Royal society of Canada COVID-19 report: Enhancing COVID-19 vaccine acceptance in Canada
Abstract
COVID-19 vaccine acceptance exists on a continuum from a minority who strongly oppose vaccination, to the “moveable middle” heterogeneous group with varying uncertainty levels about acceptance or hesitancy, to the majority who state willingness to be vaccinated. Intention for vaccine acceptance varies over time. COVID-19 vaccination decisions are influenced by many factors including knowledge, attitudes, and beliefs; social networks; communication environment; COVID-19 community rate; cultural and religious influences; ease of access; and the organization of health and community services and policies.
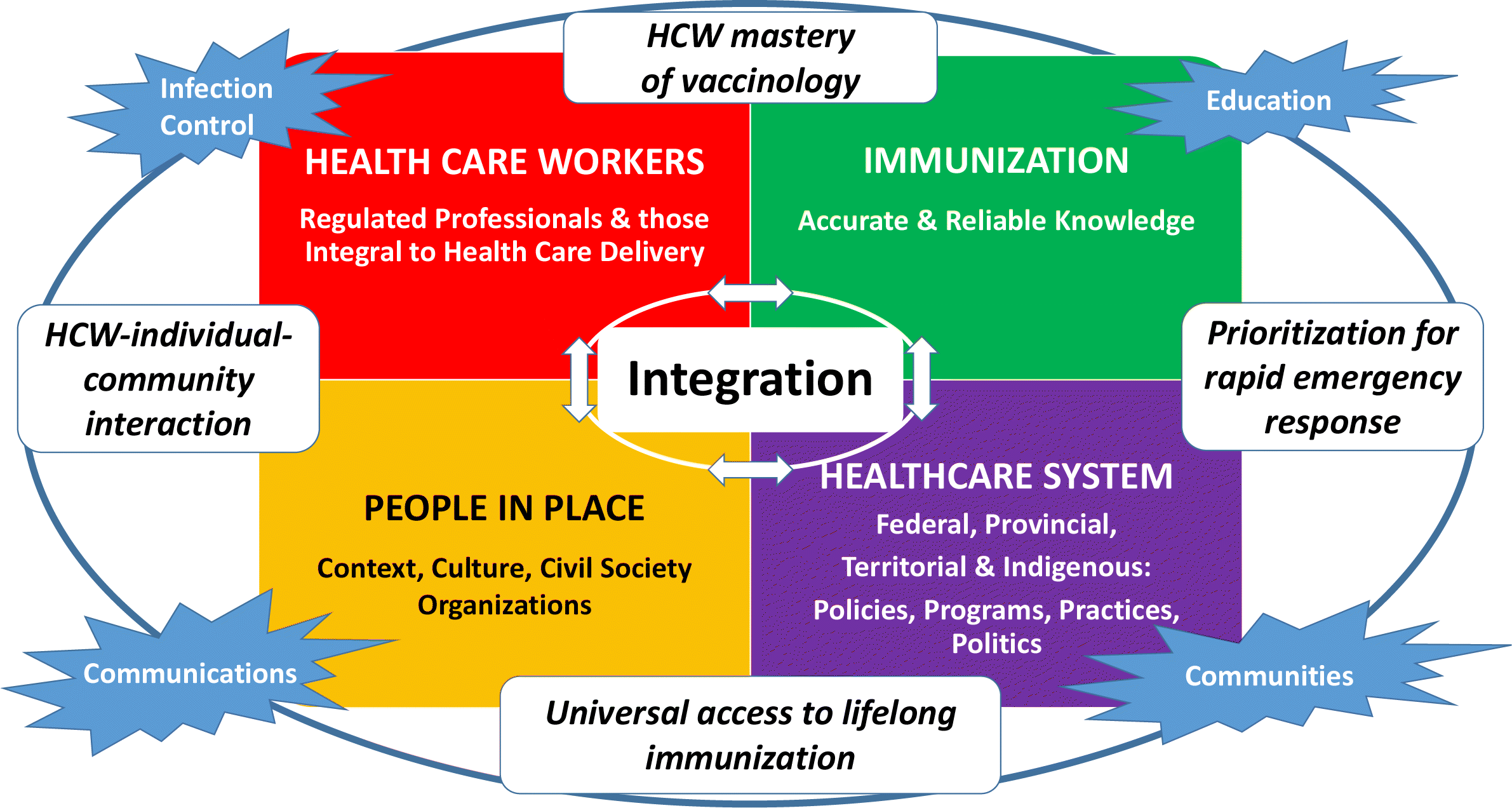
Reflecting vaccine acceptance complexity, the Royal Society of Canada Working Group on COVID-19 Vaccine Acceptance developed a framework with four major factor domains that influence vaccine acceptance (people, communities, health care workers; immunization knowledge; health care and public health systems including federal/provincial/territorial/indigenous factors)—each influencing the others and all influenced by education, infection control, extent of collaborations, and communications about COVID-19 immunization. The Working Group then developed 37 interrelated recommendations to support COVID vaccine acceptance nested under four categories of responsibility: 1. People and Communities, 2. Health Care Workers, 3. Health Care System and Local Public Health Units, and 4. Federal/Provincial/Territorial/Indigenous. To optimize outcomes, all must be engaged to ensure co-development and broad ownership.
Graphical Abstract
1. Mandate and scope
Acceptance of a vaccine and the factors that influence it is a very complex area. The urgent need for the development and deployment of COVID-19 vaccines has added even more complexity. After much deliberation, the authors developed the Royal Society of Canada Vaccine Acceptance Framework inspired by the 2021 framework of Hasnan and Tan (2021) and the World Health Assembly 2020 accepted report Immunization Agenda 2030 goal of no one left behind (WHO 2020b) to use as the backbone for the report. To limit the length of the report, overview summaries based upon our findings were developed for each section and subsection with references. The Working Group then developed recommendations for each component nested under four areas of responsibility: 1. People and Communities, 2. Health Care Workers, 3. Health Care System and Local Public Health Units, and 4. Federal /Provincial /Territorial/Indigenous. To optimize outcomes, all must be engaged to ensure co-development and broad ownership.
The complexity of vaccine acceptance, especially COVID-19 vaccine acceptance, is highlighted by Royal Society of Canada (RSC) COVID-19 Resources (rsc-src.ca/en/covid-19) including Policy Briefings and Informed Perspectives. A number of these are referenced in this report; with many of those referenced developed by this Working Group (See Supplementary Material 1).
2. Background
2.1. Defining vaccine acceptance
With the 73rd World Health Assembly’s recognition of strengthening global immunization efforts to leave no one behind in their Immunization Agenda 2030 (WHO 2020b), the COVID-19 pandemic has brought much attention to immunization across the life course. Widespread vaccine acceptance (the intent to receive a vaccine (Freemster 2013) i.e., attitude not the actual behaviour) and actual uptake (the completed action i.e., the behaviour) by an estimated 70%–80% (Anderson et al. 2020) of the population (still under debate) will be needed to generate community immunity and effectively contain the pandemic. Ensuring rapid and equitable access to vaccination services across the life-span starting with prioritizing at-risk groups, addressing concerns about the new vaccines, and countering misinformation will require an unprecedented public health, health care system, and community effort across Canada and worldwide.
To date, the definition, concept and evidence surrounding vaccine acceptance are anchored in childhood vaccination. This needs to be expanded.
What do we know about vaccine acceptance—the definition, concept, and evidence from childhood immunization?
Variolation, the practice of injecting a small preventative dose of cow pox to build antibodies against that which might otherwise cause disease, existed long before Jenner’s mythic encounter with the milkmaid (Boyleston 2018). The anti-vaccine movement has been active since Jenner’s time but more recently fanned by anti-science, anti-big corporations, anti-government groups, and the plethora of false information online (Burki 2020). Misinformation (Scheufele and Krause 2019) (unintentional inaccuracies) and disinformation (Scheufele and Krause 2019) (deliberately false or misleading content) on social media platforms now threaten the success of immunization programs, pushing scientists, clinicians, and policy makers to take a new interest in vaccine hesitancy. Misinformation that used to only rabble-rouse locally can now incite mass movements that thwart the best made plans of international efforts to control outbreaks of vaccine-preventable diseases. Hesitancy linked to delays in vaccine acceptance and vaccine refusals cannot be ignored (Larson et al. 2011).
Childhood vaccine acceptance, hesitancy, and dismissal, and their determinants have long been an interest among global health researchers, with studies exploding in the past decade. In 2012, the World Health Organization (WHO) Strategic Advisory Group of Experts (SAGE) appointed a Working Group on Vaccine Hesitancy to define this issue and undertake a review of its context-specific causes and its impact in different settings (WHO 2014). The recommendations of the Working Group were endorsed by the WHO SAGE in October 2014, including the proposed definition of vaccine hesitancy as “delay in acceptance or refusal of vaccines despite availability of vaccination services. Vaccine hesitancy is complex and context-specific, varying across time, place, and vaccines. It is influenced by factors such as complacency, convenience and confidence” (MacDonald 2015). A vaccine-hesitant person can delay; be reluctant (but still accept); or decline/accept one, some, or all vaccines. In 2019, the WHO declared vaccine hesitancy as one of the top 10 threats to global health (WHO 2019c).
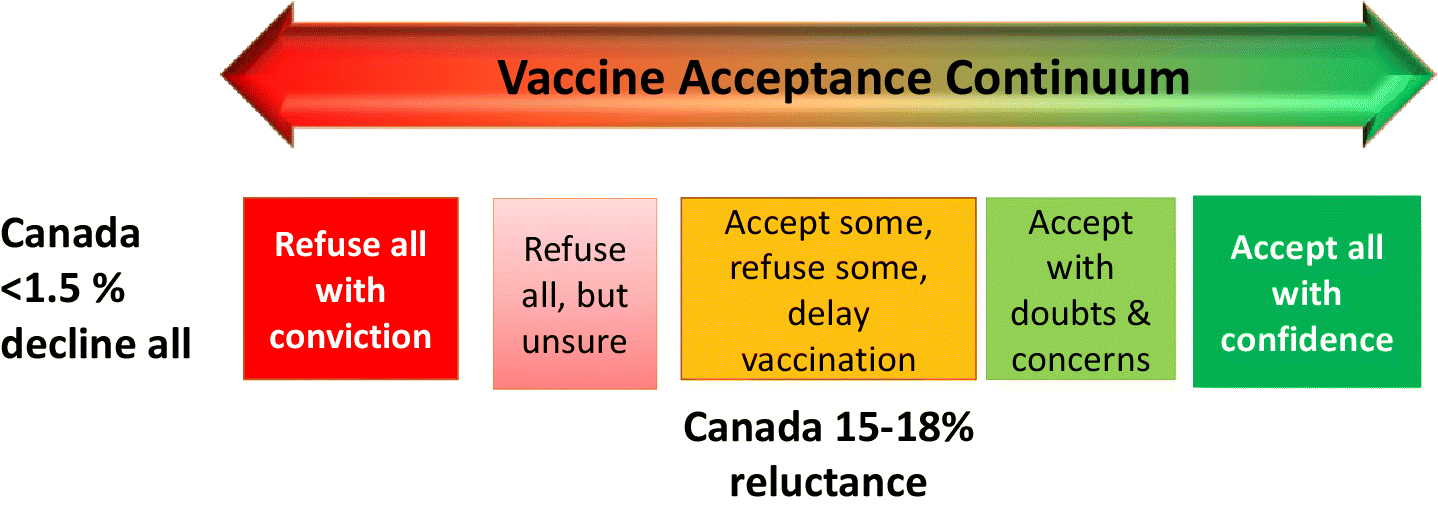
The concept of vaccine hesitancy has been criticized as being ambiguous and without sufficient theoretical background (Peretti-Watel et al. 2015)—a term that does not put enough emphasis on the practical (or access) barriers to vaccine uptake (Bedford et al. 2018). In contrast the term vaccine acceptance focuses only on the attitude. However, the hesitancy concept has prompted attention to the fact that, as for all behaviours, vaccination attitudes and decisions are best seen on a continuum, ranging from a small minority of vaccine refusal activists to the majority who accept vaccination as a vital public health provision (Hickler et al. 2017) (Fig. 1).
Fig. 1.
Growing recognition that vaccination is threatened by its critics has urged advocates into further action for vaccine acceptance and improved public health provisioning of vaccination services (Hickler et al. 2017). Vaccine acceptance highlights the multifactorial complexity of health decision-making. Reasons why a health care provider might hesitate in getting their annual influenza vaccine, for example, may be very different from the reasons why a pregnant person doesn’t get the pertussis vaccine recommended in pregnancy to protect the baby when born (Dubé et al. 2018; Mijovic et al. 2020). Ease of access is only part of the problem.
The multidimensional, complex, and persistent factors that affect vaccine acceptance and hesitancy (MacDonald 2015; Dubé et al. 2016) makes it a “wicked problem” for policymakers, a term first identified in the urban planning setting (Rittel and Webber 1973). Wicked problems defy definitive formulation and have no easy “right or wrong” solution. They can be explained in numerous ways and percolate in conditions of risk, fluidity, and uncertainty, often as symptoms of other problems (Koppenjan and Klijn 2004; Camillus 2008; Wylie and McConkey 2018).
To design effective interventions that can respond to hesitancy and enhance vaccine acceptance, it is crucial to have a critical understanding of the contexts and fluidities underlying the knowledge and beliefs about health and immunization in diverse communities, some of whom may otherwise get short shrift in scientific discourse (Dube et al. 2021). In addition to being aligned with community attitudes, values, and interests, any interventions should be tailored to the different positions held along the vaccine acceptance continuum within these communities (Fig. 1 and Table 1). These apply both to routine childhood immunizations but can also apply to immunizations across the life course including COVID-19.
Table 1.
| Vaccination intention | Intervention goal | Vaccine perceptions |
|---|---|---|
| Opposing vaccination | Reduce impact on other groups. It is not possible to stop activism; the goal is to reduce its impact using environmental strategies (e.g., restricting billboards or ads, etc.). Correcting misinformation is key, as is public education on recognizing and resisting disinformation. | May oppose all vaccines or a specific vaccine and engage in protest and related activities. This is a small but vocal group who may attract public attention, source, and share misinformation about vaccines, particularly in social networks |
| Rejecting/declining | Minimize the size of this group by managing vaccine concerns. | A significant minority intend to reject/decline a vaccine. Safety concerns are often the reason; however, there are many other factors related to experience, perceptions, and values. |
| Cautious/ hesitant | Listen to and address concerns transparently and effectively to support well-informed decisions. | Significant proportion are hesitant to accept vaccine for different reasons. Hesitancy is dynamic and can be influenced by communication with a trusted health worker. |
| Accepting | Address questions during vaccination encounters. Provide vaccine resources to share in social networks. | The majority of people will accept vaccination depending on individual motivation, social and professional influences, and the availability of, and access to, a vaccine. Acceptors may have questions about the vaccine (e.g., potential side effects) and some may want to know the risk according to age and (or) co-morbidity. |
| Demanding | Address questions during vaccination encounters. | Some people will strongly demand a vaccine. High demand with low supply can lead to conflict and perceptions of “favouritism” that may diminish trust in the overall program. |
| Vocally supporting | Support constructive advocacy with tools that accurately and transparently address concerns. | A small number of people will be strong advocates for vaccines. Advocates can be a key asset in vaccine communication, sharing information rapidly in their sometimes large social networks. Note: Adapted from Chapter 6.9 “COVID-19 vaccine safety communication” by Leask, Steffens and King in COVID-19 vaccines: Safety surveillance manual published by the World Health Organization. Available from: apps.who.int/iris/rest/bitstreams/1325611/retrieve (for direct download). |
2.2. COVID-19 vaccine acceptance: similarities and differences
The context for COVID-19 vaccine acceptance differs from routine immunization in childhood, but there are also similarities. Beyond the need to immunize across the age span, studies are showing how the pressure of rapid rollout combined with the widespread supply and demand mismatch as of March 2021 affects COVID vaccine acceptance amongst adults.
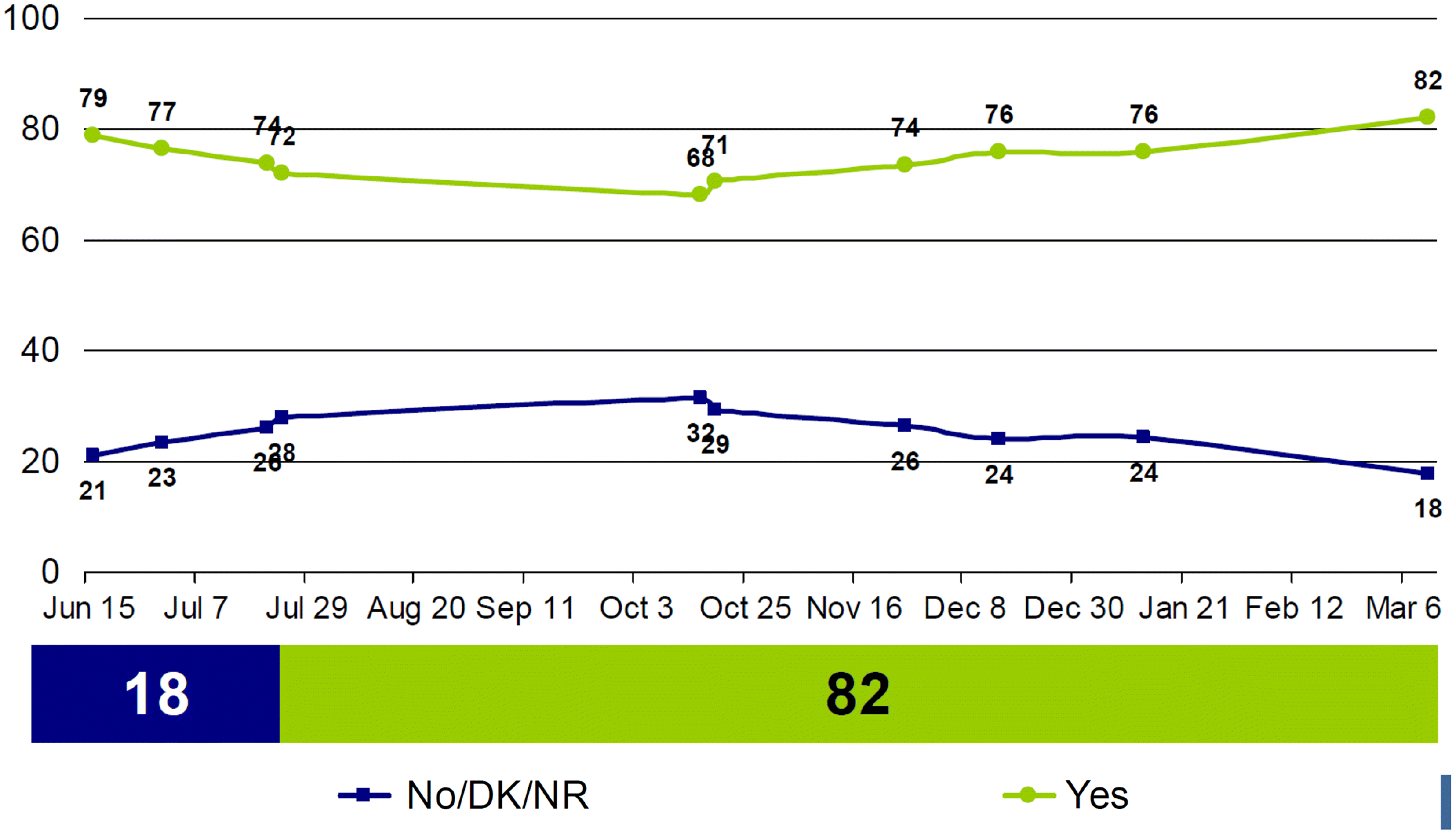
Several recently published surveys examined COVID-19 vaccine acceptance. The intention to receive vaccine varied widely between countries (from 91% in China to 54% in Russia) with acceptance intent increasing with age and being lower among visible minorities (Lin et al. 2020). A noted decline in acceptance intent between the spring and the fall of 2020 likely reflects reaction to the onslaught of confusing information about safety and efficacy reported upon vaccine approvals. In Canada, surveys and polls generally report that approximately 70%–75% of the adults are willing to be vaccinated, varying between provinces and territories and increasing to 82% in March 2021 (Statistics Canada 2020b; Leger 2021) (Fig. 2).
Fig. 2.
Some differences were observed across age groups, education levels, and sociodemographic characteristics (Figs. 3 and 4).
Fig. 3.
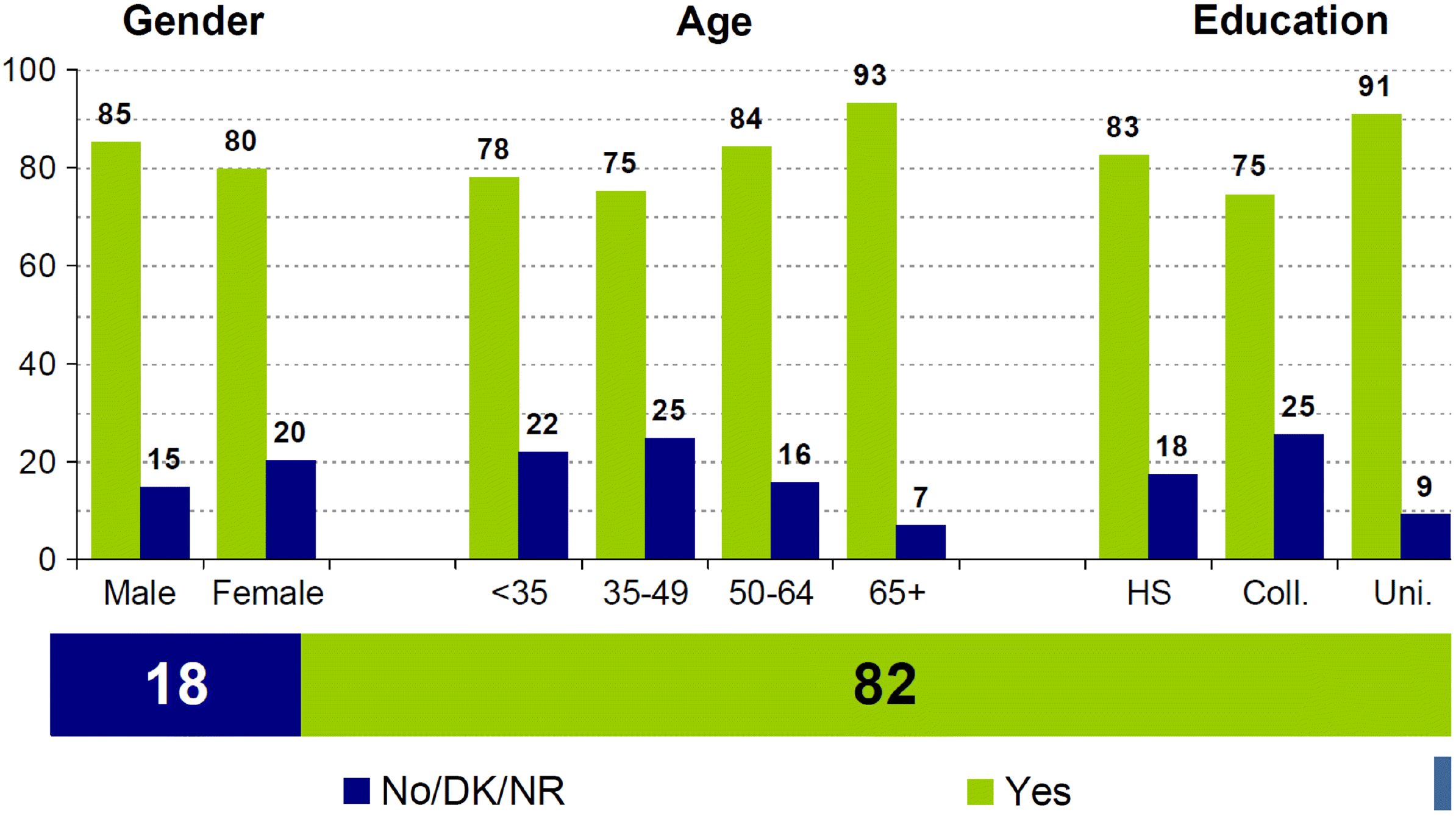
Fig. 4.
