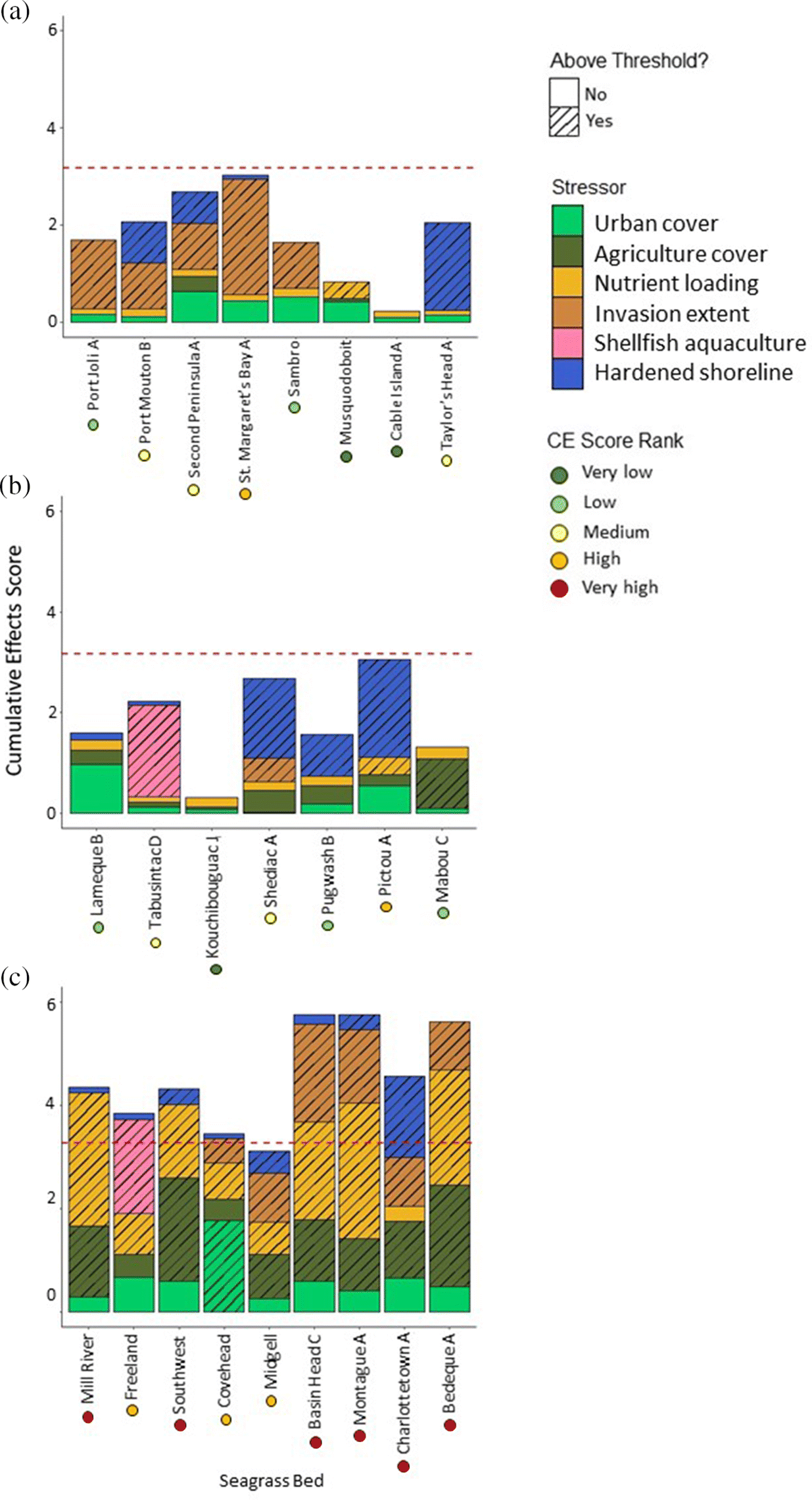
Cumulative effects scores
To calculate a CE score for each seagrass bed, we applied an additive methodology (
sensu Halpern et al. 2008) that quantifies cumulative impacts by combining the intensity of individual stressors with impact weights specific to eelgrass ecosystems, then sums these individual impacts into a single CE score. While human activities can also interact to influence ecosystems synergistically or antagonistically (
Crain et al. 2008;
Stockbridge et al. 2020), most CE studies assume additive impacts given the vast unknowns for other types of interactions (
Korpinen and Andersen 2016;
Hodgson et al. 2019). Cumulative impact scores for seagrass (
IS) were thus calculated as:
where
n is the number of human activities,
Di is the normalized value of intensity of activity
i at each seagrass bed,
Sj is the presence of seagrass ecosystems (and thus reduces to 1), and
ui,j is the impact weighting (or habitat vulnerability) of seagrass bed
j to human activity
i (
Halpern et al. 2008).
