is provided by an NSERC Discovery Grant to NEM. Thank you to two reviewers for their thoughtful revisions that have contributed to substantial improvements in the manuscript.
References
Bartoñ K. 2020. Mu-Mln: multi-model inference. R package version 1.43.17 [online]: Available from R-Forge.R-projects/mumin/.
COSEWIC. 2019. COSEWIC assessment process, categories and guidelines [online]: Available from cosewic.ca/images/cosewic/pdf/Assessment_process_criteria_Nov_2019_en.pdf.
Cooke SJ. 2008. Biotelemetry and biologging in endangered species research and animal conservation: Relevance to regional, national, and IUCN red list threat assessments. Endangered Species Research, 4(1–2): 165–185.
Creighton MJA, and Bennett JR. 2019. Taxonomic biases persist from listing to management for Canadian species at risk. Écoscience, 1–7.
DFO. 2007. Revised protocol for conducting recovery potential assessments. DFO Canadian Science Advisory Secretariat Science Advisory Report 2007/039.
DFO. 2013. Fisheries and Oceans Canada species at risk act listing policy and directive for “do not list” advice/fisheries and Oceans Canada [online]: Available from waves-vagues.dfo-mpo.gc.ca/Library/365882.pdf.
DFO. 2014. Guidance on assessing threats, ecological risk and ecological impacts for species at risk. DFO Canadian Science Advisory Secretariat Science Advisory Report. 2014/013.
Dorey K, and Walker TR. 2018. Limitations of threatened species lists in Canada: a federal and provincial perspective. Biological Conservation, 217(1): 259–268.
Favaro B, Claar DC, Fox CH, Freshwater C, Holden JJ, Roberts A, et al. 2014. Trends in extinction risk for imperiled species in Canada. PLoS ONE, 9(11): e113118.
Findlay CS, Elgie S, Giles B, and Burr L. 2009. Species listing under Canada’s Species at Risk Act. Conservation Biology, 23(6): 1609–1617.
Glass WR, Corkum LD, and Mandrak NE. 2017. Living on the edge: traits of freshwater fish species at risk in Canada. Aquatic Conservation: Marine and Freshwater Ecosystems, 27(5): 938–945.
Gehring T, and Ruffing E. 2008. When arguments prevail over power: the CITES procedure for the listing of endangered species. Global Environmental Politics, 8(2): 123–148.
[GOC 2018] Government of Canada. 2018. Consultation on Amending the list of species under the Species at Risk Act: Terrestrial species [online]: Available from sararegistry.gc.ca/default.asp?lang=En&n=335D013E-1#_02.
Gower JC. 1971. A general coefficient of similarity and some of its properties. Biometrics, 27: 857–871.
Gelman A, and Hill J. 2006. Data analysis using regression and multilevel/hierarchical models. Cambridge University Press.
Gregory R, and Long G. 2009. Using structured decision making to help implement a precautionary approach to endangered species management. Risk Analysis, 29(4): 518–532.
Himes Boor GK. 2014. A framework for developing objective and measurable recovery criteria for threatened and endangered species. Conservation Biology, 28(1): 33–43.
Hutchings JA, and Festa-Bianchet M. 2009. Canadian species at risk (2006–2008), with particular emphasis on fishes. Environmental Reviews, 17: 53–65.
Lofaro RJ. 2015. Cognitive engineering: what’s old is new again. International Symposium on Aviation Psychology, 4–7 May 2015, Dayton, Ohio. pp. 624–629.
Lundquist CJ, Diehl JM, Harvey E, and Botsford LW. 2002. Factors affecting vimplementation of recovery plans. Ecological Applications, 12: 713–718.
McDevitt-Irwin JM, Fuller SD, Grant C, and Baum JK. 2015. Missing the safety net: Evidence for inconsistent and insufficient management of at-risk marine fishes in Canada. Canadian Journal of Fisheries and Aquatic Sciences, 72: 1596–1608.
McCune JL, Harrower WL, Avery-Gomm S, Brogan JM, Csergo A, et al. 2013. Threats to Canadian species at risk: an analysis of finalized recovery strategies. Biological Conservation, 166: 254–265.
Mitchell VW. 1991. The Delphi technique: an exposition and application. Technology Analysis and Strategic Management, 3(4): 333–358.
Mooers AØ. 2004. Why did the fish miss the boat? globe and mail. [online]: Available from theglobeandmail.com/opinion/why-did-the-fish-miss-the-boat/article743291/.
Mooers AØ, Prugh LR, Festa-Bianchet M, and Hutchings JA. 2007. Biases in legal listing under Canadian endangered species legislation. Conservation Biology, 21(3): 572–575.