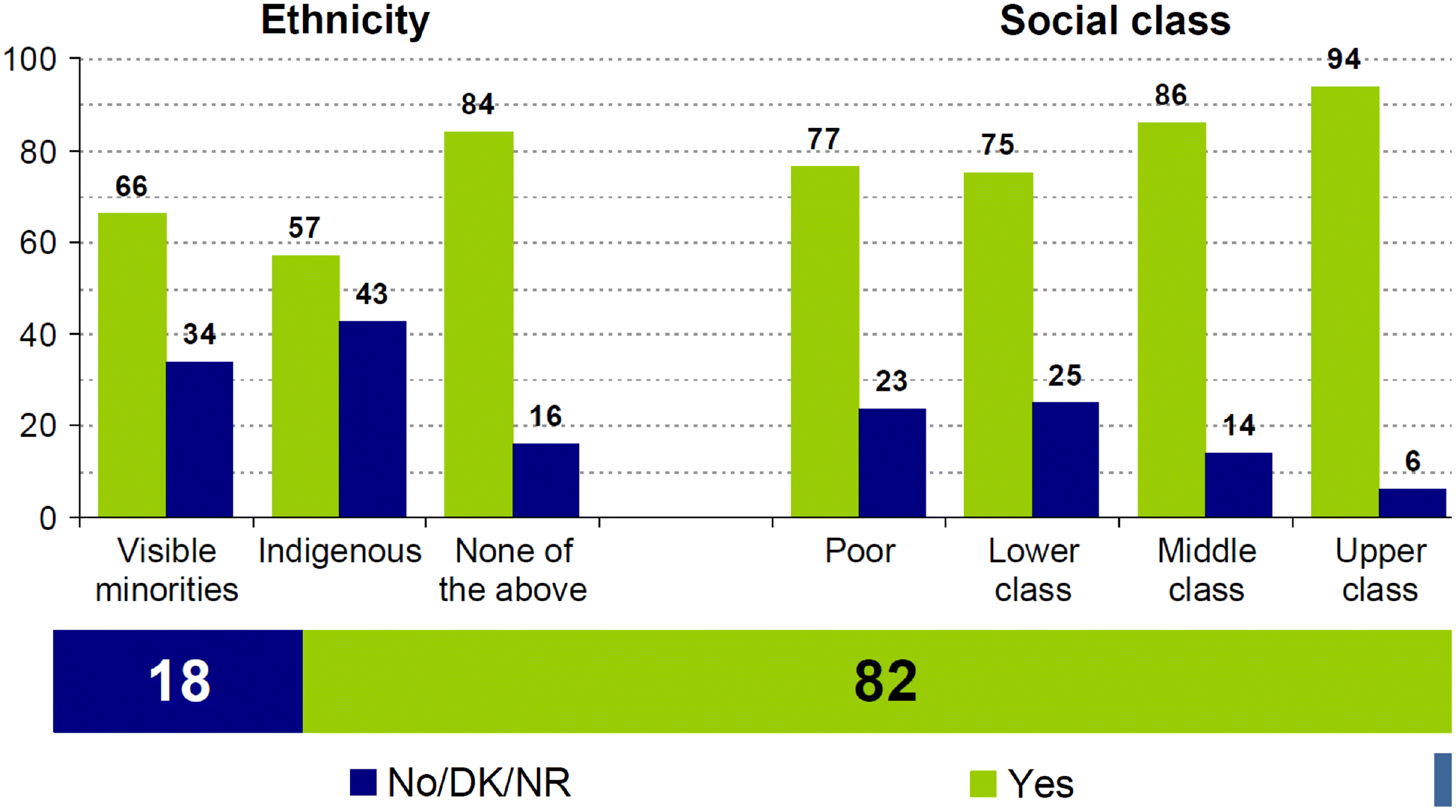
Younger Canadians, those without a university degree, newcomers, and those with a low level of trust in the federal government were less likely to indicate a willingness to get a COVID-19 vaccine (Frank and Arim 2020).
COVID-19 vaccine acceptance has evolved over 2020 into 2021. The changes as vaccines were authorized and rollouts commenced. The survey question shifted from being a theoretical ask to a reality-based ask as vaccines existed and information about them was available. The March 3–11, 2021, EKOS poll (Fig. 2) reported that most people (82%) were willing to get vaccinated, with the trend to increasing vaccine acceptance observed over time. A March 1, 2021, Leger North American tracker poll provides evidence of COVID-19 vaccine acceptance from those who have been immunized (Fig. 5). Drilling down by province, a similar trend is noted; 74% of Quebecers polled by the Institut National de Santé Publique du Québec polling since December 2020 intend to accept a COVID vaccine (INSPQ 2021).
Fig. 5.
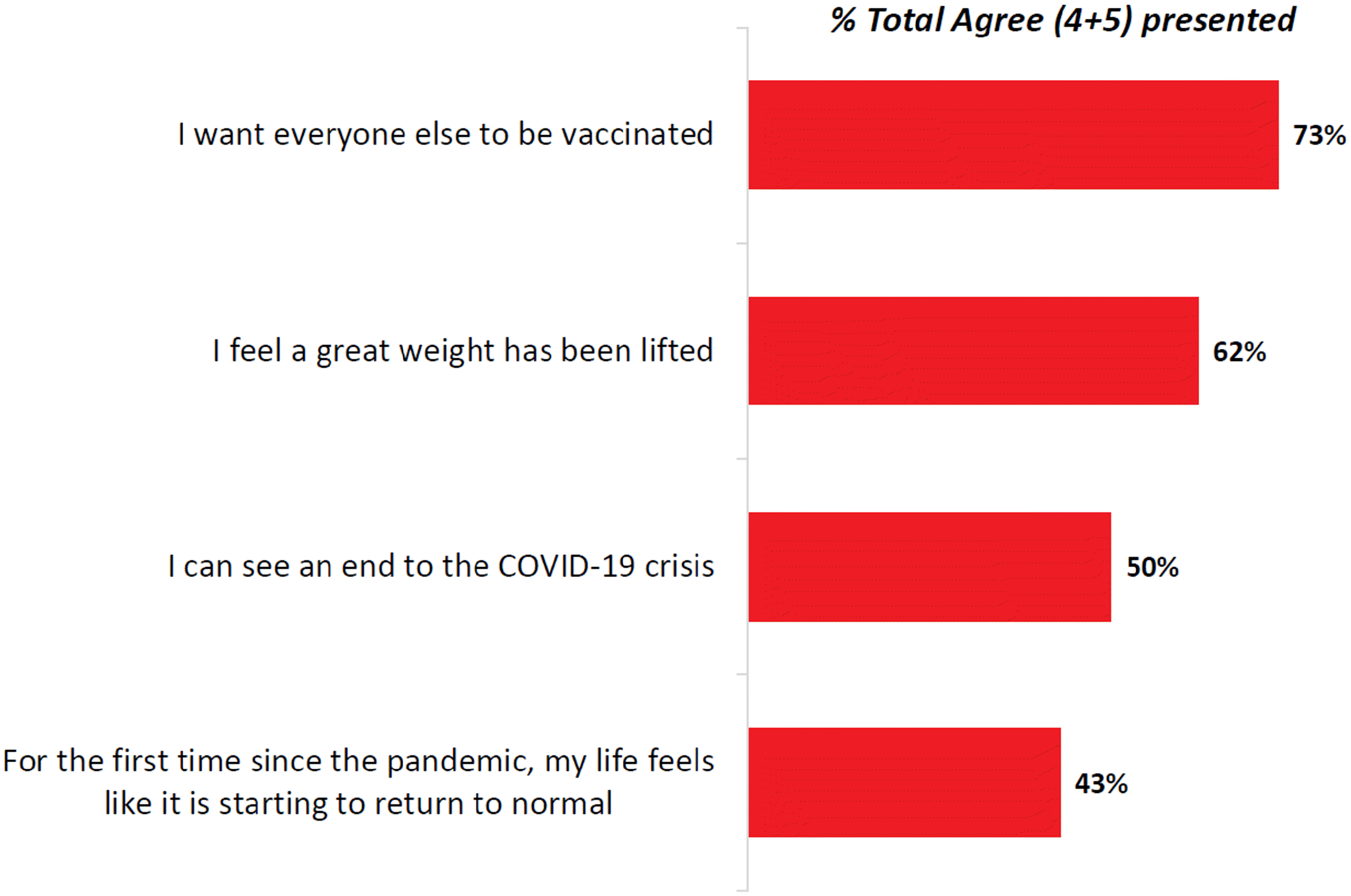
Silent barriers to access, however, make the translation of any positive intention to vaccinate into actual vaccine receipt (Brewer et al. 2017) uncertain. It is therefore critically important to systematically address the many issues in accessing vaccination and other public health services, including vaccine safety concerns, complacency, negative peer influence, and inadequate public health messaging that threaten optimal uptake.
Vaccine acceptance will undoubtedly fluctuate as evidence for vaccine safety and effectiveness grows after authorization and rollout. To date, most frail seniors in congregate living settings and many frontline health care providers in Phase 1 for priority for COVID-19 vaccine have been willing to get vaccinated, with many becoming immunization champions. However, it is not surprising that when concerns about safety were raised about anaphylaxis (CDC 2021a), eagerness to accept may falter although other factors may be more important in influencing intent to accept the vaccine (CDC 2021f). The hesitancy of some health care workers including but not limited to janitorial and personal care workers remains an issue to be addressed by public health, and others in the health care system and in their communities.
Vaccine safety is among the most prevalent causes of vaccine hesitancy (Lane et al. 2018). Concerns about rapid development amplified by relatively novel antigen-carrying platforms (e.g., mRNA vaccine, viral vector vaccine) are associated with lower intention to receive a COVID-19 vaccine (Lin et al. 2020). Potential risk of adverse events after immunization (AEFI), as well as uncertainties about immediate and long-term vaccine safety and effectiveness and how they are addressed in pharmacovigilance studies will require careful monitoring and communication with both health care providers and the public. When vaccine safety concerns emerge, real or perceived, even well-organized programs can be derailed if evidence is not provided from the beginning (Corcoran et al. 2018; Suppli et al. 2018). With COVID-19 disease and vaccines, this is a time of scientific uncertainty (Caulfield 2021). As was well said by David Heymann, the WHO’s executive director of communicable diseases during the SARS crisis—“We are building our boat and sailing it at the same time” (Rosenbaum 2015). An uncomfortable place for many.
Other barriers to COVID-19 vaccine acceptance and uptake are unique to the context of this pandemic. First, the “infodemic” (i.e., an overabundance of information, some accurate and some not, both online and offline) (WHO 2020a) makes it harder for the public to find trustworthy sources of information about these vaccines. The proliferation of misinformation (information that is false but not created with the intent of causing harm) (Scheufele and Krause 2019) and disinformation (information that is false and deliberately created to cause harm) about COVID-19 vaccines on the Internet and social media has been noted as unprecedented. This particular context makes it more difficult for governmental and public health authorities to identify and counter the mis- or disinformation in a timely manner (Burki 2020; Lancet Infectious Diseases 2020; Johnson et al. 2020). Different studies have demonstrated that being exposed to negative content about vaccination can negatively impact vaccine acceptance and uptake (Betsch et al. 2010; Wang et al. 2019).
Second, with the supply of the newly authorized COVID-19 vaccines not meeting demand and with the staggered vaccine supply dominated by some high-income countries, considerable confusion, frustration and inequity has developed surrounding vaccine delivery. In a survey conducted February 12–14, 2021, 51% of Canadians lacked confidence that the government’s stated objective to vaccinate all Canadians by the end of September 2021 will be achieved (Leger 2021) while Canadians were challenged internationally and by one another for debiting their promised portion of the COVAX initiative to buy vaccines for low-income countries.
Third, the differences in disease burden across the country influence perceived and epidemiological urgency to be vaccinated. It is well known that higher risk perceptions of a disease is a necessary motivator of preventive health behaviours, including vaccination (Brewer et al. 2007). As the number of cases of COVID-19 decline, complacency and decrease in willingness to be vaccinated may follow. Furthermore, the pandemic has generated stigmatization and discrimination against Canadians (e.g., people from Asian ethnicities have been discriminated against due to the false belief that they caused the pandemic, whereas travelers, adolescents, and young adults have been devalued for adopting irresponsible or dangerous behaviours). As with other vaccines, stigma and discrimination may negatively influence willingness to be vaccinated against COVID-19 (Quinn et al. 2017; Nyblade et al. 2011).
Fourth, the evolution of COVID-19 vaccine science knowledge is growing ever more complex. Well executed adverse event surveillance in Europe detected 37 cases of thrombosis/embolic phenomenon amongst 17 million vaccines of the Oxford AstraZeneca vaccine in March/April 2021. But these were not all the same. Twenty involved central venous sinus thrombosis with thrombocytopenia purpura. Together, these phenomenon raised enough concern that several countries halted immunization pending further clarification of the cause and relationship to the COVID-19 vaccine (Science Media Centre 2021). Despite potential disease-risk harms being much higher than harms from vaccination, the publicity has sown doubt about its safety. Careful management of risk communication is needed during vaccine rollouts so as not to amplify hesitancy. Previous suspensions of vaccine campaigns, even if temporary, have led to long-lasting impact on acceptance (e.g., HPV in Japan (Ueda et al. 2020), hepatitis B in France (Balinska 2009)). Furthermore, what is known about the effectiveness of different COVID-19 vaccines in the real world compared to that reported in clinical trials, i.e., effectiveness versus efficacy, is evolving (Fedson 1998) and can be confusing. For example Health Canada, the national drug regulatory agency for Canada, approved the AstraZeneca COVID-19 vaccine for all adults but the National Advisory Committee on Immunization (NACI) did not recommend it for use in adults over age 65 years because of limited trial data (Government of Canada 2021g). The vaccine was approved for use in older adults in the United Kingdom (UK), followed by UK Public Health’s release of real-world effectiveness data showing that this vaccine is as effective if not better than the Pfizer vaccine regarding prevention of COVID-19 hospitalizations and deaths in older adults (GOV UK 2021).Further a single-centre negative case-control study in elderly and frail adults has shown both vaccines to be very effective even with only one dose (Hyams et al. 2021). Within three weeks of issuing a recommendation for use of this vaccine NACI updated its original recommendation to now include older adults (Government of Canada 2021a) with further age lowering changes later a month later (Government of Canada 2021b). As the science evolves, recommendations may change yet again. Such changing recommendations and advice from Public Health can be confusing, unsettling, and anxiety provoking for the pubic and health care providers alike.
In addition, the not unexpected emergence of COVID-19 variants with increased transmissibility and differing vaccine effectiveness (Government of Canada 2021h) has added to the complexity, confusion, and concerns for those contemplating accepting the vaccine being offered.
Fifth and importantly, the delivery of the vaccines is complicated by logistical issues such as ultracold storage requirements for some (Ministry of Health 2021) that differ from those experienced for vaccines used in routine immunization. Among these are:
Scale of the campaign. Reaching all Canadians has important implications not only for communication strategies and tools (e.g., interventions tailored to older age groups may be less effective for young adults), but also in terms of access to vaccination services. New strategies to equitably reach and vaccinate many Canadians will be needed as usual locations of vaccines delivery (e.g., clinics, pharmacies) may not be optimal for the underserved unable to access vaccination sites. The duration and large scale of the mass COVID-19 vaccination campaign, necessary to reach an estimated 70%80% of the population, may increase the risk of immunization program errors (i.e., errors in vaccine preparation, handling, storage or administration). If not conducted carefully and communicated well, program errors can negatively impact public trust and willingness to be vaccinated.
Potential of infection transmission. Precautions will need to be taken to prevent COVID-19 transmission at vaccination sites.
Variation across Canada. Vaccination delivery is the responsibility of provincial, territorial, and Indigenous governments with some groups under the federal government e.g., inmates in federal correctional institutions. Differences in jurisdictional recommendations and priority groups, even if justified by different epidemiological contexts, can create confusion. Discrepancies in program policies (Shapiro et al. 2017), standardized information, and vaccine advice can contribute to distrust among the public (Steenbeek et al. 2012).
Availability and use of COVID-19 vaccines. The fact that different COVID-19 vaccines with different clinical trial populations, safety data, efficacy profiles, and dose schedules used in Canada is confusing. It can be expected to lead to increased anxiety about access and availability of the perceived “best” vaccines. Media reports of clinical trial results can generate hype for some vaccines as well as confusion. Without standards for reporting clinical trial results, caution is required when comparison is made between vaccines; beliefs can be hard to change once formed. Offering a vaccine that is reported as less or more effective in a particular group can exacerbate vaccine hesitancy and acceptance. For example, single-dose vaccines and limited collection of personal details may be required for acceptance by some underserved communities where both access and privacy concerns are barriers.
Lack of immunization coverage data. In some jurisdictions, the lack of population-based data on vaccine uptake will make it impossible to identify which individuals and communities have sub-optimal vaccine coverage. This hampers program adjustments and tailored interventions to ensure that all Canadians are reached by vaccination services equitably, including those living in remote rural areas, with different abilities, precarious shelter, or in congregate residential settings.
3. Factors affecting COVID-19 vaccine acceptance
3.1. Vaccine acceptance framework
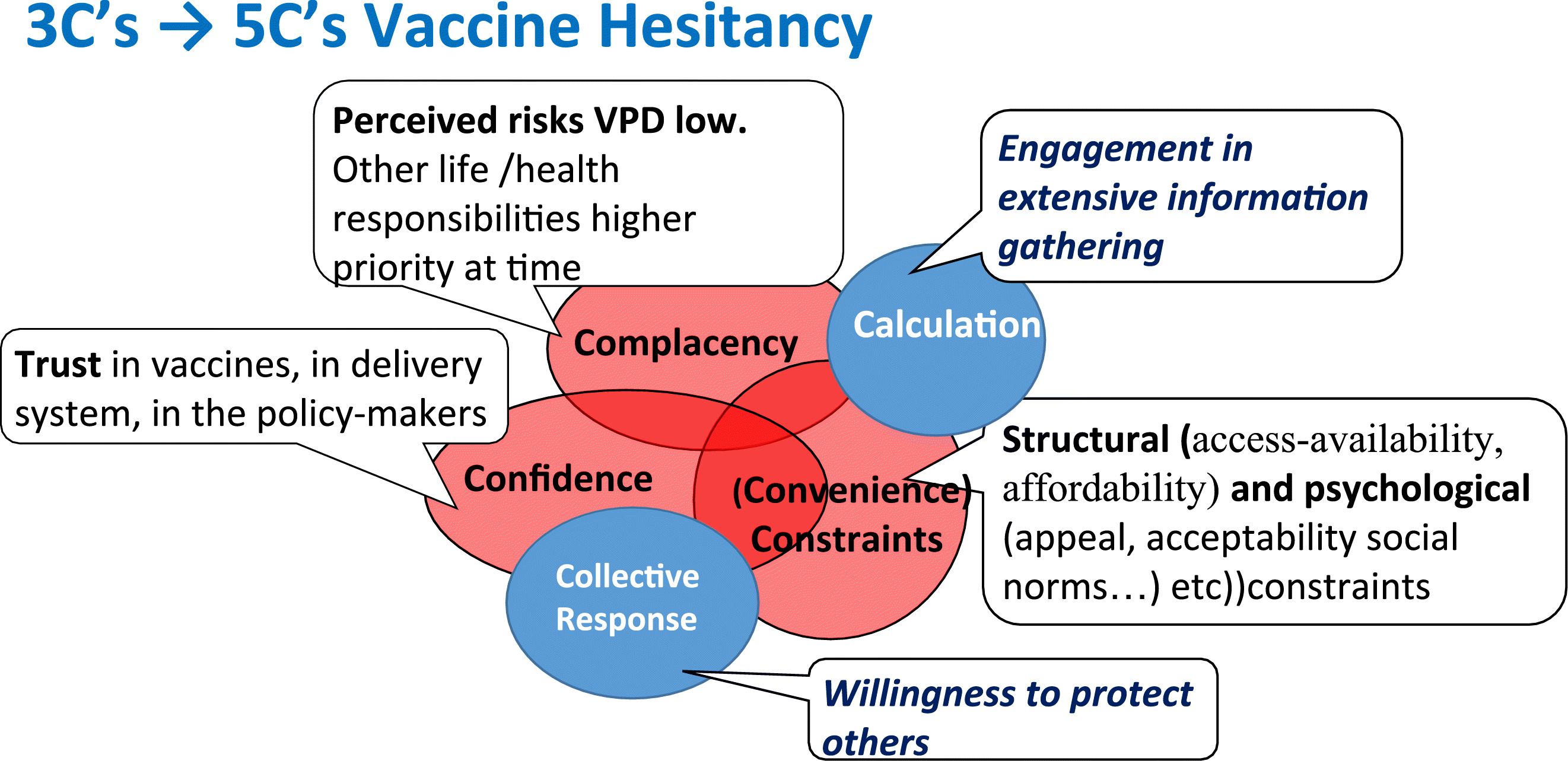
There are many factors known to influence vaccine acceptance and uptake, albeit most have been predominately examined within the context of childhood immunization. These factors have been gathered together under the term vaccine hesitancy (MacDonald 2015) and recognized as important to address if vaccine acceptance is to reach levels needed to prevent outbreaks. To help with the understanding of this complex area, the SAGE Working Group on Vaccine Hesitancy gathered the factors into three major categories; confidence, complacency and convenience (MacDonald 2015) that were later expanded to five categories with the addition of collective response and calculation (Betsch et al. 2018).
This ”5C” concept (Fig. 6), however, does not capture the multiple dimensions and complexity of vaccine hesitancy with childhood immunization (Hasnan and Tan 2021). The 5A framework by Thomson et al. (2016) (Box 1) is a step forward but still does not capture the complexity and interaction of factors.
Fig. 6.
Box 1. The 5As Framework.
Access: ability of individual to be reached by, or to reach, recommended vaccines
Affordability: ability of individual to afford vaccination, both in terms of financial and non-financial costs (i.e., ability to travel or take time off work to get vaccinated)
Awareness: degree to which individual has knowledge of the need for, and availability of, recommended vaccines and their objective benefits and risks
Acceptance: degree to which individuals accept, question, or refuse vaccination
Activation: degree to which individuals are nudged towards vaccination uptake
The WHO Expert Working Group on Behavioural and Social Drivers of Vaccination has used the model in Fig. 7 as background for developing a framework for measuring drivers of vaccine acceptance (WHO 2021i; Brewer et al. 2017) (Fig. 8).
Fig. 7.
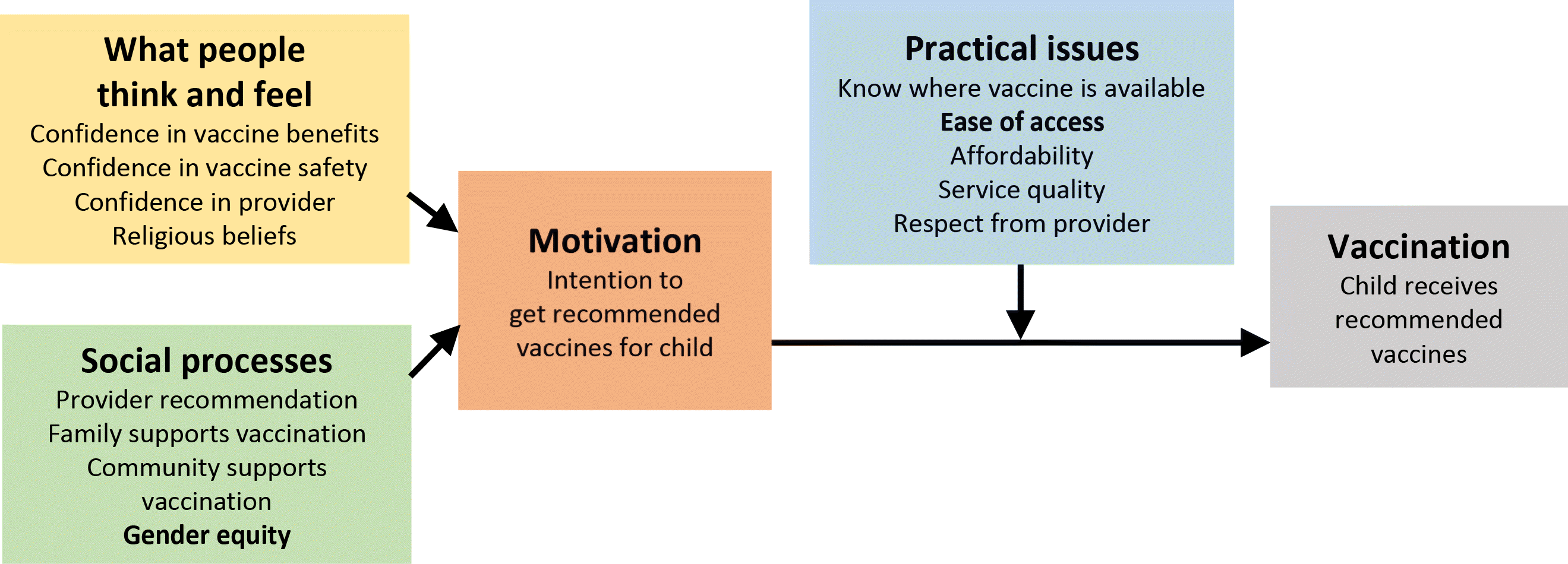
Fig. 8.