We used human activities as proxies for stressors (e.g., urban watershed land use is a proxy for pollution run-off) since in most cases direct measurements of stressors are not available for all seagrass beds in the region. Therefore, we refer to the intensity of human activities as stressor scores (i.e., the
Di). Six stressors were chosen for their expected influence on coastal biogenic habitats like seagrass: urban watershed land use, agricultural watershed land use, nitrogen loading, invasion extent, near-field shellfish aquaculture activity, and hardened shoreline (
Table 1). We did not include climate change related stressors as we chose to focus on stressors that could be managed on local or regional scales. To calculate stressor scores for urban watershed land use, agricultural watershed land use, and nitrogen loading, we used intensities from the standardized coastal human impact metric previously published in
Murphy et al. (2019). While the three land-based activities included in the CE score (urban land use, agriculture land use, and nitrogen loading) are interrelated and have similar root causes, we include each of them separately in the CE score because they each represent different stressors to seagrass (urban runoff and inorganic pollution, sediment input, and nutrient input, respectively;
Table 1) that can elicit different plant responses. While the quantity of inorganic nitrogen loading is a better predictor of seagrass coverage than total nitrogen loading (
Coffin et al. 2021), we use estimates of total nitrogen loading taken from nitrogen loading models in the CE score since nitrate concentration data were not widely available across the 187 sites included in this study. We conducted a sensitivity analysis for selected sites where both measures were available and determined that using total-N loading to represent relative anthropogenic nutrient input instead of nitrate-N loading does not result in major changes to the overall CE scores (
Supplementary Material A.2 and C).
We adjusted the calculation of two additional stressors in
Murphy et al. (2019), invasion extent and near-field shellfish aquaculture, to more closely link these stressors with previously published thresholds (see below). Invasion extent was adjusted to represent the number of established invasive biofouling species (≥3 consecutive years of sightings) as opposed to a 10-year average of invader presence/absence.
Murphy et al. (2019) included two sub-measures for shellfish aquaculture (near-field (<25 m) and mid-field (<1 km)) and only considered presence/absence of shellfish aquaculture within these ranges. Here, we measure shellfish aquaculture activity as the area of shellfish aquaculture leases within a 25 m radius surrounding each seagrass bed, standardized to the marine area within the 25 m radius. We excluded four activities included in
Murphy et al. (2019) (overwater structures, water quality, riparian land alteration, and fishing activity) from the CE score as these activities do not have published thresholds available and it is currently unclear at what levels these activities become detrimental to seagrass. Finally, we included one additional stressor not previously included in
Murphy et al. (2019) to represent the impact of hardened shorelines on seagrass beds (
Patrick et al. 2014). We used Google Earth (Google Earth Pro, 2021) and the most recent and clearest imagery data available for each site (ranging between 2014 and 2021) to measure the percentage of shoreline that had been anthropogenically hardened (including riprap, bulkheads, bridge, and wharf abutments, etc.) within 1 km of each seagrass bed.