Ng CF, Possingham HP, McAlpine CA, De Villiers DL, Preece HJ, and Rhodes JR. 2014. Impediments to the success of management actions for species recovery’. PLoS ONE, 9(4): e92430.
Oksanen J, Blanchet G, Friendly M, Kindt R, Legendre P, McGlinn D, et al. 2019. Vegan: community ecology package. R package version 2.5-6.
Otto SP, Findlay S, Hutchings JA, Kerr J, Mooers A, and Whitton J. 2016. Comments on the draft ECCC policies regarding SARA [online]: Available from sfu.ca/∼amooers/scientists4species/SARA_Policy_Comments30Nov2016.pdf.
Podani J. 1999. Extending gower’s general coefficient of similarity to ordinal characters. Taxon, 48: 331–340.
Powles H. 2011. Assessing risk of extinction of marine fishes in Canada – The COSEWIC experience. Fisheries, 36(5): 231–246.
Prugh LR, Sinclair ARE, Hodges KE, Jacob AL, and Wilcove DS. 2010. Reducing threats to species: threat reversibility and links to industry. Conservation Letters, 3(4): 267–276.
Restani M, and Marzluff JM. 2002. Funding Extinction? biological needs and political realities in the allocation of resources to endangered species recovery: An existing priority system, which should guide the fish and wildlife service in endangered species recovery, is ineffective, and current spending patterns decrease long-term viability of island species. Bioscience, 52(2): 169–177.
R Core Team. 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [online]: Available from R-project.org/.
Rohlf DJ. 1991. Six biological reasons why the Endangered Species Act doesn’t work—and what to do about it. Conservation Biology, 5(3): 273–282.
Schielzeth H, and Nakagawa S. 2013. Nested by design: model fitting and interpretation in a mixed model era. Methods in Ecology and Evolution, 4(1): 14–24.
Schultz JA, Darling ES, and Côté, IM. 2013. What is an endangered species worth? Threshold costs for protecting imperilled fishes in Canada. Marine Policy, 42: 125–132.
Walsh JC, Watson JEM, Bottrill MC, Joseph LN, and Possingham HP. 2012. Trends and biases in the listing and recovery planning for threatened species: an Australian case study. Fauna and Flora International, Oryx, 47(1): 134–143.
Waples RS, Nammack M, Cochrane JF, and Hutchings JA. 2013. A tale of two acts: Endangered species listing practices in Canada and the United States. Bioscience, 63: 723–734.
Wolf S, Hartl B, Carroll C, Neel MC, and Greenwald DN. 2015. Beyond PVA: why recovery under the endangered species act is more than population viability. Bioscience, 65(2): 200–207.
Supplementary materials
Supplementary Material 1 (DOCX / 131 KB)
- Download
- 131.52 KB
Supplementary Material 2 (DOCX / 31.6 KB)
- Download
- 31.61 KB
Supplementary Material 3 (DOCX / 22.2 KB)
- Download
- 22.29 KB
Supplementary Material 4 (DOCX / 43.3 KB)
- Download
- 43.34 KB
Information & Authors
Information
Published In

FACETS
Volume 6 • Number 1 • January 2021
Pages: 1247 - 1259
Editor: Andrea Olive
History
Received: 8 October 2020
Accepted: 18 March 2021
Version of record online: 22 July 2021
Notes
This paper is part of a collection titled “Conservation in Canada: identifying and overcoming barriers”.
Copyright
© 2021 Montgomery et al. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Data Availability Statement
All relevant data are within the paper and in the Supplementary Material.
Key Words
Sections
Subjects
Authors
Author Contributions
FAM and NS contributed equally to the manuscript and should be considered as joint first authors.
All conceived and designed the study.
FAM, NS, PAB, RAC, KG, TH, and MMK performed the experiments/collected the data.
FAM, PAB, SEC, and ESC analyzed and interpreted the data.
NEM contributed resources.
All drafted or revised the manuscript.
Competing Interests
The authors have declared that no competing interests exist.
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Fielding A. Montgomery, Noelle Stratton, Paul A. Bzonek, Sara E. Campbell, Rowshyra A. Castañeda, Emily S. Chenery, Kavishka Gallage, Tej Heer, Meagan M. Kindree, and Nicholas E. Mandrak. 2021. The role of science advice in recovery potential assessments in freshwater fish listing decisions under the Canadian Species at Risk Act. FACETS.
6(): 1247-1259. https://doi.org/10.1139/facets-2020-0091
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
Cited by
1. Transforming conservation in Canada: shifting policies and paradigms