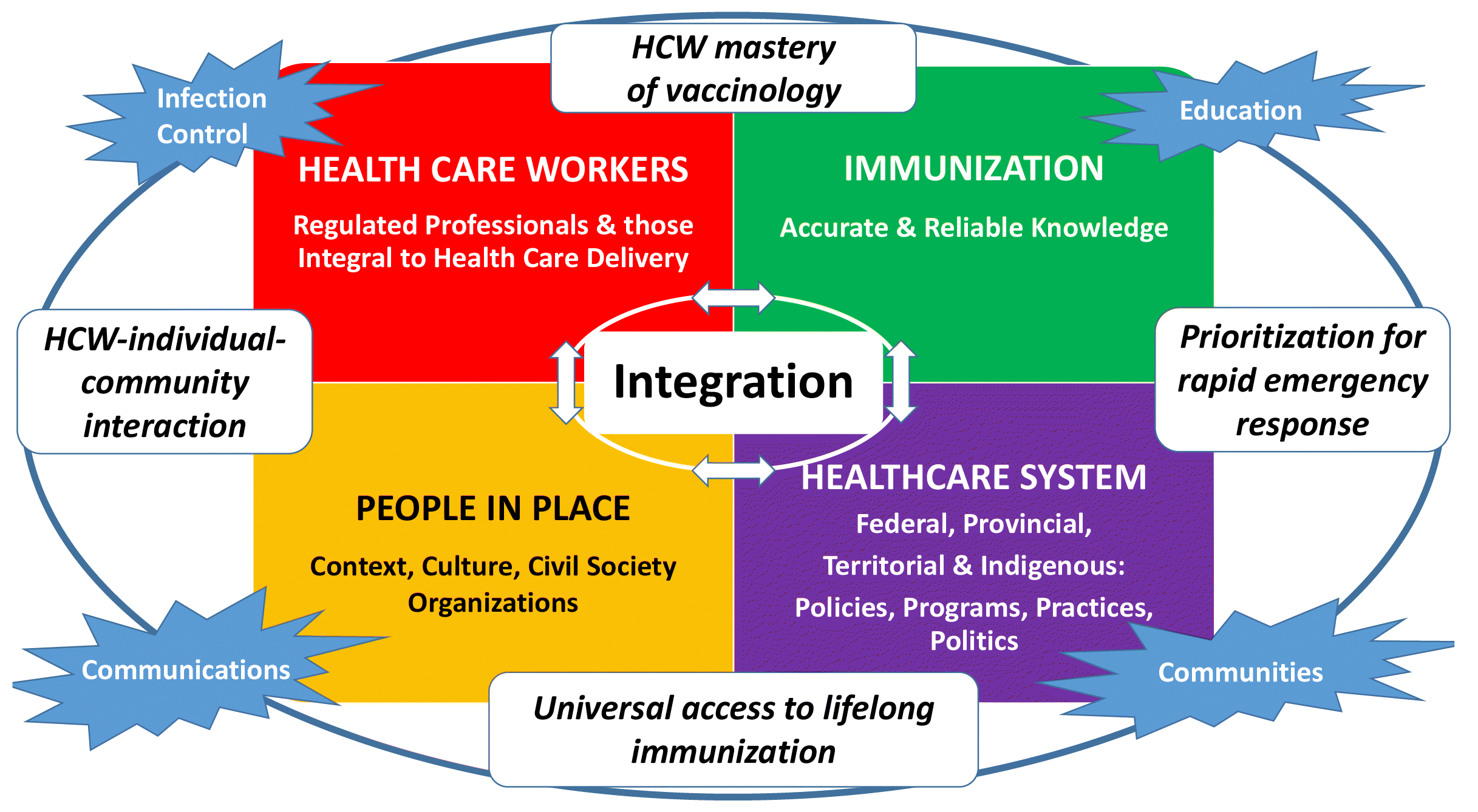
Vaccine hesitancy is even more complex during an epidemic (Dube and MacDonald 2020; Poland et al. 2021), as immunization must not just focus on children but cover the entire population. The impact of COVID-19 has driven home the costs to our health and importance of regaining control of our lives to our wellbeing. COVID-19 vaccines will only be able to help control the pandemic if acceptance is high. Bearing in mind the complexity of factors that influence vaccine acceptance, the Royal Society of Canada Working Group on COVID-19 Vaccine Acceptance has developed a framework for COVID-19 vaccines inspired by the 2021 Hasnan and Tan (2021) Framework but with many changes and additions. The titles of Hasnan and Tan’s four domains were expanded from clinician to health care workers (regulated professionals and those integral to health care delivery), infectious disease and vaccine to immunization (accurate and reliable knowledge), the terms health care system and policy to health care system (federal, provincial, territorial, and Indigenous policies, programs, practices, and politics), and child and parent/family to people in place (context, culture, civil society organizations). Four overarching areas of infection control, education, communities, and communications were added and the linking boxes for the four domains refined substantively. The framework proposed by the Working Group also took into account the World Health Assembly’s Immunization Agenda 2030 goal of leaving no one behind (WHO 2020c). It has four major domains of factors that influence vaccine acceptance (people and communities, health care workers, accurate and reliable immunization knowledge, and the health care system and public health programs)—each has implications at the federal, provincial, territorial, I or Indigenous level.
Note that none of the major domains stands alone—each influences the others and all are influenced by education, infection control, extent of collaborations and what, when, how and by whom communications about COVID-19 disease and COVID vaccines come forward.
Section 3 describes each of the four domains with examples of factors affecting vaccine acceptance within each domain and the four overarching areas emphasizing their interconnectedness across the domains.
3.2. People in place: context, culture, civil society
To control COVID-19, all vaccines must be seen to be safe and effective and their acceptance across the age span must be high (RSC-SRC 2021). Willingness to accept a COVID-19 vaccine and vaccine acceptance concerns are not static and will vary over time as shown in the EKOS poll (Fig. 2). Vaccine concerns may shift with the different COVID-19 vaccines as knowledge of their specific effectiveness (Vasileiou et al. 2021) and their specific rare serious adverse events evolves (EMA 2021b).
Vaccination decision-making, as we have seen, is complex with multiple factors influencing an individual’s decision to accept a vaccine (Dube 2016; Browne 2018; Kennedy 2019; Kata 2012; Strategic Advisory Group of Experts on Immunization 2018). Anecdotes and stories, for example, are often preferred to statistical evidence and data. Causation may be suspected even when an event is coincidental, such as was claimed in January 2021 when very frail elderly patients in Norway died after receiving the COVID vaccine—the vaccine was perceived as the cause (Torjesen 2021b). The WHO, after careful review of these cases concluded that the deaths were in line with the expected number and causes of death for this population and that they were unlikely to be related to vaccination (WHO 2021b). Importantly, careful monitoring of the side effects of these vaccines after rollout in Canada and the US has not raised any safety concerns about COVID-19 vaccination in long-term care settings. However, these deaths did raise concerns and may have made some elderly persons extra cautious about accepting a COVID-19 vaccine (CBC 2021b). Social networks affect expectations and actions, influencing decisions and choices. Negative information is heard louder and sticks (Pluviano et al. 2017), a reason why vaccine safety concerns are raised so often, especially for new vaccines. An underlying lack of trust in the health care system and (or) the government may lead to declining vaccination. The avalanche of information about COVID-19 and the COVID-19 vaccines can be overwhelming and confusing due to much misinformation, disinformation, and the changing public health recommendations as the science evolves (Section 2.2 and Section 4.4.2).
Factors affecting readiness for vaccine acceptance are substantially influenced by context, culture, recommendations, and the actions of civil society and community organizations. Reasons for some groups being disproportionately affected by COVID-19 disease in Canada vary but are rooted in the social determinants of health, local community factors, as well as other factors not yet identified. The following are examples but not an exhaustive list of disproportionately at-risk groups.
3.2.1. Indigenous communities in Canada
Vaccine confidence amongst Indigenous Peoples in Canada is also complex. Distrust is linked to the violence of colonialism (Mosby and Swidrovich 2021) where historically, Indigenous Peoples nearly met annihilation as a result of vaccine preventable diseases such as smallpox, diphtheria, polio, and tuberculosis as vaccines came late to many. Settler governments stripped Indigenous Peoples of their lands and the right to self-determination, confining many to isolated reserves. Generations of segregated substandard services were provided by a federal government who had the constitutional fiduciary responsibility for housing, education, and health care. Medical experimentation and abuse were commonplace for children in residential schools and children and adults in “Indian hospitals” and other hospitals (MacDonald et al. 2014; Collier 2017; Mosby and Swidrovich 2021).
Memories of these atrocities have been passed along generations with the legacy of colonial violence persisting today in the health and social inequities remaining (Greenwood and MacDonald 2021). Long-ignored social problems and problems stemming from government neglect of treaty and nontreaty obligations remain an emergency for Indigenous Peoples and communities.
These historic and present-day inequalities cannot be separated from Indigenous Peoples’ experiences with COVID-19 infection, initially disproportionately higher rates compared to non-Indigenous and questions about COVID-19 vaccines. Community leaders and elders have worked very hard to implement COVID-19 public health strategies for control including vaccine. Concerns have been addressed, distrust overcome to garner support for vaccine uptake on and off reserve leading to high acceptance rates in many communities and better control of COVID-19 (Government of Canada 2021e).
3.2.2. Racialized and other equity deserving communities in Canada
In the United Kingdom, United States, and Canada, racialized groups, including Black communities have been disproportionately affected by COVID-19. Canadian neighbourhoods with the largest proportions of visible minorities reported higher COVID-19-related mortality (Statistics Canada 2020a). In the Greater Toronto Area (GTA) of Ontario, as of December 2020, Black individuals accounted for 9% of the population and more than 21% of reported cases of COVID-19 during summer 2020. In contrast, the White population accounted for 48% of the population of the GTA and 17% of COVID-19 during summer 2020 (Toronto 2021). Other racialized groups are also disproportionately affected. In Ontario’s Peel region, South Asians account for 32% of the population and 59% of COVID-19 cases compared with Whites who account for 37% of the population and 13% of cases (Region of Peel 2021). Major contributing factors to these disproportionate rates for those affected by COVID-19 spill out from the social determinants of health.
3.2.3. Homeless youth in Canada
Persons experiencing homelessness have a higher prevalence of physical illness, mental health challenges, and addiction/substance abuse related concerns. Many are at higher risk for COVID-19 illness due to congregate living as well as often limited ability to utilize nonpharmacological COVID-19 prevention strategies (e.g., masks, social distancing, and frequent handwashing) (Turnbull et al. 2021; Baggett and Gaeta 2021).
Homeless youth are especially vulnerable (Karabanow et al. 2007). Approximately 20% (nearly 40,000) of Canadians experiencing homelessness are youth between 13 and 24 years of age. Most of these youth are victims of poverty and neglect, child abuse, and (or) violence. These circumstances are often due to system failures, with over 50% of youth having a long history in the child welfare system. Additionally, 12% of youth who experience homelessness have a physical disability, 18% suffer from addictions, and 39% have mental health issues. Homelessness is disproportionately higher among LGBTQ2S, Indigenous, and Black youth.
Youth who experience homelessness also experience a high incidence of infectious diseases, some of which are vaccine preventable. However, early departure from school, limited access to public health services, few youth-focused shelters, and high mobility makes these youth a vulnerable and extremely hard to reach group compared to other Canadians, including older homeless populations. Additionally, immunization may be seen as a low priority for these youth who tend to exist in a day-to-day survival mode precariously seeking out food, clothing, safety, shelter, and income. Furthermore, homeless youth may have additional concerns that push back against vaccine acceptance such as limited access to health care services (often due to a distrust of formal health systems), perceived and real discrimination by health care workers, lack of identification, worries of confidentiality breeches, and fears of being reported to law enforcement authorities.
3.2.4. Persons with differing abilities
Major challenges to access to vaccination can occur in persons living with a disability and (or) those who are reliant on home care to support their independence in the community. Factors that may affect the ability to come forward to immunization include (1) Geographic location: How far does the person have to travel to the vaccination site? (2) Transportation: Who is responsible for arranging transportation to the vaccination site? (3) Accessibility: Is there priority access to facilitate the vaccination process? Is the site wheelchair accessible? Does the site have the capacity to obtain consent to vaccination and administer the vaccine to those who are hearing or visually impaired? (4) Caregiver support: What happens if the person refuses to come forward because their care and support is provided by an unvaccinated home care worker and they are deeply concerned about the COVID-19 risks with the home care being offered?
3.2.5. Other examples of equity deserving groups
Of concern but not discussed above are those in the population who may not be large enough in any one region or jurisdiction to be recognized to receive attention as an equity deserving group e.g., migrant workers, undocumented migrants. These groups need attention and development of strategies tailored to address their concerns and fit their needs.
3.2.6. Religion: Relationship to trust and vaccine acceptance
Religious communities are among the cultural forces that socialize members into ethical frameworks that help them make both mundane and heavily freighted decisions. Religious communities are themselves so profoundly diverse that grouping them together is problematic. Nevertheless, none of the major religious traditions with which most are familiar eschew vaccines. In fact, religious institutions and leaders across communities overwhelmingly see vaccines as being consistent with internal values such as caring for others, preserving life, and having a duty to community (Grabenstein 2013). Nevertheless, religious concerns about immunization have been raised since the late 1700s when vaccination was introduced into western medicine. Recent scholarly work (Bramadat et al. 2017) and surveys by the WHO and UNICEF (Lane et al. 2018) found that religious concerns were often inextricably bound up with the delay in vaccine acceptance we see with infant and childhood vaccines globally. The impact on vaccine acceptance across the age span is unknown but likely similar to children. In Canada formal religious concerns have not been especially prominent in vaccine acceptance survey data, but there have been outbreaks of vaccine-preventable diseases in some religious communities that eschew some aspects of conventional health care, including immunization. For example, a recent study (Wilson et al. 2021) on vaccine acceptance by school age children from Ontario revealed that hot spots for under-immunization were often clustered and in some instances closely associated with specific religious communities. With respect to COVID-19 vaccines, the Canadian Conference of Catholic Bishops initially stated in early March 2021 that “parishioners should try to avoid taking viral vector vaccines like those produced by AstraZeneca-Oxford because they were developed using cell lines that may have been derived from an abortion nearly 50 years ago” (CBC 2021a). The impact of this advice on vaccine acceptance is still unknown. Of note this advice was clarified a week later stating “all COVID-19 vaccines that are medically approved by the relevant health authorities may be licitly received by Catholics” (CBC 2021a). Importantly, none of the viral vector COVID-19 vaccines contain any fetal tissue. There are concerns, however, that Canada may see similar push back to COVID-19 immunization from evangelical Christians as has been seen in the US (The Conversation 2021). Amongst these are many who are anti-science, anti-government, and believe in conspiracy theories about COVID-19 (McLaren 2020). Time will tell, but the flouting of COVID-19 public health guidance on public gatherings by some large religious congregations is not encouraging (Little 2021). This may have more basis in civil disobedience than in specific religious concerns.
Each of these community examples noted above underlines the complexity and diversity of factors influencing potential COVID-19 vaccine acceptance at the person level; the importance of who offers/recommends the vaccine, the value placed on these vaccines in the community, the knowledge about COVID-19 vaccine risks and benefits, the perception of COVID-19 risk in their context, ease of access to the vaccine, the recommendation or lack thereof by a valued civil society organization. They also highlight the importance of tailoring strategies for COVID-19 immunization for these communities. One plan does not fit all. For example for the homeless, a targeted, flexible, contextual, and culturally unique approach is needed—not a mass vaccination clinic blitz (Ghosh et al. 2021a). The outcome of a scoping review on vaccinating the homeless has highlighted key strategies (Ghosh et al. 2021b).
3.3. Health care workers: regulated professionals and those integral to health care delivery
3.3.1. Health care workers’ influence on vaccine acceptance
A major influencer of vaccine acceptance is the strength of the health care worker’s recommendation (Giambi et al. 2018) and how this is done, i.e., use of a presumptive rather than participatory introduction (Opel et al. 2013): “Today you are scheduled to receive a COVID-19 vaccine” not “What would you like to do about the COVID-19 vaccine?” Health care workers have long been seen as a most trusted source of information for vaccines and are noted to be trustworthy in surveys for COVID vaccines (Costa-Pinto et al. 2018; Nguyen et al. 2021). There are caveats, however.
Whether a health care worker is immunized for routine immunization also affects their recommendations for vaccination (Karlsson et al. 2019). This likely holds true for COVID-19 vaccines as well. Hence, ensuring high rates among health care workers is of critical importance (see below) (Zhang et al. 2010; Collange et al. 2016). All health care workers are not the same; there is a range in vaccine acceptance amongst different health care workers (Crawshaw et al. 2021; Desveaux et al. 2021; Dzieciolowska et al. 2021). Physicians and health care managers were more likely to accept vaccination compared to nurses.
In an unpublished Canadian survey in 2020, factors shown to be important in influencing health care worker intent to have a COVID-19 vaccine were vaccine safety, vaccine effectiveness, trust in the regulatory process, recommendation from NACI, personal risk factors, recommendation from their local public health authority, and recommendation from their professional association. Home care workers who are unimmunized may also impact on their clients’ decisions to accept vaccine as well as adding to their risk of contracting COVID-19, as what any health care worker says can impact patient vaccine acceptance.
Health care workers not only need regularly updated knowledge about COVID-19 disease and vaccines so they can be well placed to address queries raised by patients, but they also must also understand immunization best practices to optimize acceptance. Communities and groups who have been disrespected and (or) stigmatized when coming forward for health care by the health care workers are unlikely to trust easily. Immunization ambassadors from within the community, community members who champion vaccines, can help. Health care workers can play a role in helping to allay fears and grow trust in the vaccines by respecting lived experiences, past histories, and tailoring interaction to fit the person and the group. Training on best practices in addressing a vaccine-reluctant patient such as motivational interviewing (Gagneur 2020) is needed. For addressing specific vaccine safety concerns health care workers need ready access to up-to-date science-based answers to relevant questions. Currently this is not easy. Much time is needed for online searches, consultation on reliable and trusted web sources, as well as paying attention to the formal and social media to know what are key questions of the moment and what should be their science and evidence-informed responses. For busy frontline health care workers, this can be a major roadblock in willingness to counsel and work with vaccine reluctant patients.
3.3.2. Health care workers and the COVID-19 infodemic
The COVID-19 disease and vaccine infodemic (WHO 2021f) mis/disinformation is undermining vaccine acceptance (Section 4.4.2). Health care workers themselves may be influenced (Tomboloni et al. 2019). When patients bring up concerns raised by this mis/disinformation, health care workers need to debunk the myths (The Debunking handbook 2020), provide accurate up to date science based information (MacDonald 2020), and if able address the misinformation on social media and report this to providers. The WHO provides guidance on how to do this (WHO 2021d). Words used to discuss mis/disinformation and those promulgating it need to be chosen with care (Section 4.4.1). As noted above, a gap in easy access for health care providers to evidence-based answers to questions patients raise alerts to “hot” mis/disinformation topics and the science to address them could help health care workers to more efficiently and effectively counsel patients on COVID-19 vaccines.
3.3.3. Pain mitigation, needle fear and vaccine acceptance
Common concerns about all vaccines including COVID-19 vaccines relate not just to their safety but also to the nature of their delivery. For example, the discomfort associated with their administration—the needle stick—is an issue for many people. In Canada, about 1 in 4 adults report they are afraid of needles to some degree and about 1 in 10 report that concerns about needle stick pain and fear influence their decision about getting vaccinated (Taddio et al. 2012). Addressing needle-related pain (Taddio 2021) and fear (Meghan McMurtry 2021) using evidence-based interventions (Taddio 2021) to help make the experience as positive as possible (Taddio 2021), especially with COVID-19 vaccines as some need two separate doses, are other key knowledge components for health care workers tasked with vaccine delivery.
3.3.4. Adverse events following immunization (AEFI)
Vaccines are held to a very high safety standard because of their important role in the control of serious infectious diseases. Canada has a robust vaccine safety system with rigorous testing required in preclinical and clinical trials followed by careful pre-approval scrutiny of the clinical trial data to determine if the vaccine should be approved, and if so for whom, how, and when (MacDonald and Law 2017). These pre-approval trials must be large enough to determine efficacy, and to identify common adverse events. If the vaccine is not efficacious, or if there are common serious adverse events attributable to it, then the vaccine does not receive approval. Canada’s post-approval safety surveillance system is largely structured and undertaken at the provincial level. For children there is a paediatric hospital-based national active surveillance network for detection of vaccine failure, serious AEFIs, and selected infectious diseases that are, or will be, vaccine preventable (IMPACT) (Bettinger et al. 2014). Data collection is standardized, the nurses who collect it are well trained, and the assessment of the data is rigorous. In contrast, detection of serious AEFI in adults as needed for COVID-19 vaccines is primarily a passive system, which means that health care workers are expected to recognize a serious AEFI and then report it (Harmon and MacDonald 2020). It is unclear how many health care workers know about this responsibility and its importance. More work is needed to not only educate health care workers but also at the program level to make AEFI reporting, investigation, and causality assessment a stronger program (Harmon and MacDonald 2020).
3.3.4.1. Common AEFI and immunization stress related responses
Four COVID-19 vaccines were approved for use in Canada as of April 2021; two mRNA vaccines: Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273) and two adenovirus vector vaccines: Oxford AstraZeneca (ChAdOx1-S) and Janssen (Ad26.COV2.S) (Government of Canada 2021d). They can cause minor side effects such as injection site pain, redness and local swelling, as well as more generalized symptoms such as chills, fatigue, joint pain, headache, mild fever, and muscle aches (Government of Canada 2021d). The latter symptoms are not due to COVID-19 as none of these vaccines can transmit this virus but rather reflect the normal immune response triggered by the body to the vaccines. Not surprisingly, given the basis for these reactions, they are more common with the second dose than the first dose and are more common in women as the immune response is more brisk (Fink and Klein 2015). Over-the-counter medicines, such as ibuprofen, acetaminophen, or antihistamines, are helpful for managing any pain and discomfort that can occur after the immunization but are not recommended to be given before (CDC 2021e). In the first month of COVID-19 vaccine safety monitoring in the United States, 78.7% of reports submitted in the passive reporting system were in women. Headache (22.4%), fatigue (16.5%), and dizziness (16.5%) were the most frequently reported symptoms. Most of these generalized symptoms are gone within three to four days but may occur for up to two weeks (Chapin-Bardales et al. 2021).
Other common reactions unrelated to the vaccine and immune response may also occur at the time of vaccine injection. These are related to pain from injection and associated stress and anxiety that some people experience with an injection or anticipation of an injection, called immunization stress related responses (ISRRs), which are another type of AEFI (WHO 2019b). The vaccine recipient may turn pale, start to sweat, feel light-headed or dizzy, have numbness or tingling, start to breathe very quickly and (or) feel a loss of sensation in the face hands or feet. These symptoms may occur before, during, or after the immunization injection. The risk of ISRRs can be mitigated with attention to decreasing pain and other stressors that may augment fear and anxiety and is reviewed in a recent WHO report (WHO 2019b). An evidence-based vaccine delivery framework called the CARD (comfort, ask, relax, distract) system has been demonstrated to reduce stress-related responses in the mass immunization context (Taddio et al. 2019).
3.3.4.2. Anaphylaxis
Anaphylaxis is one example of a serious AEFI that must be rapidly and correctly recognized by health care workers who are immunizing, and it then must be clinically managed expeditiously and reported (Commeau and Top 2021). Here again, training is needed for health care workers to be able to accurately distinguish between anaphylaxis and immunization stress-related responses. How and what health care workers communicate about specific reported AEFI and the findings following causality assessment of the AEFI to determine if the vaccine was or was not the cause of the event is critical for supporting trust between the health care worker and the patient. As noted in Section 3.2.1, some serious AEFIs, like the deaths reported in the frail Norwegian elderly following a COVID-19 vaccine, were not due to the vaccine. Health care workers need the knowledge and skills to effectively discuss this if a patient is concerned.
Overall, the four authorized COVID-19 vaccines have a very good safety profile. They would not have been authorized if serious adverse events were common. Health care workers need to be well versed in their common and extremely rare adverse events so they can care for and reassure those coming forward for immunization. Immunizers also need to know and use best practices to mitigate ISRRs, including pain mitigation strategies, to make the COVID-19 vaccine experience a positive one (Taddio 2021; McMurtry 2021; WHO 2019b).
3.3.5. All health care workers need education on COVID-19 disease, vaccines, and AEFI reporting and effective vaccine communication skills
A key point to recognize is that the public looks to a wide range of health care workers for advice, not only nationally regulated health professionals such as nurses, doctors, pharmacists, etc., but also those not nationally regulated such as paramedics and personal care workers, and those in environmental services. Given the complexity of factors that can affect COVID-19 vaccine acceptance, health care workers need education about key roles they play in their interactions with all patients, education on COVID-19 disease and vaccines, as well as on AEFI and on a range of communication skills needed when addressing immunization and vaccine acceptance issues to optimize interactions with patients and support vaccine acceptance. Given the wide variation in immunization education backgrounds for different health care professionals—both regulated and unregulated—the level of knowledge and skills differ widely, hence education needs differ widely. Work is needed to develop tools and easily accessible training modules to address different health care workers’ knowledge and skills gaps as well as best practices to facilitate health care workers being up to date as the science evolves.
The large scale of the COVID-19 vaccine rollout campaign means that many health care workers will be involved, including some with limited training on immunization. It is essential that these workers be trained to competently deliver immunizations safely using best practices and equipped to adequately address questions and worries of people coming in the clinics (e.g., people refusing the Oxford AstraZeneca vaccine because it is perceived as less effective). In contrast to the clinical trials, which had different end point, in “real world effectiveness” both the Pfizer and AstraZeneca vaccines were shown to both be very effective in preventing hospitalization and death (Torjesen 2021a). To understand this health care workers must understand the difference between efficacy and effectiveness, well explained in a US National Academies of Sciences, Engineering, Medicine report that also provides communication strategies for explaining this for COVID-19 vaccines (Lowry 2021). WHO also has a report in March 2017 on COVID-19 vaccine effectiveness criteria (WHO 2019a). Similarly, the very rare serious adverse event of thrombosis with thrombocytopenia with the AstraZeneca (Government of Canada 2021b) and Jannsen (FDA 2021) vaccines needs to be explained in context with risk of serious complications including thrombosis with COVID-19 disease. Not a simple task.
3.3.6. Health care worker vaccine acceptance needs to be optimized
Given the important role health care workers have in vaccine acceptance as noted above, (Zhang et al. 2010; Collange et al. 2016) every effort needs to be made to optimize health care worker COVID-19 vaccine acceptance. Strategies need to be in place to determine the barriers and enablers. Listening is key as is co-creation of the programs. As with the general public, multi-pronged strategies are likely to be more successful than a single strategy and the reasons for hesitancy or delay should not be assumed. Local vaccine champions may be very helpful. Delay in acceptance may be linked to lived experiences (black, Indigenous, religious, etc.; Section 3.2.1) and needs appropriate respectful approaches. Barriers to access such as need for paid time off etc. need to be addressed not only for health care workers but for all employees.
3.4. Immunization—Accurate and reliable knowledge: COVID-19 disease and vaccines
3.4.1. Access to accurate and reliable information
COVID-19 disease and vaccine science are evolving. Access to needed information for health care workers, program managers, decision-makers, and the general public must be easy, and the information updated regularly as the science evolves. Health care COVID-19 vaccine program managers and health care workers need accurate, reliable, and up-to-date data and evidence on COVID-19 disease and the COVID-19 vaccines to revise programs and answer questions raised by people coming forward to be immunized. The public needs to know why, based upon what evidence, decision-makers have come to their recommendations, if these are likely to change, and if so when and why. When advice does change, care must be taken to explain the new evidence that supports this change. A major issue with COVID-19 vaccine information for vaccines approved in Canada is that the clinical trials did not all use the same end points nor end point definitions, that preliminary results are in the news even before peer review, and much of the evidence is highly technical making interpretation more difficult. Furthermore, self-proclaimed experts on COVID-19 vaccines and their best use abound. There has been a lack of coherence in recommendations for the use of vaccines (Section 2.2). Based on limited data, the regulator, Health Canada, and the independent NACI, arrived at different conclusions on whether to recommend the Oxford/AstraZeneca COVID-19 vaccine for adults over 65 years of age. This discrepancy was further compounded by different provinces then moving forward with different strategies for this vaccine with one a yes for use in those over 65 years (Quebec) the others a no. The NACI advice changed in March 2021 as real-world effectiveness data for that older age group became available (Government of Canada 2021a) and changed again in April 2021 as the science evolved (Government of Canada 2021b).
3.5. Health care system: Policies, programs, practices and politics
3.5.1. Legal frameworks: the special position of health including immunization (Harmon et al. 2021)
This subsection outlines legal frameworks critical to vaccine acceptance, equitable access, and uptake. These human rights frameworks support that, in the absence of health, individuals cannot participate in valued social activities from forming families, to performing work, to contributing to culture. Indeed, there is a link between poor health and social/political instability (Ruger 2006). Therefore, vaccines are viewed as “global public goods” meant to contribute to the equitable protection of peoples, and the WHO has offered a values framework for COVID-19 vaccine roll-outs that encompasses human wellbeing, equal respect, equity, reciprocity, and legitimacy (WHO 2020d).
3.5.2. The general international legal environment
The Universal Declaration of Human Rights 1948 (UDHR) states that the inherent dignity and equality of all members of the human family is the foundation of freedom, justice, and peace, and that all humans are born free and equal in dignity and rights, are endowed with reason and conscience, and should act towards one another in a spirit of solidarity (UN 2021a). The UDHR informs the International Covenant on Economic, Social and Cultural Rights 1966 (ICESCR) (UN 2021b), in force in Canada, and both are components of the International Bill of Human Rights (IBHR).
Two IBHR provisions are implicated by the COVID-19 pandemic. Article 12 (ICESCR) articulates the right of everyone to the enjoyment of the highest attainable standard of physical and mental health, and directs states to realize this right through multiple interventions, including those aimed at the prevention, treatment, and control of epidemic, endemic, occupational, and other diseases, and the provision of medical service. This right to health is reiterated in multiple international instruments. Article 15 (ICESCR) articulates the right of everyone to enjoy the benefits of scientific progress and its applications, imposing on States the diffusion of science and culture through multiple means. It recognizes science as a “public good”, and the benefits of science as the heritage of humanity (Morsink 1999). It places well-being at the centre of any justification for pursuing science (Chapman 2009).
3.5.3. The general Canadian legal framework
The rights that are directly enforceable in Canada must find voice in Canadian law, which should, and more or less does, reflect the above instruments. The Canadian Human Rights framework is represented by the Canadian Charter of Rights and Freedoms (Charter) (CanLII 1985), and the Canadian Human Rights Act (RSC 1985), and together with its Provincial/Territorial counterparts, seeks to eliminate discrimination by private actors.
The Charter extends to those in Canada a range of rights, and imposes on governments and government actors, and on those carrying out governmental functions, duties to refrain from unduly infringing upon those rights in pursuing their public functions (Supreme Court of Canada 1990). However, the Charter does not enumerate a right to health or health care. Having noted that, there is scope to interpret existing Charter rights—to life, liberty, and security of the person; to equality; to freedom from cruel treatment—so as to advance a right to equitable access to (reasonable levels of) health care in keeping with the IBHR.
Actions taken across Canada to overcome COVID-19 have interfered with the exercise or enjoyment of rights (e.g., mask-wearing orders (s 7), border checkpoints (ss 8 and 9), etc.). However, all of our rights and freedoms are subject to “such reasonable limits prescribed by law as can be demonstrably justified in a free and democratic society” pursuant to s 1 (Flood et al. 2020). This means that all Charter rights, regardless of whether they have internal limitations (many do), are limited by the nature of our democratic state, and by the needs associated with preserving and improving that democratic state in diverse conditions. Governments are entitled to take measures to protect public health, even if those measures infringe certain rights, so long as there is sufficient evidence supporting their actions (i.e., demonstrating that they are effective, proportionate, and minimally infringing or intrusive) (Flood et al. 2020). Essentially, public health decision-makers must base their interventions on evidence and wide-ranging risk analyses, and they are expected to communicate the basis for their actions clearly. Canadian governments have faced criticisms in this regard with respect to a wide range of their interventions.
Contrary to the Charter, human rights legislation applies to governments—in their performance of certain tasks and provision of various services—and to private actors (i.e., employers, unions, landlords, businesses, etc.). Individuals and groups can initiate discrimination claims that are then investigated by the relevant Human Rights Commission, which, if a claim is accepted, fashions a suitable remedy. Discrimination can be defined as differential treatment of an individual or group, whether intentional or not, based on grounds relating to personal characteristics, which has the effect of imposing disadvantages not imposed upon others or that withholds or limits access to opportunities available to others (Supreme Court of Canada 1989; this general definition is relevant to discrimination whether claimed under the Charter right of equality (s 15) or human rights legislation). Distinctions based on immutable personal characteristics, or aspects of the person that can only be changed at unacceptable cost, that are attributed to an individual solely on the basis of association with a group will often be classed as discriminatory.
To succeed in a discrimination claim under human rights legislation, claimants have to show (1) that they are a member of group protected under such Acts (e.g., a group characterized by religion, creed, ethnic origin, sex, genetic characteristics, disability, etc.), and (2) that they were subject to adverse treatment for which that prohibited ground was a factor (Supreme Court of Canada 1999). If an employer were contemplating mandated vaccination, for example, an employee might claim that vaccination is contrary to their religious beliefs or creed. Assuming the Human Rights Commission (or Tribunal or court) accepts this, the employer would then have to justify the mandate by showing that it is a bona fide occupational requirement (BFOR). To do this, the vaccination must be: (1) rationally connected to the performance of the job, (2) adopted in an honest and good faith belief that it is necessary to the fulfilment of that legitimate work-related purpose such as hospital and home care workers providing care to patients/clients at high risk for serious illness with COVID-19, and (3) reasonably necessary to accomplish that legitimate work-related purpose. Mandated influenza “vaccination or mask” policies for health care workers have been struck down in British Columbia (HEABC 2019) and in Ontario (Cavalluzzo 2015). In these cases, the policies were held to represent a breach of the Collective Agreement, and an unreasonable exercise of management rights. Arbitrators have also accepted that there is insufficient scientific evidence to support the effectiveness of “vaccinate or mask” in relation to asymptomatic transmission of seasonal influenza. Importantly, these arbitral decisions do not negate the validity of mandates. Instead, they speak to the fact that the mandate should have some scientific basis (re: effectiveness of the mandated action), and it cannot be contrary to already agreed working conditions.
3.5.4. Rights of Indigenous persons
Indigenous Peoples habitually face higher-than-average rates of disease burden, poor access to essential services and health care, and sub-standard health care interventions, which are often tainted by stigma and prejudice (UN Department of Economic and Social Affairs 2021). The COVID-19 pandemic poses an increased threat to Indigenous communities in Canada, who have experienced a case rate 40% higher than that in the general population (Somos 2021). This despite the fact that the UN Declaration on the Rights of Indigenous Peoples (UNDRIP) (UN 2007; Canada originally voted against the Declaration due to concerns around land claims and resource development, but acceded to it in 2010, characterizing it as an “aspirational” document) recognizes them as free and equal, and having the right to the full enjoyment of all human rights (UN 2007). The UNDRIP also states that Indigenous Peoples have the right to self-determination and self-government (UN 2007), and that Indigenous individuals have the right to life, liberty, security of the person, and physical and mental integrity (UN 2007). With respect to health, Indigenous Peoples have the right to the improvement of their social conditions, including housing, sanitation, health, and social security, and states have a duty to adopt effective measures to ensure improvement of their conditions, with particular attention the special needs of elders, women, youth, children, and persons with disabilities (UN 2007).
However, again, it is domestic law that more directly shapes the experience of Indigenous Peoples in Canada, and the Canadian government has characterized the UNDRIP as “aspirational” (Henderson and Albers 2020). Despite the constitutional recognition of self-government (see, Government of Canada 1982, which recognizes and affirms existing aboriginal and treaty rights, including land claims that exist or may be acquired), self-government advancements have been modest and incremental (i.e., there are 25 self-government agreements involving 43 Indigenous communities, and two education agreements involving 35 Indigenous communities) (Government of Canada 2020), and the resources for these communities to respond effectively to events such as the COVID-19 pandemic have not been made available (Power et al. 2020). Indeed, health data disaggregated by ethnicity is rarely collected despite such data being essential to understanding the true impact of COVID-19 on these communities and on tailoring services so that they are more equitably serviced going forward (Kauh et al. 2021).
At present, health care for Canada’s Indigenous Peoples (First Nations, Inuit, and Métis) is delivered in a piecemeal fashion, with federal and provincial governments sharing responsibilities (and often passing the buck to the other to the detriment of the patient (Blackstock 2016)). The result has been significant shortfalls and inequalities in service and health outcomes (Nader et al. 2017; Wylie and McConkey 2018). Acknowledging the significant health gap between Indigenous and non-Indigenous populations in Canada, the Government of Canada has implemented the Non-Insured Health Benefits (NIHB) program for First Nation and Inuit people to help cover the costs of medically necessary services and interventions, and some provincial programs also exist, but health disparities remain, as do significant barriers to improved outcomes (Nguyen et al. 2020).
3.5.5. Rights for identified equity-deserving groups
As noted above, discrimination can be defined as differential treatment of an individual or group, whether intentional or not, based on grounds relating to personal characteristics, which has the effect of imposing disadvantages not imposed upon others or that withholds or limits access to opportunities available to others (Supreme Court of Canada 1989). Distinctions based on immutable personal characteristics, or aspects of the person that can only be changed at unacceptable cost, that are attributed to an individual solely on the basis of association with a group will often be classed as discriminatory. Individuals from certain groups have historically been disempowered or “marginalized” by and within society and have therefore been viewed as at higher risk or as “vulnerable” to unfair treatment, both intentional and unintentional (and often systemic). Some of them now have recourse to special legal frameworks.
3.5.5.1. Rights example: older persons
The COVID-19 pandemic has been particularly hard on older persons who have suffered as a result of the virus itself and because of the failure to appropriately meet their health and support needs leading up to and during the pandemic (Informal Advocacy Group 2020). While there is no binding international legal instrument aimed specifically at age discrimination (Coalition to Strengthen the Rights of Older People 2009), there are a range of (non-binding) international policy documents and advice aimed at encouraging improved conditions for older persons and eliminating discrimination against them. For example, the Madrid Plan of Action on Aging (2002) (UN 2002) outlines an agenda for orienting policy in relation to ageing, and most of the Sustainable Development Goals (2015) address older persons either directly or indirectly (O’Sullivan 2018). Both the Charter and human rights legislation enumerate age as a prohibited ground of discrimination in Canada and other legislation seeks to protect older persons in specific contexts. Nova Scotia’s Protection for Persons in Care Act (CanLII 2017, s2), for example applies to “health facilities” (hospitals, residential care facilities, nursing homes, and homes for the aged or disabled persons; Alberta Queen’s Printer 2009, s 5(3)). It stipulates that service providers have duties to take all reasonable steps to provide for the safety and wellbeing of patients and residents (Alberta Queen’s Printer 2009, s 4(1)). Administrators also have duties to protect patients and residents from abuse (Alberta Queen’s Printer 2009). Under the Protection of Persons in Care Regulations (CanLII 2021), abuse is defined as the administration, withholding, or prescribing of medication for inappropriate purposes, and as mistreatment causing emotional harm, including threatening, intimidating, humiliating, harassing, coercing, or restricting from appropriate social contact (CanLII 2021, s 3(1)).
3.5.6. COVID-19 vaccine decision-making: consent
Targeted COVID-19 vaccination campaigns have led to high vaccination rates of those with clear capacity and ease of access to the immunization site where the vaccines are being delivered. To facilitate uptake of COVID-19 vaccines, the consent process needs to be efficient, informed, and free of coercion. It should be sensitive to the needs and context of the person and not serve as another barrier to vaccination.
Consent in most circumstances will be relatively straightforward. In the usual course, capacity to consent is presumed unless there are circumstances which make this presumption unreasonable. Individuals deemed to have capacity have the right to consent or withhold consent to any medical treatment or to withdraw consent even if it is contrary to medical recommendations (Supreme Court of Canada 1980; Supreme Court of Canada 1993). This common law right, which is legislatively embedded across Canada, stems from constitutional principles of dignity and autonomy (Supreme Court of Canada 2013).
Matters become more complicated when capacity is in issue (i.e., minors, those in long-term care where rates of dementia are relatively high, and those in community care where cognitive and physical disabilities may limit capacity). In such cases, the capacity assessment must be contextual and specific (i.e., related to the event or intervention at issue, such as COVID-19 vaccination), and they may be carried out by medical doctors, psychologists, or nurses, occupational therapists, or social workers trained and certified to carry out capacity assessments. For the factors to be considered, reference must be had to legislation specific to the province and the context because the rules around consent, and around consent on behalf of another, varies by jurisdiction and policy field.
Most provinces have a general statute that sets the age at which minors become adults with full rights to exercise autonomy. However, some Provinces/Territories set the age at 18, and others at 19, with no justification offered for the divergence (for a useful province-by-province summary, see Coughlan 2018), and the threshold age for making decisions may differ within a province depending on the setting. For example, under the NS Age of Majority Act (CanLII 1990), the minors become adults at age 19, with no stipulated age of consent for medical treatment. Under the NS Personal Directives Act (CanLII 2010), which replaced the Medical Consent Act, any person who has the capacity to make a personal care decision (i.e., is able to understand relevant information and appreciate reasonably foreseeable consequences) may make a personal directive. However, a substitute decision-maker (SDM) must be age of majority unless the SDM is a spouse or partner, in which case there is no age requirement. Under the NS Children and Family Services Act (CanLII 2020), children age 16–18 inclusive can contract with the agency for services.
By contrast, under Ontario’s Age of Majority and Accountability Act (ILO 2014), the age of majority is 18 years, again with no stipulation as to age of consent for treatment. However, under the Health Care Consent Act (Ontario 2021a), all persons are presumed to be capable of making treatment decisions, and under the Substitute Decisions Act (Ontario 2021b), persons 16 and over are capable of giving or refusing consent in connection with their own care, unless there are reasonable grounds to believe otherwise. The Health Care Consent Act recognizes an individual’s wishes, which may be expressed in a power of attorney or another written form, orally, or in any other manner, and SDMs must be at least 16 years old, unless they are parents of an incapable patient. Similarly, SDMs under the Substitute Decisions Act must be at least 16 years old.
Parents or guardians are the usual decision-makers for minors, but under both the common law and various statutory regimes, as minors mature, more weight is given to their wishes. Those assessed to be mature minors can make decisions that override those of their parents or guardian (Supreme Court of Canada 2009). In the health care context, where a court determines that a minor is sufficiently mature to understand the nature of the treatment and the consequences of accepting and refusing it, the court will permit the minor to make the decision despite the objections of parents, guardians, or care providers, though it will still consider the child’s best interests under its parens patriae jurisdiction. While it might be unusual for a court to extend autonomous decision-making to a child under 14–15, there is no hard threshold and all cases are context dependent (In CMG v. DWS 2015 ONSC 2201, the court rejected a parenting agreement in which disputing parents refused vaccination for their 10-year-old child and set the age of 12 for the child to make her own decision. Considering the child’s best interests, the court ordered vaccination and ordered that the vaccine-refusing mother was not to communicate with the child in a manner that would be negative to the child receiving vaccinations. In Re Calgary health Region 2006 CanLII 80851 (OIPC), the Commissioner gave effect to the 15.5-year-old’s refusal to release her medical records to her mother.). It has been suggested that the threshold for making decisions to accept vaccination contrary to parents’ wishes may be lower than for other more complicated medical interventions like surgery because of the reduced risk of vaccination (Glauser 2019).
For adults who are incompetent because of age-related progressive cognitive diseases, mental health problems, learning disabilities, brain injuries, etc., different legislation is implicated. For example, in Nova Scotia, the Adult Capacity and Decision-making Act (CanLII 2019), which replaced the Incompetent Persons Act, stipulates that another person can be assigned to make important decisions for an individual on matters affected by their incapacity. For a list of general factors to consider in relation to COVID-19 vaccination decisions, see Table 2.
Table 2.
| Understanding | The person can comprehend the problem, potential solutions and associated risks and benefits. Consent to vaccination: the individual recognizes that the COVID-19 pandemic is a major public health concern, that there is a vaccine available, and that the vaccine is very safe and effective. |
| Appreciation | Person recognizes how the problem might affect them. Consent to vaccination: The person recognizes that they are high risk for getting very ill with COVID-19. |
| Reasoning | Merit of the intervention, how it might affect them and logical thought process re: choice. Consent to vaccination: The person recognizes that the vaccine is safe for them and will prevent them from getting very ill with COVID-19. |
| Expressing a choice | Ability to render a clear choice for the decision to be made. Consent to vaccination: The person chooses to be vaccinated. |
| Decision-making support | Where persons may have some limitations in terms of making this decision, they should be encouraged to discuss this with and be supported by their power of attorney or essential caregiver. |
All health care providers and immunization program managers must remember that all Canadians have the right to equality, equal opportunity, fair treatment, and an environment free of discrimination (McElhaney and Andrew 2021). Automatically deferring to a substitute decision-maker when the person does indeed have decision-making ability or allowing people to miss the opportunity to be vaccinated due to access issues are two examples of potential human rights violations in the delivery of COVID-19 vaccines in Canada.
3.5.7. Vaccine injury support programs
As the most ambitious vaccination program in Canadian history ramps up, there will be occasions when individuals will experience an AEFI to the COVID-19 vaccines. Many of these will be mild and of no great consequence. However, there is the potential for very rare serious AEFI that leads to a negative outcome such as disability or death, such as the very rare but serious thrombosis with thrombocytopenia adverse events noted above. While there is no evidence that a vaccine injury support program in place leads to increased vaccine acceptance, this is the right time to consider a national program given that such programs have a an equitable and ethical rational given that immunization benefits the community not just the individual.
How should we handle those occasions when a person is harmed by a recommended vaccine taken in furtherance of the public good?
For those outside of Québec, the costs associated with a permanent or debilitating injury that is causally related to vaccination are currently borne by the injured individual (and their family), either personally as out-of-pocket expenses or through the purchase of private insurance or by the actors who bear some responsibility for the injury (e.g., vaccine manufacturers, administering health care providers, etc.) through tort litigation. However, access to these options is dependent on the individual’s social and economic conditions and capabilities. And tort litigation imposes a range of additional personal costs, including delay of benefits, financial cost, evidentiary hurdles, and emotional toll.
Surely, the concerns and sense of solidarity that drove Québec in the late 1980s to adopt its no-fault vaccine injury compensation program are shared across Canada. Canada announced in December a no-fault vaccine injury support program (VISP) to compensate and assist these individuals (Public Health Agency of Canada 2020). How this is implemented is critical. What should such a VISP look like? The seven characteristics that are essential to the fair, transparent and efficient operation of a modern VISP (Harmon 2021a) are noted in Table 3.
Table 3.
Foundation: The VISP should be grounded in a statute which articulates its purpose, operational principles, and management, ensuring transparency and accountability. |
Coverage: Benefits should be available to residents of all ages in relation to all vaccines recommended by public health authorities that are administered in Canada by authorized vaccinators, with clear instruction about the minimum or threshold injury or level of disability necessary to qualify. |
Accessibility: The claims process should be simple and clear, and the VISP administrator should have an obligation to assist claimants and families throughout the process. The enabling statute should address limitation periods, the claims process and timeframes, evidence, costs, re-assessments, and appeals, and it should result in written decisions providing claimants insight into next steps. |
Preservation of Rights: The VISP should not jeopardize a claimant’s right to pursue a civil action against potentially liable parties such as manufacturers, and it should ensure that the VISP fund is reimbursed for any benefits already provided when a court award is made in such litigation. |
Causality: It is essential to determine when an AEFI is, in fact, caused by a vaccine, as this will impact on vaccine safety and efficacy profiles, on recommendations for routine use, and on general acceptance of the vaccine. While claimants must prove that their injury was caused by the vaccine, this issue needs to be handled with care and sensitivity. |
Compensation: When a serious AEFI is caused by a vaccine, it is important for us—society—to compensate and support the injured individual. A national VISP should compensate: funeral expenses; income-replacement costs; medical expenses; physical, social, occupational rehabilitation expenses; personal assistance expenses; home alteration expenses; bodily injury indemnity, including compensation for pain and suffering; nominal damages for pain and suffering of the immediate family. |
Funding: The VISP should be funding through the national treasury with contributions to the fund coming from a manufacturers’ levy. |
3.5.8. COVID-19 vaccine certificates and mandates
Given that COVID-19 pandemic has curtailed attendance at many activities and limited travel, the rollout of effective COVID-19 vaccines has raised the issue of COVID-19 vaccine certificates (Oguamanam 2021). This is not a new concept. The WHO-approved international certificates of vaccination or prophylaxis (ICVP), the yellow card, has been widely used since 2007 for documentation of immunization against diseases that are limited in geographical spread such as yellow fever (WHO 2021j). While there are points in its favour for documenting COVID-19 immunization (Flood and Thomas 2021) such as decreasing risk by not allowing those not immunized to participate in certain activities such as air travel, in-door restaurant dining, or work opportunities in high-risk settings, concerns have been raised that such certificates might be easily falsified, can create a false sense of security as COVID-19 vaccines do not prevent infection with all variants of concern (WHO 2021k), could increase health and social inequities, and are a distraction to even discuss at this point in the pandemic (WHO 2021l). There is no evidence to date that these would be an incentive for COVID-19 vaccine acceptance as it has not been studied. There is concern that the attraction would be of benefit only for those contemplating travel or indoor dining, i.e., the relatively elite. The legal and ethical defensibility of vaccination certificates is contingent on ensuring equitable access to the vaccine as well as the merit of having these, i.e., impact on COVID-19 disease transmission. The Royal Society of Canada Working Group on Vaccine Acceptance did not see this as a major incentive to consider for improving vaccine acceptance at this time; however, this might become more relevant for consideration as the COVID-19 vaccines rollout moved beyond the older age cohorts.
With respect to mandating COVID-19 vaccines, a number of factors would need to be in place before such an action could even be contemplated (MacDonald et al. 2018; Harmon 2021b). Views toward government mandating COVID-19 vaccines have been surveyed, with support for them falling from July to September 2020 (IPSOS 2020). However, while governments may for a variety of reasons decline to mandate, there remains the possibility driven by the desire of businesses to quickly return to full operational status—that employers may turn to mandates in relation to their employees. This begs the question: Should or can private enterprises stipulate COVID-19 vaccination as a condition of continued employment? If an employer is considering mandates, then it must be circumspect in doing so because employees forced to accept a vaccine or vacate the workplace could launch actions under the Charter (if the employer were a government actor), file complaints of discrimination under human rights legislation, or file labour grievances through their union if a collective agreement is breached. The example of mandated vaccination in the workplace has been addressed in Section 3.5.1.
These arbitrary decisions noted do not negate the validity of mandates but rather speak to the fact that mandates should have some scientific basis (re: effectiveness of the mandated action) and should not be contrary to already agreed working conditions.
A much more beneficial approach for employers—particularly large employers or employers in critical sectors (e.g., health care, social care, transport and shipping, education, etc.)—is to actively partner with public health authorities in the delivery of vaccines, helping to ensure ease of access as well as ease of obtaining necessary information to make the vaccination decision. There are a number of strategies that can help such as support positive vaccine decision-making (Harmon 2021b) (Table 4)
Table 4.
Solicit information. On a voluntary basis solicit information relevant to vaccinators about their employees’ desire for a vaccination and history with reactions to vaccines. |
Carve out vaccination times. In the workday set aside vaccination times that are convenient for their employees. |
Set up vaccination spaces. In cooperation with public health authorities, create vaccination spaces at the workplace that are comfortable for employees. |
Vaccination navigators. Ensure that there is someone present to speak to employees, answering questions, allaying fears, comforting them (i.e., a well-known hurdle to people getting vaccinated is fear of needles,a or fear of pain from needle pricks; having someone present to talk them through that, or to distract them is helpful (Taddio 2021; Taddio et al. 2021)). |
Information. Distribute to employees in manageable amounts and useful formats, reliable, evidence-based information from health authorities. Promote immunization with local champions, positive reinforcement for vaccine acceptance, and visual feedback that acceptance is normative with pins, stickers, pledge forms, etc. |
a
The Royal Society of Canada Working Group on Vaccine Acceptance did not see this as a major issue to consider for improving vaccine acceptance at this time. However, as the context changes and access to COVID-19 vaccine eases, this complex and potentially fraught area might be considered.
3.5.9. Transparency and equity: What’s needed to engender trust that vaccines are safe and effective?
Eleven COVID-19 vaccines have been authorized or are in review (as of 14 April 2021) by the WHO following unprecedented collaborations made possible by a world threatened by the SARS-CoV2 virus (WHO 2021h). Canada has approved four of these under its priority review process as of April 2021; Pfizer-BioNTech’s BNT162b2, Moderna’s mRNA-1273, AstraZeneca-Oxford’s ChAdOx 1, and the Johnson & Johnson’s (Jannsen) AD26.CoV2.S. All have terms and conditions (Government of Canada 2021c) attached, signalling they can be quickly withdrawn should there be serious AEFI. Surveillance of their safety and effectiveness has received a funding boost by the Public Health Agency of Canada (,) and Canadian Institutes of Health Research (CIHR) (Government of Canada 2021h; CIRN 2021a; CIRN 2021b) along with efforts to return capacity for international disease surveillance (Robertson 2021) and national biomanufacturing (McQuaig 2020; The Globe and Mail 2021).
3.5.9.1. Transparency in a field of ghosts
Combinations of biomedical, public health, and social interventions are generating data that will contribute to better systems and technologies to control disease transmission in future outbreaks. Knowledge and independent access to the clinical trial evidence for new vaccine approvals (MacDonald and Law 2017) builds trust in their safety and efficacy (Morten et al. 2020; Herder and Graham 2021). Trust, however, “arrives on foot and leaves on horseback”; it demands open and accessible evidence (O’Neill 2014) and genuine longitudinal community engagement (Ryan et al. 2019). Some fundamental principles would help ensure confidence and trust in a vaccine ecosystem that emphasizes open data, strict conflict of interest guidelines, and equity in global response and rollout (Table 5) (Graham 2021).
Table 5.
| 1 | Clear disclosure guidelines that exclude those with perceived conflict of interest (personal and professional) from being involved vaccine related decisions. |
| 2 | Transparency and open access to all trial protocols (including any modifications) and data (including manufacturing quality control (Tinari 2021), vaccine components, i.e., adjuvants, decision-making processes and rationales for amendments, adaptations and contracts for procurement). |
| 3 | In adaptive trials, no increased participant risk from modifications made to compress testing phases or add new arms in response to new findings. If, as GAVI, The Global Vaccine Alliance states “[t]hese changes aren’t guesswork—they are based on clearly defined rules that have been set up by scientists who are experts at evaluating uncertainty” (GAVI 2021a), then these modifications should be made immediately known with rationale and data/evidence supporting those decisions. |
| 4 | Independent appraisal of all clinical trial evidence and decision-making rationale, advisory committee recommendations, and vaccine promotion to address perception of conflict of interest. |
| 5 | Properly designed and sufficiently powered trials to address efficacy. Disease prevention rather than surrogate (e.g., serological) endpoints are a higher standard that must be encouraged in Phase III trials. Trails must be large enough so that rare (occurrence rate ≥0.01% and <0.1%) are detected; very rare but serious AEs that can result in morbidity may only be detected post-approval with very large populations, many million, have been vaccinated); efforts should be made to encourage continuation of such trials after emergency expedited authorizations. |
| 6 | Robust post-approval monitoring and active surveillance must be in place to detect very rare but serious AEFIs (e.g., AstraZeneca COVID-19 vaccine and very rare unusual blood clots with low blood platelets (EMA 2021a) and possibly with the Janssen COVID-19 vaccine (CDC. 2021d)) as well as vaccine failures. |
| 7 | Locating Phase III trials where the disease is prevalent, including areas with variants of concern, is critical to disease exposure and the determination of disease prevention; with no available cures for COVID-19, human challenge trials are unethical. |
| 8 | Equitable participation in research of under-represented communities at risk for serious disease (i.e., older adults, racial/ethnic groups, pregnant and immunocompromised persons) with ongoing monitoring after trial completion. |
| 9 | “Universal, timely and equitable access to, and fair distribution of” (EMA 2021c) approved vaccines following principles of allocation and prioritization. Intellectual property protections that cite confidential business information clauses that prevent these rights need to be actively challenged rather than reinforced by governments. |
| 10 | Strengthening and sustaining community-based public health including immunization programs in communities. To support trust in public health recommendations and processes to track, assess., and treat serious AEFI through causality assessment; those making these decisions must be supported by robust scientific knowledge and expertise. The Public Health Agency of Canada, Health Canada, and all provincial, territorial and Indigenous public health facilities need to have a local community presence and the scientific and technical expertise to test, treat and respond to any emergency linked to wider provincial and national health systems of detection and response (Graham et al. 2018). |
3.5.9.2. Equity and politics
Controlling COVID-19 is a complex, wicked problem that involves exceptional scientific effort, public health engagement, and governance for its resolution. COVID morbidity and mortality ride geopolitical rogue waves. The global economic impact of COVID-19 as of January 2021 was $16.2 trillion (Koop 2021). Much has been learned in Canada and elsewhere, including: (i) dense (congregate) living and working conditions are “grim reapers”, especially for those already suffering the consequences of structural inequities, the marginalized, racialized, and older and frailer (Section 3.2.2); (ii) stigmatization and disinformation are rampant (Section 4.4.2); (iii) reducing severe disease and transmission is the primary goal; and (iv) vaccines for everyone everywhere are a necessary part of a pandemic endgame. In addressing the consequences of structural inequities, program development must be co-created with these communities as noted in Section 3.2.1. Community engagement and involvement is crucial (Ortiz et al. 2021).
For Canada, a country established preconfederation on the principles of peace, welfare, and good government, equitable access to COVID vaccines remains a valued principle but in practice, a hoary beast. In the years since medicare made its way from Saskatchewan to the rest of Canada, international trade agreements have co-mingled with austerity measures to fracture the health care system (Mijovic et al. 2020). Successive governments have not always protected the public good; public institutions, infrastructure, and health care services have been undermined, chipped away, and sold in the guise of collaboration, free trade, and public–private partnerships. National vaccine manufacturing capabilities were neglected. Hollowed out social structures and services means those most in need have overwhelming been the victims of COVID-19 disease.
Internationally, other equity imbalances are clear. Dozens of COVID-19 vaccine candidates were expedited for clinical trials by public funding (Knowledge Ecology International 2021). Nations that could afford it entered into bilateral negotiations with vaccine manufacturers (KHN 2020; The Guardian 2021; Rauhala 2021), some despite commitments made to the principles of Access to COVID-19 Tools (ACT) Accelerator and to COVAX (GAVI 2021b) to ensure equitable and fair access to COVID vaccines. As of mid-March 2021, 16% of the global population had secured access to 70% of the COVID-19 vaccines available (Lancet 2021). March 1, 2021 marked the beginning of the COVAX rollout in the African continent, while several high income countries were touting already having immunized more than 30% to over 60% of their own population (Ritchie et al. 2021).
Amidst these egregious global inequalities, science in Canada was also under siege. In Canada government scientific and clinical experts experienced cuts. Universities became increasingly dependent on corporate partnerships (McQuaig 2020). Researchers across the country were persuaded to collaborate with industry in enterprises that privatized the intellectual property of their findings (Herder et al. 2021). By 2020, Canada’s world class Global Public Health Intelligence Network (GPHIN) (Robertson 2021) had been so dismembered that when WHO declared a Public Health Emergency on 30 January 2020, the federal government lacked capacity to meet its 2005 International Health Regulations (IHR) commitment to pandemic preparedness. With a lack of manufacturing capacity, Canada was left scrambling for personal protective equipment (PPE) and experts to negotiate the procurement of vaccine candidates (Tumilty 2021). That successive austerity measures had reduced national capacity to the point where Canadian security was so exposed was not lost on Royal Society of Canada members who, in April 2020, recommended immediate retooling to fix these gaps (Graham and Manca 2021).
Canada was not alone. Governments worldwide, responding to political rather than public health appeals, were slow to lockdown their borders; they concentrated on international trade rather than disease transmission (CBC 2020c). Some communities and provinces acted swiftly when the virus first appeared, with organized public health responses led by medical officers of health sensitive to epidemiology rather than to politicians. They prioritized and enforced the public health measures that proved most effective: hand hygiene, masking, and social distancing. Those public health interventions, sufficiently reinforced by both government and its citizens, held the virus at bay before there were vaccines. Estimates of the economic costs of keeping economies open model the complexities of compliance in dollars and deaths (Chen et al. 2020). The goodness of time will determine whether communities that were locked down fared better than those with open businesses and borders. We have learned that compliance with public health for social distancing, hand hygiene, and masking is influenced by both micro and macroeconomics, and that access to scarce resources, from PPE to vaccines, can be uncertain and discriminatory. With community immunity still a ways away, Canadians are, perhaps characteristically, somewhere in the middle in the world of statistics and politics for COVID infection, vaccination, and response.
3.5.9.3. Variation in immunization across Canada: policies, politics, and tradition
In contrast to other OECD countries, Canada, being a federation with the provinces and territories having jurisdiction over health (MacDonald and Bortolussi 2011), does not have one harmonized immunization schedule across the country. Canada has a schedule for each of the 10 provinces, one for each of the 3 territories, plus federal ones for those under their care such as those living on reserves. This has led to a lack of coherence in routine immunization across the country. The National Immunization Strategy that was established in 2003 to provide a framework for effective inter-jurisdictional collaboration to improve the relevance, effectiveness, and efficiency of immunization programing across Canada (Government of Canada 2017) is a failure. There is still no harmonized national immunization schedule in 2021. This is not safe and not equitable. This is a problem for parents and for health care providers—who is missing what vaccine when they move (MacDonald and Bortolussi 2011)—a difficult question to answer. While one might argue Canada’s geography and different contexts could/should influence schedules, they more often differ based upon, autonomy, local capacity and resources, and tradition rather than a scientific evidence-based rationale (MacDonald and Bortolussi 2011). If we even had one national immunization registry where all vaccinated and nonvaccinated were noted, we might be able to adapt but we lack this as well as a fully integrated patient-centred health information system where not only would immunization status be available but also risk factors for complications with vaccine preventable diseases. Currently with our very fragmented and disjointed-data health systems it is not possible in real time to know how many over 50-year olds with diabetes and or hypertension have or have not been immunized against COVID-19.
Despite pleas to correct these problems before the COVID-19 vaccines were approved (MacDonald et al. 2020), COVID-19 vaccine disharmony still prevails. Some provinces initiated prolonged interval between mRNA vaccine doses, while others did not; at the time NACI recommended adherence to the 21- or 28-day second dose. This has now changed as NACI recommendations have changed (Government of Canada 2021g)—but again not all provinces are acting in a similar fashion. As noted earlier, 9 provinces limited Oxford AstraZeneca vaccine to those under 65 years as NACI initially recommended while another did not. The provinces changed yet again when concerns about the very rare unusual blood clots with low blood platelet adverse events were detected in Europe (EMA 2021a). British Columbia and Saskatchewan changed to use this vaccine only in those over 55 years, Quebec opened the vaccine for anyone over 55 years but it is a choice (many have accepted the vaccine), and Nova Scotia has targeted this vaccine for those 55 to 64 years. Why does this matter? It can undermine vaccine acceptance and trust in public health advice. For example while uptake of the AstraZeneca vaccine has been strong in Quebec, this is not so in Saskatchewan despite significant cases of COVID-19 (White-Crummey 2021).
There is also discordance in access by priority group as well. Some differences make sense, others do not. How quickly those in long-term care had access and those over 80 years varied across the country leaving some older people and their families anxious and concerned. Examination of postal codes and vaccine status in Toronto in early April 2021 showed marked disparities by socioeconomic status (Westoll 2021).
3.5.10. Jurisdictional discordance in access—examples: corrections and migrants
Discordance between jurisdictions within an institution is very problematic because policy change is hard. Persons working and living in federal correctional institutions share many factors that increase the probability of spread of COVID-19 if introduced and more serious illness amongst inmates due to the high prevalence of underlying conditions (RSC 2021). Outbreaks in correctional facilities have raised much concern and fear in the communities around them. Access to COVID-19 vaccine is problematic—prisoners in federal institutions are covered by a federal program, but the correction officers fall under the province in which the institution is located. This means incoherence in vaccine rollout; prisoners being offered vaccine but not the correction officers is undermining trust in the vaccines on both sides (R. Ricardelli, personal communication, 8 March 2021). Furthermore, there is no scientific rationale to support such a disjointed disconnect on access (see examples below).
Migrant workers both coming to Canada and within Canada provide more examples of jurisdictional disconnects. Neither are being coherently managed based upon scientific risk assessment and potential impact on communities. In the first COVID-19 wave in Ontario, 12% of migrant agricultural workers were infected after arriving in Canada and three died (Faraday et al. 2021). These are essential service workers that help ensure our food supply yet currently, Canada, neither federally nor provincially, has a coherent integrated plan for access to COVID-19 immunization for these migrant food workers (Faraday et al. 2021).
Workers in the oil sands in Alberta, many of whom are rotational migrant workers inside Canada, have also seen significant COVID-19 infections and even deaths (McDermott 2021). This is not surprising giving the congregate living at many sites. Many of these rotational workers have homes and families in other parts of Canada that they return to every month. This increases the risk of spreading COVID-19 to other communities and importing it as well when they return. As yet, there is currently no coherent interprovincial vaccine access program for these rotational workers nor for their families. Neither migration to Canada for an essential service nor migration within Canada count for vaccine access priority even if the risks of disease are higher and the potential impact on communities increased.
3.5.11. Programs and policies to support immunization
As noted in Section 2 there are many factors that influence vaccine acceptance including COVID-19 vaccine acceptance. Many of these factors are under the control of the health care program. For example, ease of access matters—where clinics are set up, who can come, distance to travel, cost for parking, hours of operation, etc. all influence attendance and acceptance. Currently many COVID-19 clinics have been set up to fit the system—initial concerns regarding transport of vaccine or plans for mass program. Little, if any, attention has been paid to working collaboratively with the communities these are intended to serve to ensure they fit the communities needs not just the programs. How will those with mobility problems manage, what about those that are blind, hearing impaired, and (or) have limited language skills in English or French. For example, almost three-quarters of all persons over 80 years in Ontario as of 23 March 2021 had been immunized or had appointments but nearly 200,000 had not signed up (CBC 2021b). Not surprisingly, the major issues identified included: reluctance among some seniors to go to mass-vaccination sites for their vaccinations, either because of transportation or mobility issues or because they fear it will increase their contact with others and therefore their risk of contracting the virus; limited opportunity to get vaccinated by their family doctor; language, literacy, and technological barriers to booking a vaccination appointment; lingering concerns about the efficacy rate of the AstraZeneca-Oxford vaccine and reluctance to take it because of initial guidance from NACI that it should not be used for adults 65 and older; and vaccine hesitancy, in particular among seniors worried about interaction with their prescribed medications. The program needs to address these issues but not blame or shame those who have not as yet come forward.
As noted in Section 3.2.1 programs need to be tailored to fit the group being served or low vaccine uptake will be the outcome (e.g., adult homeless example (Ghosh et al. 2021b)). Who attends and how attendees and are welcomed matters. Accepting a COVID-19 vaccine depends on trust—in the vaccine, in the immunizer, and in the immunization program. Immunization ambassadors from the community, those who actively champion vaccine, may be very helpful in garnering trust and in encouraging potential vaccinees to come forward (Habersaat and Jackson 2020). The experience must be as positive as possible (Taddio 2021) to ensure return for the second dose which may now be months later.
For many groups, bureaucracy must be minimized. Requiring a computer or phone to set up an appointment is a step too far for many equity deserving groups. Similarly, as noted above policies must not preclude access to vaccine because paperwork is missing—no health card, no identification etc. Even in rural remote parts of Afghanistan and Pakistan amongst populations who are illiterate, receipt of polio vaccine is checked with a blue dipped finger not by a note in a computer. The immunization program set up must be stretchable enough to serve all and not be the barrier.
All steps in the program need to be scrutinized through yet another lens. Is it needed or simply tradition? Using an alcohol swab to “cleanse” the area pre-injection is one example of an unnecessary procedure (Pakes and Taddio 2021). This not only does not add value for infection prevention, the smell can be off putting for some and these swabs cost money and add to medical waste and resources (time/personnel) for vaccination that could be deployed to improve access. Remember literally the COVID-19 vaccine program will likely swab over 70,000,000 arms if two doses of vaccine are needed.
A major gap in our current immunization programs and in health care across Canada is data (Ling 2021). We lack a fully integrated patient-centred electronic health information in all provinces and territories. Hence, knowing who should be prioritized because of underlying conditions is unknown at the provincial level. Such a system would also better support detection of serious AEFI and vaccine failures (MacDonald et al. 2020). Our current piecemeal system is not helpful at all and is impeding quality public health and acute, chronic, and preventive health care. While implementation of such systems takes time, COVID-19 can be the impetus. Such systems can improve health outcomes as well as decreasing costs if program management is built in (Graven et al. 2013). We cannot afford to ignore this data gap.
4. Overarching themes
There are four overarching themes: education, control of infection, communities, and communication that act on all four domains: people in place—context, culture and civil society organizations; health care workers—regulated, beyond regulated, and environmental services; immunization—diseases and vaccines; and the health care system—policies, programs, and real-world practices and are also need to be interwoven amongst themselves.
4.1. Education: COVID-19 disease, vaccines, and coping
The COVID-19 pandemic has exposed the inadequacy of the collective knowledge and confidence of Canadians (and others) in immunization. A significant percent of the population is reluctant about getting vaccinated, including COVID-19 vaccination, even in the face of the pandemic (Section 2.2). People have many questions about COVID-19 vaccination and about immunization more generally. This includes concerns about how vaccines work, how quickly they were developed, how effective they are, and how safe they are.
While public health experts have been talking openly and often about COVID-19 vaccination, the messages have not been easy for everyone to understand. For many, terms such as clinical trials, viral mutations, immunogenicity, and community immunity are new. COVID-19 disease and vaccine mis/disinformation has only compounded the problem (Section 4.4.2).
Several communication strategies on the “basics” of vaccines are being employed to help us through the COVID-19 pandemic. Public health officials and other experts need to ensure everyone has access to online information that address questions about vaccination even as these keep changing. Health providers across the country need access to up-to-date information that they can share with patients as well as be able to correct mis/disinformation (Section 3.2.3). Multi-pronged strategies are needed such as easily accessible accurate and up-to-date vaccine information rolled out using tools that fit the targeted subgroups (Section 4.4), vaccine education in schools, and attractive online games that teach not just vaccine facts but more importantly critical thinking skills and science literacy.
There is evidence that the public can be taught about immunization whether adults or school children with good effect. Canada has been a leader with the I Boost Immunity online game developed in partnership with Public Health Association of British Columbia, the BC Ministry of Health, and the BC Centre for Disease Control (I Boost Immunity 2021) and is approved by the Global Vaccine Safety net of WHO (2021c) This has been updated to contain COVID-19 disease and vaccine questions. The version for school aged children—Kids Boost Immunity—that also has teachers support elements, is now being used in schools across the country as well as in Scotland and Ireland (Kids Boost Immunity 2021a). This has been evaluated and shown to increase student immunization knowledge, critical thinking as well as fostering a positive attitude to vaccines (Kids Boost Immunity 2021b).
The CARD (C = comfort, A = ask, R = relax, D = distract) system is an evidence-based framework for planning and delivering vaccinations. Each letter category of CARD includes strategies that different stakeholders in the vaccination process—including health care providers and vaccine recipients—can “play” to improve the vaccination experience for everyone. CARD involves a series of steps and measures that health providers and institutions follow, including education, communication, and collaboration of important stakeholders, including vaccine recipients. It was initially developed for use in school-based immunization programs; however, it is relevant more broadly across immunization populations and settings to improve vaccination attitudes and reduce negative vaccination experiences (e.g., fear and dizziness) (Taddio 2021). Such approaches should be integrated in COVID-19 vaccine programs to support vaccination and reduce AEFI.
To help combat disinformation albeit in general not just for vaccines, the United Kingdom has online games for different ages—the Badnews games designed for adults and for students (Bad News 2021; Go Viral 2021). Evaluation of these has again shown that teaching the techniques used by those spreading disinformation can work to help protect against future disinformation (Roozenbeek and van der Linden 2019).
It’s Contagious! (Its Contagious Game 2021), It’s Infectious! (Its Infectious 2021), and Know It Or Not! (Know It or Not 2021) are gamified educational experiences developed by a not for profit (Digital Public Square 2021) that evolved from a project at the Munk School of Global Affairs and Policy at the University of Toronto. These games give people the opportunity to test their knowledge on common COVID-19 and vaccine misinformation narratives. A distinguishing feature of this educational platform is that it intentionally sought to involve individuals and communities vulnerable to misinformation and health disparities. Vulnerable segments of the population were identified and invited to opt-in to play the games via targeted advertisements on social media. As such, the digital games successfully captured and maintained attention of vulnerable individuals within the contemporary infodemic media system: in just five months (2020/21), over 200,000 Canadians and Americans engaged with the COVID-19 educational games and once interacting with the game, 41.5% of individuals played until completion, with the average player viewing 11.6 claims and corrections for approximately 2.33 million claims evaluated over the lifecycle of the projects. Additionally, 14.5% of players opted to “Read more” and 10% shared the game on their social media accounts, indicating deeper informational seeking and multiplier potential for intervention dissemination. Games also led to growth and retention of correct knowledge: a randomized control trial among a nationally representative Canadian sample showed a 15% gain in knowledge.
While all of these programs noted above are important steps forward, a national plan for immunization education, so that everyone understands the immunization process and how vaccines can contain a serious preventable disease like COVID-19 is critical given the importance of immunization to health, well-being, and our economy and quality of life (WHO 2021e).
One important step would be to educate the next generation—and the ideal place to do this is at school (Taddio and MacDonald 2021). Immunization is a topic that can be integrated into many courses, in health (learning about diseases and how to keep your body healthy), science (learning about how vaccines work and clinical trials), math (understanding risks of disease and risks of vaccination), mental health (learning how to cope with fear and pain from needles (McMurtry 2021)), and history (learning how vaccines and vaccine preventable diseases have shaped history). Immunization is a subject all need to know and care about, just like diet and exercise, so it is time to include it in the school curriculum Teaching children about immunization can shape their understanding and thus support vaccine acceptance, by them and their families, as they share what they learn.
Education for health providers should also be strengthened. This will empower them to be advocates for vaccination and engage patients in more conversations about the subject (Section 3.2.2). It will also lead to more consistent information being provided, which will reduce confusion. For health professions that have national licensing examinations, ensuring they include questions about immunization is an important step.
4.2. Control infections—COVID-19 disease: Infection prevention and control (IPAC) impact on vaccine acceptance (Comeau 2021)
Experience has shown that COVID-19 infections ebb and flow depending on local nonpharmacological infection control practices (Chu et al. 2020). Adherence to these practices matters. Travelers from away, whether from outside Canada or from a different region in Canada, coming into a community can bring in new infections including new variants. Isolation and testing of travelers until now are not infected can help decrease risk of spread if importation occurs.
The community context with COVID-19 vaccine rollout varies across the country. Some regions in Atlantic Canada have relatively fewer infections while other provinces have higher daily rates of new cases can influence perception of COVID-19 risk (Government of Canada 2021a). Rates vary even within a province with regions of hotspots. Context influences perception of need to be immunized as well as comfort in coming forward to be immunized, i.e., perception of risk of getting COVID-19 in the community and perception of risk while at the immunization venue (Comeau 2021). Regardless of the venues for vaccine delivery, attention is needed to ensure appropriate infection control practices for the safety of the health care worker (Government of Canada 2019b) and the public (Government of Canada 2019a). Some potential recipients may be reluctant to come to the venue if they see it as risky in terms of exposure to others who might be infected with COVID-19. The system must be in place so both the public and health care workers are reassured the health system has put best-practice infection control guidance into practice (Public Health of Ontario 2021).
Over the past year, the public has become familiar with the importance of personal protective equipment (PPE) as a means of reducing the spread of COVID-19. However, for those working in Infection Protection and Control (IPAC), multiple other upstream considerations and controls should be made and observed to ensure that individuals at mass immunization clinics do not contract COVID-19 while administering or receiving the vaccine. In order of effectiveness (and also importance) (see Fig. 9) these include: elimination of exposure, substitution of activities, engineering controls, administrative controls, and PPE (CDC 2021b).
Fig. 9.
4.2.1. Elimination of exposure and substitution of activities
In the context of immunization clinics, prescreening vaccine recipients well before entering the clinic (and, if they are not well, asking them to return only when they are well again) serves as a form of local elimination.
4.2.2. Engineering controls
Considerable attention has been placed on ventilation systems when people are indoors, and meeting outside has been recommended when possible. While immunization clinics cannot necessarily be outdoors, they should in large rooms or drive-through facilities that provide space for physical distancing and capacity for adequate ventilation both while the vaccine is administered and during the post-immunization observation period. Ideally traffic should be unidirectional.
4.2.3. Administrative controls
Carefully documented policies and procedures should include checklists of the IPAC measures implemented at each site. It is also necessary to have a system in place that screens and tracks both immunizers and immunized, so if an inadvertent exposure occurs, individuals can be quickly notified.
4.2.4. PPE
In a medical setting PPE is designed to protect the wearer, and individual users put on and take off their PPE (such as masks, face shields, gowns, and gloves) when encountering a potentially contagious patient. It is essential that this is done properly to avoid the possibility of self-contamination. In the context of the pandemic, masking has become an important measure in protecting others, so immunization clinics must have adequate supplies for both the health care providers and those who present for immunization but do not have their own (Comeau 2021).
The American Centers for Disease Control (CDC) has developed comprehensive advice for those providing COVID-19 immunization. This guidance holds true in the Canadian context (CDC 2021c). Of note, while it may seem contradictory to infection prevention, however, the common but unnecessary practice of using an alcohol swab on the arm prior to injecting the vaccine is not advised because it can increase anxiety for some and does not have medical evidence to support its need for infection prevention (Pakes and Taddio 2021).
Policy makers and program managers need to ensure that the immunization delivery sites are following these best practices and have the required equipment to do this. Health care workers need to adhere to best practices and address concerns of those who are anxious. The safety of attending a COVID-19 vaccine clinic needs to be emphasized.
4.3. Communities
Partnering with communities, defined as a group of people with diverse characteristics who are linked by social ties, share common perspectives, and engage in joint action in geographical locations or settings (may also be social media linked) (MacQueen et al. 2001), is a well-known element that improves childhood vaccine acceptance as it leads to increased trust in the immunization program, the vaccines, and the health care workers delivering the program (Hardt et al. 2013). As WHO outlines, partnering with communities for immunization refers to “supportive, coordinated action that can be taken by health workers and community members towards achieving their shared goal of providing accessible, reliable and friendly services that are used appropriately by all. It is based on the principle that when communities are involved in planning, providing and evaluating services, they will develop stronger trust and ownership of those services” (WHO 2021a). This is a critical element for developing support for COVID-19 vaccines for the public as well as equity-seeking populations such as the homeless, Black, Indigenous, etc. Most importantly partnering does not mean co-opting or simply consulting with. It means active collaborative planning, setting up conditions for buy in, and working with community leaders. This must also not be perceived as a one off. Such partnership is critical for continuing support for COVID-19 control measures as they evolve. Of note, community partnership does not mean long or extended delays for vaccine rollouts. With skill and collaborative motivation on both sides, this can be done quickly and more effectively.
Sadly, many of the early vaccine rollout programs have not had community partnership. For example, in some provinces couples of different ages cannot be immunized at the same time through adjoining appointments, and distance to clinics precludes attendance for those with limited transportation options.
Community partnership helps foster community demand and acceptance of vaccines and resiliency as the vaccine programs evolve and recommendations change. All too often partnering with communities has been token instead of core. Partnership must not be perceived or just be a one off.
The breadth of potential communities for partnership is wide and deep: by geographic locale, by equity deserving needs, by age cohort, by relationship with a civil society organization, by private or public business etc. When collaboration is real, truly creative and insightful suggestions may pop up. To optimize the value of the community engagement strategy in supporting vaccine acceptance attention must be paid to timing, leadership, feedback, and ability to support ownership. In the longer term, taking care in building many community partnerships will yield sustained demand for COVID-19 vaccines and vaccine in general. When a community supports immunization, this helps nudge those who are fence sitting to accept (Atwell and Smith 2018). It helps support resiliency in the face of access issues or misinformation strikes because vaccine acceptance is seen as normative behaviour.
4.4. Communications
4.4.1. What’s in a word?
Concerns about words used in discussing vaccines and those reluctant to accept a vaccine have been raised pre-COVID-19 (Dudley et al. 2020). Words can dramatically affect how a message about COVID-19 vaccines is heard, how an invitation to be vaccinated is received, and the decision to accept or not. Labels may also over simplify complex dynamics and not lead to changes needed (Walcott 2020), and they may also obscure the impact of history and lives lived on the vaccine acceptance decision (Section 3.2.1). The Royal Society of Canada Working Group on COVID-19 Vaccine Acceptance has contemplated the words issue, discussing potential alternatives for terms that may be perceived as disrespectful, disparaging, and off putting in communication materials and discussions with individuals as well as words used when immunizing a patient (McMurtry et al. 2021). Such words add yet another barrier to vaccine acceptance. Table 6 presents some of these terms with potential alternatives. The list is not exhaustive but a start for those developing communication plans and materials for the public and for health workers interacting with the public about COVID-19 and COVID-19 vaccines .
Table 6.
| Word | Problem/comments | Potential Alternative |
|---|---|---|
In communication materials and discussion with individuals | ||
Vaccine hesitancy (Dudley et al. 2020) | Variable meanings Polarizing Sometimes compressed as “confidence” when far broader in scope | Vaccine acceptance Factors affecting vaccine acceptance |
Vaccine demand | Unclear meaning: for some means at community level; for others also at individual level Also note, that acceptance does not equate with demand as “an individual or community may fully accept vaccination without hesitancy but may not demand vaccination or a specific vaccine” (MacDonald 2015) | Community and individual support for vaccine and the vaccine program |
Marginalized, vulnerable, people living with health and social inequities | An important concept with respect to vaccine equity. Some see these terms as disrespectful and even hurtful, i.e., as if this is their choice. | Underserved Equity-deserving Equity-requiring Equity-plus (requiring attention to needed supports to achieve equity) |
Vaccine refuser | On the continuum from full acceptance to non-acceptance. Refuser implies active and possibly loud refusal. Some see this term as pejorative and hurtful. | (Person who) declines vaccination |
Anti-vaxxer | May be vocal in concerns raised and active in spreading vaccine disinformation. Some see this term as derogatory. Adds a “them versus us divide” ethos that may be off-putting to those who are still in contemplation stage of vaccine acceptance. Not constructive in a patient-health care worker relationship. | Person opposed to vaccinations |
Herd immunity | Technical jargon. For some this is off putting as term used in livestock farming etc. | Community immunity Community protection |
Seniors, old, the elderly | Many do not want to be labelled as “old” as may imply “no longer useful”. “Elderly” is an adjective not a noun (as in “the elderly”) | Older adults Elders (older wise persons who carry the teachings) Persons above a specified age (e.g., over 65 years) |
In the context of data collection: gender identity (male, female) and racial identity | Why the information is being collected may not be clear. Some programs may request people to identify (self-label) with racial or gender identity categories that individuals find pejorative or inappropriate. For example, asking someone to indicate whether they are male or female doesn’t fit for someone who identifies as non-binary. | Be clear about why the information is being gathered and provide an option not to respond and (or) “select all that apply” Support flexible options when collecting these data (e.g., male, female, non-binary, two-spirit, other, prefer not to answer, etc.) |
Communication during vaccination | ||
“Here comes the sting.” “Here comes the jab.” | Words perceived as threatening may elicit fear. | Use neutral words when signaling the procedure and during interactions “Here I go.” “One, two, three.” |
“Don’t worry… It’s ok… You’ll be fine.” “It’s almost over, it’s almost over.” | Repetitive statements of reassurance can increase distress by bringing attention to the procedure. | “Would you like to talk about something else? Or would you like to take a few slow deep breaths?” If yes to distraction, consider question: “What music/book/sports do you like?” |
“It won’t hurt.” | False suggestions are ineffective and promote mistrust. | Provide information in a balanced manner. “Some people say they feel a pinch or some pressure. Others don’t feel much at all. We do not know how it will feel for you. You can let me know.” |
“Look away now.” “Take a deep breath now.” | Imposing coping strategies that are not aligned with an individual’s preferred strategies can increase distress. | Invite individuals to exercise their preferences. “Do you like to look or to look away?” “Do you want to take a deep breath?” |
Recovery area: zone where wait for 15 minutes following immunization | Exaggerates the risk and seriousness of the immunization procedure by using a term used post-surgery—a potentially, negative connotation | Departure lounge Celebration site |
4.4.2. Infodemic
In the spring of 2020, the WHO declared an “infodemic” was accompanying the COVID-19 pandemic (WHO 2021f). An onslaught of harmful, inaccurate information now pollutes the information ecosystem, causing confusion, stoking division in communities, and undermining public health messaging (Kurasawa 2020; Abdul-Mageed 2021; Swire-Thompson and Lazar 2021). As an example, Twitter exploded with COVID-19 tweets in 2020 (Abdul-Mageed 2021). Both COVID-19 vaccine misinformation (Scheufele and Krause 2019) (unintentional inaccuracies) and disinformation (Scheufele and Krause 2019) (deliberately false or misleading content) have been spreading rapidly and widely (Ryerson University 2021). Strategies to combat this have been outlined by WHO (2021g) and include timely dissemination of accurate information based upon science and evidence to all communities, particularly those at high risk and steps to prevent the spread of mis/disinformation education. Educate the public to recognize techniques being used. Public health and service providers must make it easier for lay people to find accurate information and check rumours for truth, providing opportunities for the public to query public health experts on confusing topics and enacting legislation to require better control accuracy of information on social media etc. (MacDonald 2020). All those recognizing COVID-19 online misinformation and disinformation are also encouraged to report this to the social media platforms so they can take action can address these (WHO 2021d). Sometimes even well-intended interventions by recognized institutions can contribute to the infodemic by adding to the confusion (MacDonald 2021). WHO has provided step by step guidance on how to report (MacDonald 2021).
4.4.3. Effective communication strategies
The key to successful public communication to address factors affecting vaccine acceptance lies in careful targeting of messages about vaccine effectiveness, safety, and availability. This requires attention to 12 points of communication strategy (Table 7):
Table 7.
| Strategy | Principle | |
|---|---|---|
| 1 | Segmentation strategy | An understanding of the prevailing views of the different target groups to design the appropriate message |
| 2 | Avoid/reduce confusion strategy | Consistency of the underlying vaccine and health message |
| 3 | Communications clarity strategy | Use of target group accessible language in its communication material |
| 4 | Balanced stimulus strategy | Messaging that while delivering a consistent main message varies to achieve optimal response by use of the intended target audience; use an appropriate blend of sensory, rational, and emotional communication |
| 5 | Communications inoculation strategy | Application of arguments that anticipate objections |
| 6 | Listen more, talk less strategy | Enable conversation, two- way communication, and follow up as much as possible |
| 7 | Traditional plus social media mix strategy | Use of media that the target groups can access with minimal effort |
| 8 | Source credibility strategy’ | Use of spokespeople and source material that have credibility amongst the target group |
| 9 | Repetition strategy | High frequency and ongoing timing of follow-up messages but delivered in a variety of formats to offset “message burn out”’ |
| 10 | Feedback loop strategy | Ongoing measurement of message response and vaccination experience by the target group |
| 11 | Word-of-mouth strategy | Follow up communication to those vaccinated to reassure them and to generate social media positives |
| 12 | Availability strategy | That the strategy to provide easy but controlled vaccinations enables both location and time convenience and that these are extensively communicated |
4.4.4. Bites, snacks, lunch
As noted above, the COVID-19 infodemic is overwhelming—not only for the public but also for health care workers. There is growing recognition that in terms of COVID-19 vaccine information tailored for a targeted subgroup using the above strategies, all within the subgroup are not looking for the same amount of information. Some want just a brief answer to the query—the bites—others want the bite plus some explanation—the snack—and still others want this plus the evidence—full lunch. This applies across the spectrum from one on one patient interactions with health care workers to public health materials for the public and (or) health care worker consumption. This means as evidence is prepared, the sources need to be referenced and where possible linked for those who want more than a bit or snack. Trustworthy evidence websites need to be highlighted. The WHO Vaccine Safety Net (VSN) helps Internet users find reliable vaccine safety information tailored to their needs (WHO 2021c). There are many VSN-approved websites in Canada in English and in French.
4.4.5. Consistent, accurate, and up to date
In the midst the cacophony of mis/disinformation, public health and health care worker messages must stand out as being consistent, accurate (i.e., evidence based), and up to date. Telling the truth, noting that the science has evolved, and this is what the new findings mean, must shine through the messaging. Correcting mis/disinformation is crucial. In the past concerns were raised that correcting a myth might make the myth stick even more. Recent research has shown that debunking the myth and presenting the correct evidence-based facts is helpful (Lewandowsky et al. 2020). While the Debunking Handbook was developed by experts from 20 universities (Australia, Canada, Germany, United Kingdom and the United States) to address climate change misinformation, the principles apply to addressing vaccine mis/disinformation as well (Lewandowsky et al. 2021).
4.5. Consistency of communications across jurisdictions in Canada
Coherent communication across the country is key to the public as they navigate the complex COVID-19 landscape (MacDonald et al. 2020). Having each province and territory presenting different program policies and different information on the same vaccine, as occurred in Canada with HPV vaccine, leads to confusion and undermines trust (Shapiro et al. 2017; Steenbeek et al. 2012). As noted in Section 3.2.3 there are already examples of confusion that has undermined vaccine confidence because of differing advice from Health Canada versus the NACI and between different provinces and territories on use of the Oxford-AstraZeneca COVID-19 vaccine. Better co-ordination and unity in messaging is much needed. If programs are to differ, then the messages must highlight why a different context warrants a different strategy. Core content on the available COVID-19 vaccines must be presented in a similar manner, although what and how messages are conveyed may vary by community targeted, i.e., message tailoring (Dube et al. 2020). Uncertainties about vaccines in general and their specifics need to be acknowledged—the science of what is known, what is not known, and what is being done to close those gaps to nurture individual and community trust in the program and in these vaccines needs to be conveyed (MacDonald et al. 2020).
5. Research
As noted in Section 2, vaccine acceptance is complex and many factors influence it including but not limited to vaccine safety concerns, access issues, and trust in health care providers. COVID-19 disease and COVID-19 vaccines have only made this an even more complex area. Most of the research in this area prior to COVID-19 focused on routine vaccine acceptance in childhood with limited studies in adults. Hence COVID-19 vaccine acceptance issues have showcased many gaps in our knowledge and in the science of vaccine acceptance in adults. The research needs concerning vaccine acceptance have been gathered under data gaps, education, equity, and lessons learned.
5.1. Data gaps
Many of these data gaps arise because there is a dearth of research in adult vaccines and acceptance, in vaccine acceptance in specific areas and subgroups in Canada, and (or) in COVID-19 vaccine acceptance and strategies for increasing acceptance.
What are the relationships between vaccine acceptance and social determinants of health across Canada?
What are specific concerns/objections to vaccine acceptance and how do these vary by age, subgroup, and context of COVID-19 disease in area?
Which strategies have been most effective for which subgroups? Contexts? Ages?
How should front-line workers and essential workers be defined in a pandemic—on what evidence? What factors should be in the risk analysis? Is the precautionary principle still valid and helpful for addressing extremely rare vaccine events during a pandemic? What is the evidence, and the analysis?
What is the goal of the COVID-19 vaccine program (control versus prevention of serious disease (hospitalization and death)) and how does this affect vaccine acceptance?
How can silos of information and advice from different experts be made more coherent?
What strategies are most effective in combating the infodemic and its impact on vaccine acceptance? How does this vary by subgroup, by age, by context of COVID-19 disease?
Have patient health information gaps hindered vaccine rollout planning and acceptance? How can these gaps best be adjusted to prevent problems with future vaccines?
5.2. Education
What are the most effective strategies for educating health care workers, the general public, equity deserving groups, children and adolescents, and communities about COVID-19 vaccines? How does this affect acceptance and resilience?
5.3. Equity
What are the best strategies by equity subgroup and by context for optimizing vaccine acceptance?
What are the most and least effective strategies for community engagement that lead to increased vaccine acceptance?
5.4. Lessons learned on COVID-19 vaccine acceptance
What are the lessons learned to date on vaccine acceptance? What changes are needed now to increase acceptance? How can these lessons be applied to routine immunization and future immunization programs?
What strategies can increase coherence for immunization across Canada, decrease confusion for the public and increase vaccine acceptance?
What lessons from COVID-19 vaccine programs and vaccine acceptance can be applied to the to 2020 World Health Assembly accepted Immunization Agenda 2030 (WHO 2021e) that focuses on immunization across the life course globally?
6. Recommendations
As this overview has noted, COVID-19 vaccine acceptance is complex with many factors influencing the outcomes of COVID-19 vaccination decisions such as knowledge, attitudes and beliefs; social networks; the communication environment; the rate of COVID-19 in a community (i.e., the context); cultural and religious influences; and the organization of health and community services and policies. Carefully designed interventions that are evidence based and tailored to community needs (MacQueen et al. 2001) and concerns are needed to engage and empower people to make informed choices about COVID-19 vaccines, to build trust in health authorities and those delivering vaccines and to promote acceptance.
Following their deliberations on this complex issue the RSC COVID-19 Vaccine Acceptance Working Group proposes the following recommendations for each of the four categories noted in Fig. 1. category for responsibility. There are 18 pressing recommendations requiring immediate attention, 8 rapid recommendations to be addressed in the next 3 to 6 months, and the 17 longer-term ones to be addressed within the next year.
| Pressing | Rapid | Longer Term |
As these recommendations are inter-related, the more traditional siloed approaches to vaccine acceptance will not be effective. To optimize outcomes it is essential that people and communities (MacQueen et al. 2001), health care workers, health care systems and public health programs, and Federal/Provincial/Territorial/Indigenous health programs are all engaged to ensure co-development and broad ownership.
People and communities: responsibilities
People and communities must work with the other partners to actively support COVID-19 vaccine acceptance. We, therefore, recommend:
| 1. | That COVID-19 vaccine programs are tailored through active engagement and co-creation by the community to meet local needs, incorporating the best evidence. | |
| 2. | That each local programme foster development of immunization ambassadors (such as religious leaders, community leaders) who will work with subgroups in the community to increase COVID-19 vaccine acceptance. | |
| 3. | That individuals and communities advocate for the immunization needs of underserved communities being prioritized. | |
| 4. | That paid time off be provided to all workers to facilitate COVID-19 immunization. | |
| 5. | That access to vaccination be facilitated through mobile clinics, transportation to vaccination sites and help provided for booking appointments. | |
| 6. | That education initiatives under a National Immunization Framework be co-developed with communities including equity deserving groups. |
Health care workers (regulated professionals and those integral to health care delivery)
Health care workers have a shared responsibility to actively support COVID-19 vaccine acceptance themselves, and within their communities. We, therefore, recommended:
| 1. | That all health care workers have access to education about COVID-19 disease, COVID-19 vaccines and immunization best practices that have been co-developed and tailored to fit their needs. | |
| 2. | That all health care workers involved in immunization programs be properly trained in vaccine acceptance, immunization pain mitigation and immunization stress related responses. This includes the CARD system (Section 4.1) and using appropriate words (Table 6) and other factors that will ensure a more positive immunization experience, thereby fostering vaccine acceptance. | |
| 3. | That health care workers support each other by rapidly getting the COVID-19 vaccine and becoming immunization ambassadors. | |
| 4. | That health care workers support each other through the uptake and use of twice-weekly briefing notes/updates (see Federal/Provincial/Territorial/Indigenous Responsibilities below) on current COVID-19 disease and vaccine issues to expediate quality responses to patient queries. |
Health care system and public health: responsibilities
The health care systems and public health programs have a shared responsibility to work collaboratively with other partners that include health care workers, communities, and Federal, Provincial, Territorial, and Indigenous governments, to actively support COVID-19 vaccine acceptance across their jurisdictions. We, therefore, recommend:
| 1. | That the health care system and public health COVID-19 vaccine programs support active listening in diverse communities for COVID-19 disease and vaccine acceptance and access issues. | |
| 2. | That vaccine acceptance issues among health care workers be addressed using evidence-based strategies and that this is continuous quality improvement in the programs. | |
| 3. | That real time assessment of the progress on vaccination uptake in populations and diverse subgroups be done and program adjustments made to fill any existing gaps. | |
| 4. | That COVID-19 immunization programs implement best infection control practices and best pain and fear mitigation practices (i.e., CARD system; Section 4.1). | |
| 5. | That health care systems and public health programs support twice-weekly evidence based briefing notes/updates (see Federal/Provincial/Territorial/Indigenous Responsibilities below). | |
| 6. | That the COVID-19 vaccine program optimize data collection systems (see Federal/Provincial/Territorial/Indigenous Responsibilities below) so that they are user friendly for health care workers, for those doing health planning, and for the public. | |
| 7. | That COVID-19 vaccine programs implement appropriate models that strengthen preventive care within the health system (see also Federal/Provincial/Territorial/Indigenous Responsibilities below) even beyond the pandemic. | |
| 8. | That health care systems and public health programs foster and support COVID-19 vaccine and more general immunization education. | |
| 9. | That health care systems and public health programs use COVID-19 vaccine experiences, and lessons learned, to strengthen routine immunization programs. |
Federal/provincial/territorial/Indigenous responsibilities
There Federal, Provincial, Territorial, and Indigenous governments have a shared responsibility to work collaboratively with other partners including communities, health care workers, the health care systems, and public health programs and each other to actively support COVID-19 vaccine acceptance across the country. We, therefore, recommend:
| 1. | That the Federal, Provincial, Territorial, and Indigenous governments ensure immunization equity for both COVID-19 vaccines and all other ones recommended by NACI. | |
| 2. | That all jurisdictions support acceptance of COVID-19 vaccines and other vaccines across communities through extensive public engagement with communities. | |
| 3. | That, if not covered by the employer, the federal government provide/cover the salary when an individual takes off when to receive a COVID-19 vaccine. | |
| 4. | That all jurisdictions develop a strategy to provide evidence based twice-weekly briefing notes for health system and public health programs, health care workers and the media. | |
| 5. | That all jurisdictions recognize the importance of clear, concise, country-wide public communication about COVID-19 disease and vaccines. This includes acknowledging and explaining why things may change in light of new knowledge. | |
| 6. | That coherence and transparency in communication be fostered across all levels of government and public health to support trust and vaccine acceptance using language that is culturally and community appropriate. It should be made clear that messages/advice are based on the best science/evidence available. | |
| 7. | That all jurisdictions support the removal of intellectual property protections for manufacturers that interfere with human rights for equitable access to health care, including vaccines. | |
| 8. | That Federal, Provincial, Territorial, and Indigenous governments work to ensure that all aspects of all parts of the vaccination process, from approval to the vaccination programmes, adhere to fundamentals that engender the development of trust (Table 5). | |
| 9. | That all jurisdictions recognize immunization as a legally enforceable right by publicly recommending vaccinations in public health or equivalent statutes and remove barriers that inhibit equitable access. | |
| 10. | That all jurisdictions put laws in place that support the development and implementation of a National Immunization Framework that includes equitable access to vaccines and immunization education for citizens of all ages, as well as support for immunization research. | |
| 11. | That government departments, including departments of Health and Education, work together to optimize immunization acceptance strategies and integrate education about immunization (e.g., IBoost, CARD system). | |
| 12. | That all jurisdictions use the experiences gained during the COVID pandemic to strengthen preventive care country wide. | |
| 13. | That the Federal/Provincial/Territorial/Indigenous governments aggressively support upgrading electronic health information systems across country to ensure they are all patient centred and fully integrated. | |
| 14. | That the Federal/Provincial/Territorial/Indigenous jurisdictions review the risks of corporatization of immunization. | |
| 15. | That the Federal/Provincial/Territorial/Indigenous governments enhance scientific expertise and infrastructure within agencies and programs to better support all programs, including those relating to vaccines. | |
| 16. | That lessons learned from the COVID-19 immunization program be applied to improve all immunization programs at all levels of government. | |
| 17. | That the Federal and Provincial/Territorial governments agree upon, and statutorily entrench, a common Canadian age of majority. | |
| 18. | That jurisdictions implement the no-fault Vaccine Injury Support Program. |
Acknowledgements
The authors would like to formally acknowledge the health care workers, program managers, peers and members of the public who have generously provided their input in the creation of this Policy Briefing with special acknowledgement of the contributions from: Upton Allen, University of Toronto; Melissa Andrew, Dalhousie University; Benjamin L. Berger, York University; Andrew Bond, Inner City Health Associates in Toronto; Paul Bramadat, University of Victoria; Terence Flynn, McMaster University; Monty Ghosh, University of Alberta; Devon Greyson, University of Massachusetts, Amherst; Jeff Karabanow, Dalhousie University; Aaron Orkin Inner City Health Associates in Toronto; Karina Top, Dalhousie University; and Jeff Turnbull, University of Ottawa. We would also like to thank the peer-review monitor Tom Marrie, FRSC Dalhousie University, and Laryssa Laurignano and Michael Boivin who helped copy edit the report.
References
Abdul-Mageed M. 2021. Negotiating the pandemic twitterverse. [online]: Available from rsc-src.ca/en/voices/negotiating-pandemic-twitterverse.
Alberta Queen’s Printer. 2009. Protection for Persons in Care Act.
Anderson RM, Vegvari C, Truscott J, and Collyer BS. 2020. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet, 396(10263): 1614–1616.
Atwell K, and Smith DT. 2018. Hearts, minds, nudges and shoves: (how) can we mobilise communities for vaccination in a markitised world? Vaccine, 36: 6506–6508.
Bad News. 2021. [online]: Available from getbadnews.com/#intro.
Baggett TP, and Gaeta JM. 2021. COVID-19 and homelessness: when crises intersect. Lancet Public Health, 6: e193–e194.
Balinska MA. 2009. Hepatitis B vaccination and French Society ten years after the suspension of the vaccination campaign: how should we raise infant immunization coverage rates? Journal of Clinical Virology, 46(3): 202–205.
Bedford H, Attwell K, Danchin M, Marshall H, Corben P, and Leask J. 2018. Vaccine hesitancy, refusal and access barriers: the need for clarity in terminology. Vaccine, 36(44): 6556–6558.
Betsch C, Renkewitz F, Betsch T, and Ulshofer C. 2010. The influence of vaccine-critical websites on perceiving vaccination risks. Journal of Health Psychology, 15: 446–455.
Betsch C, Schmid P, Heinemeier D, Korn L, Holtmann C, and Bohm R. 2018. Beyond confidence: Development of a measure assessing the 5C psychological antecedents of vaccination. PLoS One, 13(12): e0208601.
Bettinger JA, Halperin SA, Vaudry W, Law BJ, and Scheifele DW. 2014. Canadian IMPACT members. The Canadian Immunization Monitoring Program, ACTive (IMPACT): Active surveillance for vaccine adverse events and vaccine-preventable diseases. Canada Communicable Disease Report, 40(Suppl 3): 41–44.
Blackstock C. 2016. Toward the full and proper implementation of Jordan’s Principle: An elusive goal to date. Paediatrics & Child Health, 21(5): 245–246.
Boyleston AW. 2018. The myth of the milkmaid. NEJM, 378: 414–415. nejm.org/doi/full/10.1056/NEJMp1715349.
Bramadat P, Guay M, Bettinger JA, and Roy R, eds. 2017. Public health in the age of anxiety: religious and cultural roots of vaccine hesitancy in Canada. University of Toronto Press.
Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, and Weinstein ND. 2007. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychology, 26(2): 136–145.
Brewer NT, Chapman GB, Rothman AJ, Leask J, and Kempe A. 2017. Increasing vaccination: Putting psychological science into action. Psychological Science in the Public Interest: A Journal of the American Psychological Society, 18(3): 149–207.
Browne M. 2018. Epistemic divides and ontological confusions: the psychology of vaccine skepticism. Human Vaccines and Immunotherapeutics, 14(10): 2540–2542.
Burki T. 2020. The online anti-vaccine movement in the age of COVID-19. The Lancet Digital Health, 2(10): e504–e505.
Camillus J. 2008. Strategy as a wicked problem. Harvard Business Review, 86: 98–106.
CanLII. 1985. Constitution Act, 1982, Part I, being Schedule B to the Canada Act 1982, 1982, c. 11 (U.K.), in force on 17 April 1982.
CanLII. 1990. Age of Majority Act, RSNS 1989, c. 4.
CanLII. 2010. Personal Directives Act, SNS 2008, c. 8.
CanLII. 2017. Protection for Persons in Care Act, SNS 2004, c 33.
CanLII. 2019. Adult Capacity and Decision-making Act, SNS 2017, c. 4.
CanLII. 2020. Children and Family Services Act, SNS 1990, c. 5.
CanLII. 2021. Protection for persons in care regulations, NS Reg 364/2007 [online]: Available from canlii.ca/t/543nj.
Caulfield T. 2021. Science and the uncertainty dance [online]. Available from: rsc-src.ca/en/voices/science-and-uncertainty-dance.
Cavalluzzo. 2015. Sault Area Hospital & Ontario Nurses’ Association, [2015] OLAA No. 339 (Arb.); St. Michael’s Hospital v Ontario Nurses’ Association, 2018 CanLII 82519 (ON LA).
CBC. 2020c. The WE Charity controversy explained [online]: Available from cbc.ca/news/canada/we-charity-student-grant-justin-trudeau-testimony-1.5666676.
CBC. 2021a. Bishops dial back advice to Canadian Catholics about choosing alternatives to AstraZeneca vaccine [online]: Available from cbc.ca/news/politics/catholic-bishops-astra-zeneca-vaccine-1.5945928.
CBC. 2021b. Nearly 200,000 Ontarians aged 80 and older have not signed up for a COVID-19 vaccination [online]: Available from cbc.ca/news/canada/toronto/covid-19-vaccine-ontario-booking-appointments-1.5958792.
CDC. 2021a. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine — United States, December 14–23, 2020 [online]. Available from: cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm.
CDC. 2021b. Hierarchy of controls [online]: Available from cdc.gov/niosh/topics/hierarchy/default.html.
CDC. 2021c. Interim guidance for routine and influenza immunization services during the COVID-19 pandemic [online]: Available from cdc.gov/vaccines/pandemic-guidance/index.html?deliveryName=USCDC_7_3-DM34944.
CDC. 2021d. Joint CDC and FDA statement on Johnson & Johnson COVID-19 vaccine [online]: Available from cdc.gov/media/releases/2021/s0413-JJ-vaccine.html.
CDC. 2021e. Possible side effects after getting a COVID-19 vaccine [online]: Available from cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html.
CDC. 2021f. Some health-care workers still hesitant to get COVID-19 vaccine [online]. Available from: cbc.ca/radio/whitecoat/some-health-care-workers-still-hesitant-to-get-covid-19-vaccine-1.5872004.
Chapin-Bardales J, Gee J, and Myers T. 2021. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA.
Chapman A. 2009. Towards an Understanding of the right to enjoy the benefits of scientific progress and its applications. Journal of Human Rights, 8: 1–36.
Chen J, Vullikanti A, Hoops S, Mortveit H, Lewis B, and Venkatramanan S, et al. 2020. Medical costs of keeping the US economy open during COVID-19 [online]: Available from nature.com/articles/s41598-020-75280-6.
Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, and Schünemann HJ. 2020. COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet, 395(10242): 1973–1987.
CIRN. 2021a. Canadian national vaccine safety network (CANVAS) [online]: Available from cirnetwork.ca/network/national-ambulatory-network/.
CIRN. 2021b. Serious outcomes surveillance (SOS) network [online]: Available from cirnetwork.ca/network/serious-outcomes/.
Coalition to Strengthen the Rights of Older People. 2009. Strengthening Older People’s Rights: Towards a UN Convention [online]: Available from social.un.org/ageing-working-group/documents/Coalition%20to%20Strengthen%20the%20Rights%20of%20Older%20People.pdf.
Collange F, Verger P, Launay O, and Pulcini C. 2016. Knowledge, attitudes, beliefs and behaviors of general practitioners/family physicians toward their own vaccination: a systematic review. Human Vaccines & Immunotherapeutics, 12(5): 1282–1292.
Collier R. 2017. Reports of coerced sterilization of indigenous women in Canada mirrors shameful past. Canadian Medical Association Journal, 189(33): E1080–E1081.
Comeau J. 2021. Keeping everyone safe: infection prevention & control in COVID-19 vaccine clinics [online]: Available from rsc-src.ca/en/voices/keeping-everyone-safe-infection-prevention-control-in-covid-19-vaccine-clinics.
Commeau J, and Top K. 2021. Anaphylaxis and COVID-19 vaccines. [online]: Available from rsc-src.ca/en/voices/anaphylaxis-and-covid-19-vaccines.
Corcoran B, Clarke A, and Barrett T. 2018. Rapid response to HPV vaccination crisis in Ireland. Lancet, 391(10135): 2103.
Costa-Pinto JC, Willaby HW, Leask J, Hoq M, Schuster T, Ghazarian A, et al. 2018. Parental immunisation needs and attitudes survey in paediatric hospital clinics and community maternal and child health centres in Melbourne, Australia. Journal of Paediatrics and Child Health, 54: 522–529.
Coughlan K. 2018. Medical decision-making in paediatrics: infancy to adolescence. Paediatric & Child Health, 23: 138–146.
Crawshaw J, Castillo G, Grimshaw JM, and Presseau J. 2021. Factors affecting healthcare worker COVID-19 vaccination acceptance and uptake: a living behavioural science evidence synthesis (v1.0, March 31st, 2021). Ottawa Hospital Research Institute, Ottawa [online]: Available from drive.google.com/file/d/10W5XDumdcwta5YMLWrXMb5UI3mHhKISa/view.
Desveaux L, Savage RD, Kithulegoda N, Thai K, Stall NM, and Ivers NM. 2021. Beliefs associated with intentions of non-physician healthcare workers to receive the COVID-19 vaccine in Ontario, Canada [online]: Available from medrxiv.org/content/10.1101/2021.02.19.21251936v1.
Digital Public Square. 2021. Healthy communities enabled by good technology [online]: Available from digitalpublicsquare.org/.
Dube E. 2016. MacDonald NE Managing the risks of vaccine hesitancy and refusals. Lancet Infectious Diseases, 16(5): 518–519.
Dube E, and MacDonald NE 2020. How can a global pandemic affect vaccine hesitancy? 10, 2020. Expert Review of Vaccines, 19: 899–901.
Dubé E, Bettinger J, Fisher WA, Naus M, Mahmud SM, and Hilderman T. 2016. Improving vaccination rates: vaccine acceptance, hesitancy and refusal in Canada: challenges and potential approaches. Canada Communicable Disease Report, 42, 246–251.
Dubé E, Gagnon D, MacDonald N, Bocquier A, Peretti-Watel P, and Verger P. 2018. Underlying factors impacting vaccine hesitancy in high income countries: a review of qualitative studies. Expert Review of Vaccines, 17(11): 989–1004.
Dube E, Gagnon D, and Vivion M. 2020. Optimizing communication material to address vaccine hesitancy. CCDR, 46 (2/3): 48–45.
Dube E, Ward JK, Verger P, and MacDonald NE. 2021. Vaccine hesitancy, acceptance and anti-vaccination: trends and future prospects for public health. Ann Review Public Health, 42: 175–191.
Dudley MZ, Privor-Dumm L, Dube E, and MacDonald NE. 2020. Words matter: Vaccine hesitancy, vaccine demand, vaccine confidence, herd immunity and mandatory vaccination. Vaccine, 38: 709–711.
Dzieciolowska S, Hamel D, and Gadio S, et al. 2021. Covid-19 vaccine acceptance, hesitancy and refusal among Canadian healthcare workers: a multicenter survey. American Journal Infection Control, S0196-6553(21)00274-1.
EMA. 2021a. AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets [online]: Available from ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood.
EMA. 2021b. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 6–9 April 2021 [online]: Available from ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-6-9-april-2021.
EMA. 2021c. Signal assessment report on embolic and thrombotic events (SMQ) with COVID-19 Vaccine (ChAdOx1-S [recombinant]) – Vaxzevria (previously COVID-19 Vaccine AstraZeneca) (Other viral vaccines) [online]: Available from ema.europa.eu/en/documents/prac-recommendation/signal-assessment-report-embolic-thrombotic-events-smq-covid-19-vaccine-chadox1-s-recombinant_en.pdf.
Faraday F, Fudge J, Hanley J, McLaughlin J, Ramsaroop C, Tungohan E, et al. 2021. Migrant workers need priority access to the COVID-19 vaccine [online]: Available from rsc-src.ca/en/voices/migrant-workers-need-priority-access-to-covid-19-vaccine.
FDA. 2021. FDA and CDC lift recommended pause on Johnson & Johnson (Janssen) COVID-19 vaccine use following thorough safety review [online]: Available from fda.gov/news-events/press-announcements/fda-and-cdc-lift-recommended-pause-johnson-johnson-janssen-covid-19-vaccine-use-following-thorough.
Fedson DS. 1998. Measuring protection: efficacy versus effectiveness. Journal of Biological Standardization, 95: 195–201.
Fink AL, and Klein SL. 2015. Sex and gender impact immune responses to vaccines among the elderly. Physiology, 30(6): 408–416.
Flood C, MacDonnell V, Thomas B, and Wilson K. 2020. Reconciling civil liberties and public health in the response to COVID-19. [online]: Available from rsc-src.ca/en/research-and-reports/covid-19-policy-briefing/reconciling-civil-liberties-and-public-health-in.
Flood C, and Thomas B. 2021. The case for a COVID19 vaccination certificate [online]: Available from rsc-src.ca/en/voices/case-for-covid-19-vaccination-certificate.
Frank K, and Arim R. 2020. Canadians’ willingness to get a COVID-19 vaccine: Groupe differences and reasons for vaccine hesitancy [online]: Available from www150.statcan.gc.ca/n1/pub/45-28-0001/2020001/article/00073-eng.htm.
Freemster K. 2013. Overview: Special focus vaccine acceptance. Human Vaccines & Immunotherapeutics, 9: 1752–1754.
Gagneur A. 2020. Motivational interviewing: a powerful tool to address vaccine hesitancy. Canada Communicable Disease Report, 46(4): 93–97.
GAVI. 2021a. Can vaccine clinical trials be sped up safely for COVID-19? [online]: Available from gavi.org/vaccineswork/how-covid-19-leading-innovation-clinical-trials.
GAVI. 2021b. What is Covax? [online]: Available from gavi.org/covax-facility.
Ghosh M, Trunbull J, MacDonald N, Bond A, and Orkin A. 2021a. Time to innovate for vulnerable people and vulnerable points in society: COVID immunization for people experiencing homelessness [online]: Available from rsc-src.ca/en/voices/time-to-innovate-for-vulnerable-people-and-vulnerable-points-in-society-covid-immunization.
Ghosh M, Trunbull J, MacDonald N, Bond A, and Orkin A. 2021b Key strategies to vaccinate homeless populations. Royal Society of Canada [online]: Available from rsc-src.ca/en/voices/key-strategies-to-vaccinating-homeless-populations.
Giambi C, Fabiani M, D’Ancona F, Ferrara L, Fiacchini D, Gallo T, et al. 2018. Parental vaccine hesitancy in Italy—Results from a national survey. Vaccine, 36: 779–787.
Glauser W. 2019. Teens, vaccines and the age of consent, CMAJ News [online]: Available from cmajnews.com/2019/03/07/teens-vaccines-and-the-age-of-consent-cmaj-109-5730/.
Go Viral. 2021. [online]: Available from goviralgame.com/en/share/.
GOV UK. 2021. New data show vaccines reduce severe COVID-19 in older adults. New data show both Pfizer-BioNTech and Oxford-AstraZeneca vaccines significantly reduce severe COVID-19 in older adults [online]: Available from gov.uk/government/news/new-data-show-vaccines-reduce-severe-covid-19-in-older-adults.
Government of Canada. 1982. Constitution Act, 1982, Schedule B to the Canada Act 1982 (UK), 1982, c 11, s 35.
Government of Canada. 2017. National Immunization Strategy: Objectives 2016–2021 [online]: Available from canada.ca/en/public-health/services/publications/healthy-living/national-immunization-strategy-objectives-2016-2021.html.
Government of Canada. 2019a. Coronavirus disease (COVID-19): Prevention and risks [online]: Available from canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/5wprevention-risks.html?utm_campaign=hc-sc-coronavirus2021-ao-20-21&utm_medium=sem&utm_source=ggl&utm_content=ad-text-en&utm_term=%2Bcovid%20%2B19%20%2Bcanada&adv=2021-0005&id_campaign=10020125402&id_source=107800103024&id_content=434525470206&gclid=EAIaIQobChMI9Ju-iqTE7wIVDotaBR0NVgKbEAAYASAAEgJdZ_D_BwE&gclsrc=aw.ds#p.
Government of Canada. 2019b. COVID-19 for health professionals: Infection prevention and control [online]: Available from canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/infection-prevention-control.html.
Government of Canada. 2020. Self-Government [online]: Available from rcaanc-cirnac.gc.ca/eng/1100100032275/1529354547314.
Government of Canada. 2021a. Archive 6: National Advisory Committee on Immunization (NACI): Summary of updated vaccine statement of March 16, 2021 [online]: Available from canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/summary-updated-statement-16-march-2021.html.
Government of Canada. 2021b. Archived 7: National Advisory Committee on Immunization: Summary of updated vaccine statement of April 23, 2021 [online]: Available from canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/summary-updated-statement-april-23-2021.html.
Government of Canada. 2021c. COVID-19 daily epidemiology update [online]: Available from health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html.
Government of Canada. 2021d. Drug and vaccine authorizations for COVID-19: list of applications received [online]: Available from canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/authorization/applications.html.
Government of Canada. 2021e. Epidemiological summary of COVID-19 cases in First Nations communities [online]: Available from sac-isc.gc.ca/eng/1589895506010/1589895527965.
Government of Canada. 2021f. Preparations of COVID-19 vaccines authorized and available for use in Canada [online]: Available from canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html#t2.
Government of Canada. 2021g. Recommendations on the use of COVID-19 vaccines [online]. Available from canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html#a7.
Government of Canada. 2021h. Reported side effects following COVID-19 vaccination in Canada [online]: Available from health-infobase.canada.ca/covid-19/vaccine-safety/.
Government of Canada. 2021i. USA advisory committee on immunization practices. emerging SARS-CoV-2 variants: considerations for vaccines. CDR Heakth Scobie March 1 2021 [online]: Available from cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/28-03-01/07-COVID-Scobie.pdf.
Government of Canada. 2021j. Vaccines for COVID-19: authorized vaccines [online]: Available from canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines.html.
Grabenstein JD. 2013. What the World’s religions teach, applied to vaccines and immune globulins. Vaccine, 31: 2011–2023.
Graham J. 2021. What measures ensure safe vaccines? On [online]: Available from canvax.ca/covid-19-vaccine-questions-and-answers-healthcare-providers.
Graham J, and Manca T. 2021. Return to a new normal: Royal society members identify key societal challenges posed by COVID-19 [online]: Available from rsc-src.ca/en/voices/return-to-new-normal-royal-society-members-identify-key-societal-challenges-posed-by-covid-19.
Graham JE, Lees S, Le Marcis F, Faye S, Ronse M, Lorway R, et al. 2018 Prepared for the “unexpected”? Lessons from the 2014–16 Ebola epidemic in West Africa on integrating emergent theory designs into outbreak response. BMJ Global Health, 3: e000990.
Graven M, Allen P, Smith I, and MacDonald NE. 2013. Decline in mortality with the Belize integrated patient-centred country wide health information system (BHIS) with embedded program management. International Journal of Medical Informatics, 82(10): 954–963.
Greenwood M, and MacDonald NE. 2021. Vaccine mistrust: a legacy of colonialism [online]: Available from rsc-src.ca/en/voices/vaccine-mistrust-legacy-colonialism.
Habersaat K, and Jackson C. 2020. Understanding vaccine acceptance and demand—and ways to increase them. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz, 63: 32–39.
Hardt K, Schmidt-Ott R, Glismann S, Adegbola RA, and Meurice FP. 2013. Sustaining vaccine confidence in the 21st century. Vaccines, 1(3): 204–224.
Harmon S. 2021a. Characteristics of a fair vaccine injury compensation program for Canada [online]: Available from rsc-src.ca/en/voices/characteristics-fair-vaccine-injury-compensation-program-for-canada.
Harmon S. 2021b.Vaccine rollouts and the role of employers [online]: Available from rsc-src.ca/en/voices/vaccine-rollouts-and-role-employers.
Harmon SH, MacDonald NE, Petite W, and Graham JE. 2021. Invigorating public health in canada: new governance for immunization in the post-COVID-19 era. Royal Society of Canada.
Harmon SHE, and MacDonald NE. 2020. COVID-19 vaccines and serious adverse events following immunization: action needed! [online]: Available from rsc-src.ca/en/voices/covid-19-vaccines-and-serious-adverse-events-following-immunization-action-needed.
Hasnan S, and Tan NC 2021. Multi-domain narrative review of vaccine hesitancy in childhood. Vaccine.
HEABC. 2019. Changes to provincial influenza prevention policy [online]: Available from phsa.ca/staff-resources-site/Documents/Occupational%20Health%20Documents/FAQ%20Amended%20provincial%20policy%202019.pdf.
Henderson W and Albers G. 2020. Indigenous Self-Government in Canada. Canadian Encyclopedia [online]: Available from thecanadianencyclopedia.ca/en/article/aboriginal-self-government.
Herder M, Graham JE, and Gold R. 2021. The public science behind the ‘Merck’ Ebola vaccine [online]: Available from statnews.com/2020/01/16/public-science-behind-merck-ebola-vaccine/.
Herder M, and Graham JE. 2021. Opinion: Herder and Graham: Canadians need and deserve transparency on COVID-19 vaccines. Ottawa Citizen Sept 15.
Hickler B, MacDonald NE, Senouci K, and Schuh HB. 2017. Efforts to monitor Global progress on individual and community demand for immunization: Development of definitions and indicators for the Global Vaccine Action Plan Strategic Objective 2. Vaccine, 35(28): 3515–3519.
Hyams C, Marlow R, Maseko Z, King J, Ward L, Fox K, et al. 2021. Assessing the effectiveness of BNT162b2 and ChAdOx1nCoV-19 COVID-19 vaccination in prevention of hospitalizations in elderly and frail adults: a single centre test negative case-control study. The Lancet [online]: Available from papers.ssrn.com/sol3/papers.cfm?abstract_id=3796835.
I Boost Immunity. 2021. Earn a vaccine for someone in need by answering a quiz! [online]: Available from iboostimmunity.com/.
ILO. 2014. Age of Majority and Accountability Act, RSO 1990, c. A.7.
Informal Advocacy Group. 2020. Time for a UN convention on the rights of older persons [online]: Available from age-platform.eu/sites/default/files/Discussion_Paper_COVID-19_Time_for_a_UN_Convention-Aug2020.pdf.
INSPQ. 2021. Available from: inspq.qc.ca/covid-19/sondages-attitudes-comportements-quebecois/9-mars-2021.
IPSOS. 2020. Many Canadians aren’t in a hurry to receive COVID-19 vaccine [online]: Available from ipsos.com/en-ca/news-polls/many-canadians-arent-in-a-hurry-to-receive-covid-19-vaccine.
Its Contagious Game. 2021. [online]: Available from itscontagiousgame.com/.
Its Infectious. 2021. [online]: Available from itsinfectious.com/.
Johnson NF, Velásquez N, Restrepo NJ, Leahy R, Gabriel N, El Oud S, et al. 2020. The online competition between pro- and anti-vaccination views. Nature, 582(7811): 230–233.
Karabanow J, Hopkins S, Kisely S, Parker J, Hughes J, Gahagan J, and Campbell LA. 2007. Can you be healthy on the street?: Exploring the health experiences of Halifax street youth. Canadian Journal of Urban Research, 16(1): 12–32.
Karlsson LC, Lewandowsky S, Antfolk J, Salo P, Lindfelt, M, Oksanen T, et al. 2019. The association between vaccination confidence, vaccination behavior, and willingness to recommend vaccines among Finnish healthcare workers. PLoS ONE, 14(10): e0224330.
Kata A. 2012. Anti-vaccine activists, Web 2.0, and the postmodern paradigm – an overview of tactics and tropes used online by the anti-vaccination movement. Vaccine, 30: 3778–3789.
Kauh TJ, Read JG, Scheitler AJ. 2021. The critical role of racial/ethnic data disaggregation for health equity. Population Research and Policy Review, 1–7.
Kennedy J. 2019. Populist politics and vaccine hesitancy in Western Europe: an analysis of national-level data. European Journal of Public Health, 29(3): 512–516.
KHN. 2020. They pledged to donate rights to their COVID vaccine, then sold them to pharma [online]: Available from khn.org/news/rather-than-give-away-its-covid-vaccine-oxford-makes-a-deal-with-drugmaker/.
Kids Boost Immunity. 2021a. Free Science, Social Studies and Health lessons developed by teachers to inspire digital-age students in support of UNICEF Canada! [online]: Available from kidsboostimmunity.com/.
Kids Boost Immunity. 2021b. [online]: Available from youtube.com/watch?v=UZLm_gna2go.
Know It or Not. 2021. [online]: Available from knowitornot.com/.
Knowledge Ecology International. 2021. COVID-19 contracts [online]: Available from keionline.org/covid-contracts.
Koop A. 2021. Putting the cost of COVID-19 in perspective [online]: Available from visualcapitalist.com/putting-the-cost-of-covid-19-in-perspective/.
Koppenjan J and Klijn E. 2004. Managing uncertainties in networks. Routledge, London.
Kurasawa F. 2020. #COVID19: Social media both a blessing and a curse during coronavirus pandemic. [online]: Available from rsc-src.ca/en/voices/covid19-social-media-both-blessing-and-curse-during-coronavirus-pandemic.
Lancet. 2021. Access to COVID-19 vaccines: looking beyond COVAX. Lancet, 397: 941.
Lancet Infectious Diseases. 2020. The COVID-19 infodemic. Lancet Infectious Diseases, 20(8):875.
Lane S, MacDonald NE, Marti M, and Dumolard L. 2018. Vaccine hesitancy around the globe: analysis of three years of WHO/UNICEF joint reporting form data-2015–2017. Vaccine, 36(26): 3861–3867.
Larson HJ, Cooper LZ, Eskola J, Katz SL, and Ratzan S. 2011. Addressing the vaccine confidence gap. Lancet, 378(9790): 526–535.
Leger. 2021. Leger’s North American Tracker – February 16, 2021 [online]: Available from leger360.com/surveys/legers-north-american-tracker-february-16-2021/.
Lewandowsky S, Cook J, Ecker UKH, Albarracín D, Amazeen MA, Kendeou P, et al. 2020. The Debunking Handbook 2020 [online]: Available from climatechangecommunication.org/wp-content/uploads/2020/10/DebunkingHandbook2020.pdf.
Lewandowsky S, Cook J, Schmid P, Holford DL, Finn A, Leask J, et al. 2021. The COVID-19 vaccine communication handbook. A practical guide for improving vaccine communication and fighting misinformation [online]: Available from repository.essex.ac.uk/29625/1/The%20COVID-19%20Vaccine%20Communication%20Handbook.pdf.
Lin C, Tu P, and Beitsch LM. 2020. Confidence and receptivity for COVID-19 vaccines: a rapid systematic review. Vaccines, 9(1).
Ling J. 2021. Canada’s public health data meltdown [online]: Available from macleans.ca/news/canada/canadas-public-health-data-meltdown/?utm_source=nl&utm_medium=em&utm_campaign=mme_daily&sfi=cb2875a77ac4cec0219658e665f53d1a.
Little S. 2021. B.C. churches flout COVID-19 health restrictions, proceed with indoor Easter services [online]: Available from globalnews.ca/news/7738119/b-c-churches-flout-covid-19-health-restrictions-proceed-with-indoor-easter-services/.
Lowry M. 2021. New rapid expert consultation shares insights from social science on communicating COVID-19 vaccine efficacy, effectiveness, and equity [online]: Available from nationalacademies.org/news/2021/04/new-rapid-expert-consultation-shares-insights-from-social-science-on-communicating-covid-19-vaccine-efficacy-effectiveness-and-equity.
MacDonald NE. 2015. Vaccine hesitancy: definition, scope and determinants. Vaccine, 33(34): 4161–4164.
MacDonald NE. 2020. Fake news and science denier attacks on vaccines. What can you do? Canada Communicable Disease Report, 46(1112): 432–435.
MacDonald NE. 2021. COVID-19, public health and constructive journalism in Canada. Canadian Journal of Public Health, 112: 179–182.
MacDonald NE, and Bortolussi R. 2011. A harmonized immunization schedule for Canada: A call to action. Paediatric & Child Health, 16(1): 29–31.
MacDonald NE, Stanwick R, and Lynk A. 2014. Canada’s shameful history of nutrition research on residential school children: The need for strong medical ethics in Aboriginal health research. Paediatrics & Child Health, 19(2): 64.
MacDonald NE, and Law BJ. 2017. Canada’s eight-component vaccine safety system: a primer for health care workers. Paediatric & Child Health, 22(4): e13–e16.
MacDonald NE, Comeau J, Dubé E, Bucci L, and Graham JE. 2020. A public health timeline to prepare for COVID-19 vaccines in Canada. Canadian Journal of Public Health, 111(6): 945–952.
MacDonald NE, Harmon S, Dube E, Steenbeek A, Crowcroft N, Opel DJ, et al. 2018. Mandatory infant & childhood immunization: rationales, issues and knowledge gaps. Vaccine, 36(39): 5811–5818.
MacQueen KM, McLellan E, Metzger DS, Kegeles S, Strauss RP, Scotti R, et al. 2001. What is community? An evidence-based definition for participatory public health. American Journal of Public Health, 91(12): 1929–1938.
McDermott V.2021. More than 1,000 oilsands workers infected with COVID-19 since beginning of pandemic [online]: Available from edmontonjournal.com/business/energy/covid-oilsands-alberta.
McElhaney J, and Andrew M. 2021. Reducing barriers to vaccination: decision-making and access [online]: Available from rsc-src.ca/en/voices/reducing-barriers-to-vaccination-decision-making-and-access.
McLaren P. 2020. Religious nationalism and the coronavirus pandemic: soul-sucking evangelicals and branch covidians make America sick again. Postdigital Science and Education, 2, 700–721.
McMurtry CM. 2021. High needle fear and COVID-19 vaccines [online]: Available from rsc-src.ca/en/voices/high-needle-fear-and-covid-19-vaccines.
McMurtry MC, Taddio A, and MacDonald NE. 2021. COVID-19 vaccines, vaccine aceptance: words matter [online]: Available from rsc-src.ca/en/voices/covid-19-vaccines-vaccine-acceptance-words-matter.
McQuaig L. 2020. When Canada was a world leader in vaccine research and production [online]: Available from thestar.com/opinion/contributors/2020/11/30/when-canada-was-a-world-leader-in-vaccine-research-and-production.html.
Meghan McMurtry C. 2021. High needle fear and COVID-19 vaccines [online]: Available from rsc-src.ca/en/voices/high-needle-fear-and-covid-19-vaccines.
Mijovic H, Greyson D, Gemmell E, Trottier M-E, Vivion M, Graham JE, et al. 2020. Perinatal health care providers’ perceptions of facilitators and barriers influencing pertussis vaccination in pregnancy: a qualitative study. CMAJ Open, 8(2): E377–E382.
Ministry of Health. 2021. COVID-19: vaccine storage and handling guidance [online]: Available from health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/vaccine/vaccine_storage_handling_pfizer_moderna.pdf.
Morsink J. 1999. The universal declaration of human rights: origins, drafting and intent. Philadelphia U Press.
Morten CJ, Kapczynski A, Krumholz HM, and Ross JS. 2020. To help develop the safest, most effective coronavirus tests, treatments, and vaccines, ensure public access to clinical research data. Health Affairs.
Mosby I, and Swidrovich J. 2021. Medical experimentation and the roots of COVID-19 vaccine hesitancy among Indigenous Peoples in Canada. Canadian Medical Association Journal, 193: E381–E383.
Nader F, Kolahdooz F, and Sharma S. 2017. Assessing health care access and use among Indigenous peoples in Alberta: a systematic review. Journal of Health Care for the Poor and Underserved, 1286–1303.
Nguyen KH, Srivastav A, Razzaghi H, Williams W, Lindley MC, Jorgensen C, et al. 2021. COVID-19 vaccination intent, perceptions, and reasons for not vaccinating among groups prioritized for early vaccination — United States, September and December 2020 [online]: Available from cdc.gov/mmwr/volumes/70/wr/mm7006e3.htm.
Nguyen NH, Subhan FB, Williams K, and Chan CB. 2020. Barriers and mitigating strategies to healthcare access in indigenous communities of Canada: a narrative. Healthcare, 8(2): 112.
Nyblade L, Singh S, Ashburn K, Brady L, and Olenja J. 2011. “Once I begin to participate, people will run away from me”: Understanding stigma as a barrier to HIV vaccine research participation in Kenya. Vaccine, 29(48): 8924–8928.
Oguamanam C. 2021. Vaccine certificates should not come at expense of other COVID priorities [online]: Available from policyoptions.irpp.org/magazines/march-2021/vaccine-certificates-should-not-come-at-expense-of-other-covid-priorities/.
O’Neill O. 2014. Trust, trustworthiness, and accountability. In Capital failure: rebuilding trust in financial services. Edited by N Morris and D Vines. Oxford University Press, Oxford. pp. 172–192.
Ontario. 2021a. Health Care Consent Act, SO 1996, c. 2.
Ontario. 2021b. Substitute Decisions Act, SO 1992, c. 30.
Opel DJ, Heritage J, Taylor JA, Mangione-Smith R, Salas HS, Devere V, et al. 2013. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics, 132: 1037–1046.
Ortiz K, Nash J, Shea L, Oetzel J, Garoutte J, Sanchez-Youngman S, and Wallerstein N. 2021. Partnerships, processes, and outcomes: a health equity–focused scoping meta-review of community-engaged scholarship. Annual Review of Public Health, 41: 177–199.
O’Sullivan M. 2018. 2018 Update on the UN Convention on the Rights of Older Persons. O’Sullivan Estate Lawyers [online]: Available from osullivanlaw.com/blog/2018/07/2018-update-on-the-un-convention-on-the-rights-of-older-persons/?utm_source=mondaq&utm_medium=syndication&utm_campaign=linkedin-integration.
Pakes BN, and Taddio A. 2021. Wiping the alcohol swab away from COBID-19 vaccine program [online]: Available from rsc-src.ca/en/voices/wiping-alcohol-swab-away-from-covid-19-vaccine-program.
Peretti-Watel P, Larson HJ, Ward JK, Schulz WS, and Verger P. 2015. Vaccine hesitancy: clarifying a theoretical framework for an ambiguous notion. PLoS Currents, 7.
Pluviano S, Watt C, and Della Sala S. 2017. Misinformation lingers in memory: failure of three pro-vaccination strategies. PLoS ONE, 12(7): e0181640.
Poland CM, Matthews AKS, and Poland GA. 2021. Improving COVID-19 vaccine acceptance: Including insights from human. Vaccine, 39: 1547–1550.
Power T, Wilson D, Best O, Brockie T, Bearskin LB, Millender E, and Lowe J. 2020. COVID-19 and indigenous peoples: an imperative for action. Journal of Clinical Nursing, 29: 2737–2741.
Public Health Agency of Canada. 2020. Government of Canada announces pan-Canadian vaccine injury support program [online]: Available from canada.ca/en/public-health/news/2020/12/government-of-canada-announces-pan-canadian-vaccine-injury-support-program.html.
Public Health of Ontario. 2021. IPAC recommendations for use of personal protective equipment for care of individuals with suspect or confirmed COVID-19 [online]: Available from publichealthontario.ca/-/media/documents/ncov/updated-ipac-measures-covid-19.pdf?la=en.
Quinn SA, Jamison A, Freimuth VS, An J, Hancock GR, and Musa D. 2017. Exploring racial influences on flu vaccine attitudes and behavior: results of a national survey of White and African American adults. Vaccine, 35(8): 1167–1174.
Rauhala E. 2021. ‘’Moderna agreed to ‘equitable access’ for its coronavirus vaccine, but most of its doses are going to wealthy countries [online]: Available from washingtonpost.com/world/coronavirus-vaccine-access-poor-countries-moderna/2021/02/12/0586e532-6712-11eb-bf81-c618c88ed605_story.html?fbclid=IwAR1kxT08_FuAnfnVPLC2w1P_UQniL4R1bnOOtqDopaOhiKQy2EEnSrEvLlg.
Region of Peel. 2021. COVID-19 in Peel [online]: Available from peelregion.ca/coronavirus/case-status/.
Ritchie H, Ortiz-Ospina E, Beltekian D, Mathieu E, Hasell J, Macdonald B, et al.2021. Coronavirus (COVID-19) vaccinations [online]: Available from ourworldindata.org/covid-vaccinations.
Rittel H, and Webber M. 1973. Dilemmas in a general theory of planning. Policy Sciences, 4: 155–169.
Robertson G. 2021. ‘Without early warning you can’t have early response’: How Canada’s world-class pandemic alert system failed [online]: Available from theglobeandmail.com/canada/article-without-early-warning-you-cant-have-early-response-how-canadas/.
Roozenbeek J, and van der Linden S. 2019. Fake news game confers psychological resistance against online misinformation. Palgrave Communications, 5: 65.
Rosenbaum L. 2015. Communicating uncertainty-Ebola, public health and the scientific process. The New England Journal of Medicine, 372: 7–9.
RSC. 1985. c. H-6.
RSC. 2021. Correctional Services during and beyond COVID-19 [online]: Available from rsc-src.ca/en/research-and-reports/covid-19-policy-briefing/correctional-services-during-and-beyond-covid-19.
RSC-SRC. 2021. The vaccine will only work if enough people take it [online]: Available from rsc-src.ca/en/voices/vaccine-will-only-work-if-enough-people-take-it.
Ruger J. 2006. Ethics and governance of global health inequalities. Journal of Epidemiology and Community Health, 60: 998–1003.
Ryan M, Giles-Vernick T, and Graham JE. 2019. Technologies of trust in epidemic response: openness, reflexivity and accountability during the 2014–2016 Ebola outbreak in West Africa. BMJ Global Health, 4: e001272.
Ryerson University. 2021. [online]: Available from covid19misinfo.org/.
Scheufele DA, and Krause NM. 2019. Science audiences, misinformation, and fake news. Proceedings of the National Academy of Sciences of the United States of America, 116: 7662–7669.
Science Media Centre. 2021. Expert reaction to some European countries pausing the Oxford-Astrazeneca vaccine over fears of blood clotting [online]: Available from: sciencemediacentre.org/expert-reaction-to-some-european-countries-pausing-the-oxford-astrazeneca-vaccine/.
Shapiro GK, Guichon J, and Kelaher M. 2017. Canadian school-based HPV vaccine programs and policy considerations. Vaccine, 35(42): 5700–5707.
Somos C. 2021. A year later, Indigenous communities are fighting twin crises: COVID-19 and inequality. CTV News [online]: Available from ctvnews.ca/health/coronavirus/a-year-later-indigenous-communities-are-fighting-twin-crises-covid-19-and-inequality-1.5280843.
Statistics Canada. 2020a. COVID-19 mortality rates in Canada’s ethno-cultural neighbourhoods [online]: Available from www150.statcan.gc.ca/n1/pub/45-28-0001/2020001/article/00079-eng.htm.
Statistics Canada. 2020b. Majority of Canadians intend to get the COVID-19 vaccine, September 2020 [online]: Available from www150.statcan.gc.ca/n1/daily-quotidien/201217/dq201217c-eng.htm.
Steenbeek A, MacDonald N, Downie J, Appleton M, and Baylis F. 2012. Ill-informed consent? A content analysis of physical risk disclosure in school-based HPV vaccine programs. Public Health Nursing, 29(1): 71–79.
Strategic Advisory Group of Experts on Immunization. 2018. Global vaccine action plan assessment report 2018: [online]: Available from apps.who.int/iris/bitstream/handle/10665/276967/WHO-IVB-18.11-eng.pdf?ua=1.
Suppli CH, Hansen ND, Rasmussen M, Valentiner-Branth P, Krause TG, and Mølbak K. 2018. Decline in HPV-vaccination uptake in Denmark – the association between HPV-related media coverage and HPV-vaccination. BMC Public Health, 18(1): 1360.
Supreme Court of Canada. 1980. hopp v. Lepp, [1980] 2 SCR 192.
Supreme Court of Canada. 1989. Andrews v Law Society of British Columbia, [1989] 1 SCR 143.
Supreme Court of Canada. 1990. McKinney v. University of Guelph, [1990] 3 SCR 229.
Supreme Court of Canada. 1993. Ciarlariello v. Schacter, [1993] 2 SCR 119.
Supreme Court of Canada. 1999. British Columbia v. BCGSEU, [1999] 3 SCR 3.
Supreme Court of Canada. 2009. AC v. Manitoba (Director of Child and Family Services), [2009] 2 SCR 181.
Supreme Court of Canada. 2013. Cuthbertson v. Rasouli, [2013] 3 SCR 341.
Swire-Thompson B, Lazar D. 2021. Public health and online misinformation: challenges and recommendations. Annual Review of Public Health, 41: 433–451.
Taddio A. 2021. What cards will you play to improve your COVID-19 vaccination experience? [online]: Available from rsc-src.ca/en/voices/what-cards-will-you-play-to-improve-your-covid-19-vaccination-experience.
Taddio A, MacDonald NE. 2021. Building knowledge about immunization to promote good health [online]: Available from rsc-src.ca/en/voices/building-knowledge-about-immunization-to-promote-good-health.
Taddio A, Ipp M, Thivakaran S, Jamal A, Parikh C, Smart S, et al. 2012. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine, 30: 4807–4812.
Taddio A, McMurtry CM, Bucci LM, MacDonald N, Ilersich ANT, Ilersich ALT, et al. 2019. Overview of a knowledge translation (KT) project to improve the vaccination experience at school: the CARD™ System. Paediatrics & Child Health, 24(Suppl 1): S3–S18. :
Taddio A, Ilersich A, McMurtry CM, Bucci LM, and MacDonald NE. 2021. Managing pain and fear: Playing your CARDs to improve the vaccination experience. Canada Communicable Disease Report, 47(1): 87–91.
The Conversation. 2021. Christian nationalism is a barrier to mass vaccination against COVID-19 [online]: Available from theconversation.com/christian-nationalism-is-a-barrier-to-mass-vaccination-against-covid-19-158023.
The Debunking handbook. 2020. [online]: Available from who.int/health-topics/infodemic#tab=tab_1.
The Globe and Mail. 2021. Other countries are making vaccines. Why can’t Canada? [online]: Available from theglobeandmail.com/opinion/editorials/article-other-countries-are-making-vaccines-why-cant-canada/.
The Guardian. 2021. South Africa paying more than double EU price for Oxford vaccine [online]: Available from theguardian.com/world/2021/jan/22/south-africa-paying-more-than-double-eu-price-for-oxford-astrazeneca-vaccine.
Thomson A, Robinson K, Vallée-Tourangeau G. 2016. The 5As: a practical taxonomy for the determinants of vaccine uptake. Vaccine, 34(8): 1018–1024.
Tinari S. 2021. The EMA covid-19 data leak, and what it tells us about mRNA instability. BMJ, 372: n627.
Tomboloni C, Tersigni C, de Martino M, et al. 2019. Knowledge, attitude and disinformation regarding vaccination and immunization practices among healthcare workers of a third-level paediatric hospital. Italian Journal of Pediatrics, 45(1): 104.
Torjesen I. 2021a. COVID-19: first doses of vaccines in Scotland led to a substantial fall in hospital admissions BMJ, 372: n523.
Torjesen I. 2021b. Covid-19: Norway investigates 23 deaths in frail elderly patients after vaccination. BMJ, 372: n149.
Toronto. 2021. COVID-19: status of cases in Toronto [online]: Available from toronto.ca/home/covid-19/covid-19-latest-city-of-toronto-news/covid-19-status-of-cases-in-toronto/.
Tumilty R. 2021. Seven vaccine-makers rejected our request to produce COVID shots in Canada, Anand says [online]: Available from nationalpost.com/news/politics/every-covid-vaccine-manufacturer-passed-on-making-them-in-canada-federal-procurement-minister.
Turnbull J, Baral S, Bond A, Boozary A, Bruketa E, Elmi N, et al. 2021. Seeking shelter: homelessness and COVID-19. Royal Society of Canada. .
Ueda Y, Yagi A, Abe H, Nakagawa S, Minekawa R, Kuroki H, et al. 2020. The last strategy for re-disseminaiton of HPV vaccination in Japan while still under suspension of the governmental recommendation. Scientific Reports, 10: 16091.
UN. 2002. Second world assembly on aging, Madrid, 8–12 April 2002 [online]: Available from un.org/esa/socdev/documents/ageing/MIPAA/political-declaration-en.pdf.
UN. 2007. General Assembly Resolution 61/295, 13 September 2007, voting 144-4-11 [online]: Available from un.org/development/desa/indigenouspeoples/wp-content/uploads/sites/19/2018/11/UNDRIP_E_web.pdf. Articles 1 and 2; Articles 3-5 and 19-20; Article 7; Article 21.
UN. 2021a. United Nations General Assembly Resolution 217A of 10 December 1948 [online]: Available from un.org/en/universal-declaration-human-rights/, Preamble, Articles 1 and 2.
UN. 2021b. United Nations General Assembly Resolution 2200A (XXI) of 16 December 1966, in force 3 January 1976 [online]: Available from ohchr.org/en/professionalinterest/pages/cescr.aspx.
UN Department of Economic and Social Affairs. 2021. COVID-19 and indigenous peoples [online]: Available from un.org/development/desa/indigenouspeoples/covid-19.html.
Vasileiou E, Simpson CR, Robertson C, Shi T., Kerr S., and Agrawal U. 2021. Effectiveness of first dose of covid-19 vaccines against hospital admissions in Scotland: national prospective cohort study of 5.4 million people [online]: Available from ed.ac.uk/files/atoms/files/scotland_firstvaccinedata_preprint.pdf.
Walcott R. 2020. Data or politics? Why the answer still remains political [online]: Available from rsc-src.ca/en/covid-19/impact-covid-19-in-racialized-communities/data-or-politics-why-answer-still-remains.
Wang Y, McKee M, Torbica A, and Stucler D. 2019. Systematic literature review on the spread of health-related misinformation on social media. Social Science & Medicine, 240(112552): 1–12.
Westoll N. 2021. COVID-19 in Toronto neighbourhoods: HOW postal code data highlights vaccine inequities [online]: Available from globalnews.ca/news/7741950/covid-toronto-vaccines-cases-ices-postal-code-data/.
White-Crummey A. 2021. Scott Moe laments vaccine ‘dilemma’ as AstraZeneca lines shrink [online]: Available from leaderpost.com/news/saskatchewan/scott-moe-laments-vaccine-dilemma-as-astrazeneca-lines-shrink.
WHO. 2014. SAGE working group dealing with vaccine hesitancy (March 2012 to November 2014) [online]: Available from who.int/immunization/sage/meetings/2014/october/SAGE_working_group_revised_report_vaccine_hesitancy.pdf.
WHO. 2019a. Evaluation of COVID-19 vaccine effectiveness [online]: Available from who.int/publications/i/item/WHO-2019-nCoV-vaccine_effectiveness-measurement-2021.1.
WHO. 2019b. Immunization stress related responses [online]: Available from who.int/publications/i/item/978-92-4-151594-8.
WHO. 2019c. Ten threats to global health in 2019 [online]: Available from who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
WHO. 2020a. An ad hoc WHO technical consultation managing the COVID-19 infodemic: call for action, 7–8 April 2020. World Health Organization [online]: Available from apps.who.int/iris/bitstream/handle/10665/334287/9789240010314-eng.pdf?sequence=1&isAllowed=y.
WHO. 2020b. 73rd World Health Assembly Decisions WHA73/9. Strengthening global immunization efforts to leave no one behind [online]: Available from who.int/news-room/feature-stories/detail/73rd-world-health-assembly-decisions.
WHO. 2020c. Immunization AGENDA 2030: a global strategy to leave no one behind [online]: Available from who.int/publications/m/item/immunization-agenda-2030-a-global-strategy-to-leave-no-one-behind.
WHO. 2020d. WHO SAGE values framework for the allocation and prioritization of COVID-19 vaccination [online]: Available from apps.who.int/iris/bitstream/handle/10665/334299/WHO-2019-nCoV-SAGE_Framework-Allocation_and_prioritization-2020.1-eng.pdf?sequence=1&isAllowed=y.
WHO. 2021a. 7 Partnering with communities [online]: Available from who.int/immunization/documents/IIP2015_Module7.pdf?ua=1.
WHO. 2021b. GACVS COVID-19 Vaccine Safety subcommittee meeting to review reports of deaths of very frail elderly individuals vaccinated with Pfizer BioNTech COVID-19 vaccine, BNT162b2 [online]: Available from who.int/news/item/22-01-2021-gacvs-review-deaths-pfizer-biontech-covid-19-vaccine-bnt162b2.
WHO. 2021c. Global vaccine safety [online]: Available from who.int/vaccine_safety/initiative/communication/network/ibi/en/.
WHO. 2021d. How to report misinformation online [online]: Available from who.int/campaigns/connecting-the-world-to-combat-coronavirus/how-to-report-misinformation-online?gclid=EAIaIQobChMI4IevluCq7wIVxt7ICh0KmwO2EAAYASAAEgLV_PD_BwE.
WHO. 2021e. Immunization Agenda 2030: Global strategy to leave no one behind [online]: Available from who.int/teams/immunization-vaccines-and-biologicals/strategies/ia2030.
WHO. 2021f. Infodemic [online]: Available from who.int/health-topics/infodemic#tab=tab_1.
WHO. 2021g. Managing the COVID-19 infodemic: Promoting healthy behaviours and mitigating the harm from misinformation and disinformation [online]: Available from who.int/news/item/23-09-2020-managing-the-covid-19-infodemic-promoting-healthy-behaviours-and-mitigating-the-harm-from-misinformation-and-disinformation.
WHO. 2021h. Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process [online]: Available from extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_14April2021.pdf.
WHO. 2021i. Strategic advisory group of experts on immunization, 22–25 March 2021 [online]: Available from terrance.who.int/mediacentre/data/sage/SAGE_eYB_Mar2021.pdf.
WHO. 2021j. Strengthening health security by implementing the International Health Regulations (2005) [online]: Available from who.int/ihr/ports_airports/icvp/en/.
WHO. 2021k. The effects of virus variants on COVID-19 vaccines [online]: Available from who.int/news-room/feature-stories/detail/the-effects-of-virus-variants-on-covid-19-vaccines?gclid=EAIaIQobChMItujX17Ke8AIVjf7jBx29oAFjEAAYASAAEgL7rPD_BwE.
WHO. 2021l. Vaccine certificates should not come at expense of other COVID priorities [online]: Available from policyoptions.irpp.org/magazines/march-2021/vaccine-certificates-should-not-come-at-expense-of-other-covid-priorities/.
Wilson SE, Bunko A, Johnson S, Murray J, Wang Y, Deeks SL, et al. 2021. The geographic distribution of un-immunized children in Ontario, Canada: Hotspot detection using Bayesian spatial analysis Vaccine, 39(8): 1349–1357.
Wylie L, and McConkey S. 2018. Insiders’ insight: discrimination against indigenous peoples through the eyes of health care professionals. Journal of Racial and Ethnic Health Disparities, 6: 37–45.
Zhang J, While AE, and Norman IJ. 2010. Knowledge and attitudes regarding influenza vaccination among nurses: a research review. Vaccine, 28: 7207–7214.
Supplementary material
Supplementary Material 1 (DOCX / 14.5 KB)
- Download
- 14.55 KB
Information & Authors
Information
Published In

FACETS
Volume 6 • Number 1 • January 2021
Pages: 1184 - 1246
Editor: Jules M. Blais
History
Received: 31 March 2021
Accepted: 11 May 2021
Version of record online: 22 July 2021
Notes
Note: This paper is part of the Royal Society of Canada’s COVID-19 Task Force Collection.
Copyright
© 2021 MacDonald et al. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Data Availability Statement
All relevant data are within the paper and Supplementary Material.
Key Words
Sections
Subjects
Authors
Author Contributions
All conceived and designed the study.
All performed the experiments/collected the data.
All analyzed and interpreted the data.
All contributed resources.
All drafted or revised the manuscript.
Competing Interests
The authors have declared that no competing interests exist.
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Noni E. MacDonald, Jeannette Comeau, Ève Dubé, Janice Graham, Margo Greenwood, Shawn Harmon, Janet McElhaney, C. Meghan McMurtry, Alan Middleton, Audrey Steenbeek, and Anna Taddio. 2021. Royal society of Canada COVID-19 report: Enhancing COVID-19 vaccine acceptance in Canada. FACETS.
6(): 1184-1246. https://doi.org/10.1139/facets-2021-0037
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
Cited by
1. Hesitancy for COVID-19 Vaccines and Its Implications for Routine Immunisation
2. Proposing a New Conceptual Syndemic Framework for COVID-19 Vaccine Hesitancy: A Narrative Review
3. The second year of pandemic in the Arctic: examining spatiotemporal dynamics of the COVID-19 “Delta wave” in Arctic regions in 2021
4. An equitable vaccine delivery system: Lessons from the COVID-19 vaccine rollout in Canada
5. Measuring inequalities in COVID-19 vaccination uptake and intent: results from the Canadian Community Health Survey 2021
6. “
I don’t think there’s a point for me to discuss it with my patients”
: exploring health care providers’ views and behaviours regarding COVID-19 vaccination
7. COVID-19 vaccine hesitancy
8. Barriers to adult vaccination in Canada: A qualitative systematic review
9. Have vaccine hesitancy models oversimplified a complex problem to our detriment? The Adapted Royal Society of Canada vaccine uptake framework
10. Between persuasion and compulsion: The case of COVID-19 vaccination in Canada
11. Impact of the CARD (Comfort Ask Relax Distract) system on school-based vaccinations: A cluster randomized trial