Vulnerability scores for seagrass habitats (i.e., the
ui,j) have not been developed for Atlantic Canada; thus, we used a vulnerability matrix developed for the nearby New England region that includes vulnerability scores (also called impact weights) for 14 marine ecosystems, including seagrass (
Kappel et al. 2012a,
2012b). Current evidence supports the application of vulnerability matrices developed in a specific area to different regions (
Kappel et al. 2012a). These vulnerability scores were developed using a standardized survey design to gather knowledge from experts on criteria that are expected to influence the vulnerability of an ecosystem to a stressor (
Halpern et al. 2007;
Teck et al. 2010). The five vulnerability criteria considered by experts in the calculation of a single vulnerability score for each ecosystem–stressor combination included: (
i) the spatial scale that a stressor will impact an ecosystem, (
ii) the frequency of the stressor, (
iii) the trophic impact and extent of marine life affected by a stressor within an ecosystem, (
iv) the degree to which the ecosystem’s “natural” state is changed by the stressor, and (
v) the time it will take for the affected components of the ecosystem to return to their former state following the impact (
Kappel et al. 2012a). The vulnerability matrix developed by
Kappel et al. (2012a) provides vulnerability scores for 58 stressors. We use the vulnerability scores specific to seagrass habitat from
Kappel et al. (2012a) and link each of the six human activities included in the CE score to the dominant stressor expected to be caused by that human activity (
Table 1). Nutrient pollution was further separated into region-specific stressors with separate vulnerability scores since the PEI bays and estuaries are generally considered eutrophic while those in NS and NB are generally considered oligotrophic.
Before weighting stressors based on vulnerability, the intensities of the six individual stressors were normalized (i.e., adjusted to range between 0 and 1), with zero representing the absence of the stressor and one representing the highest observed value within the region plus 10%. We added 10% to the maximum observed value for each impact in our dataset as this range has been suggested as appropriate to capture current and likely near-term future levels of the stressors and are often used in CE analyses (
Halpern and Fujita 2013). An example of the CE score calculation can be found in
Supplementary Material A,
Table S1.
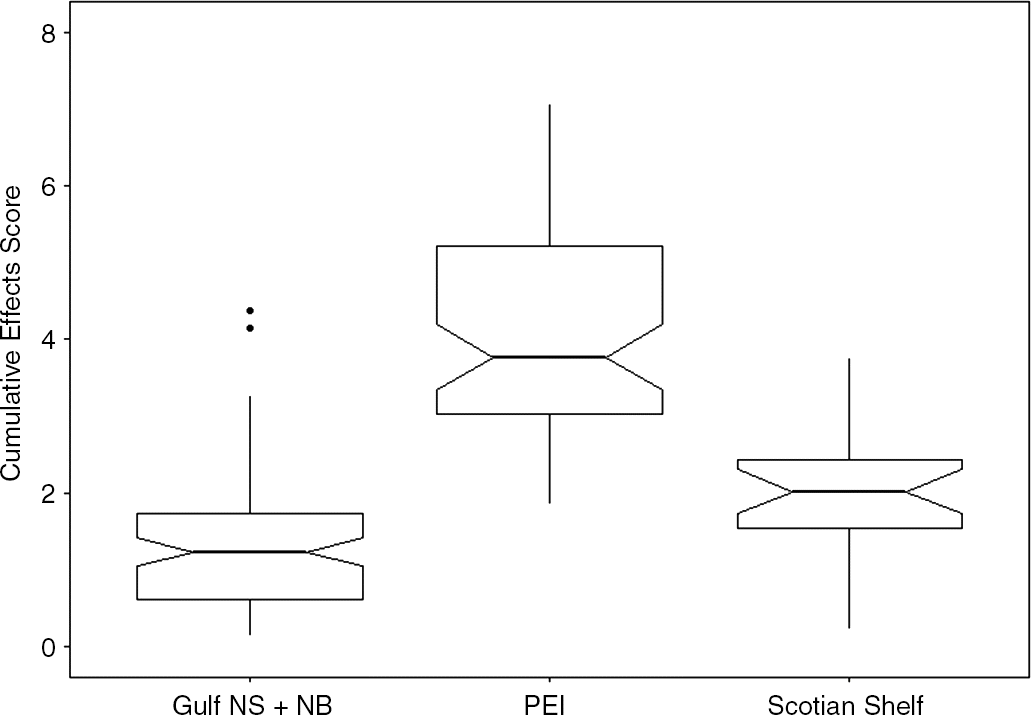
We assessed differences in CE scores among and within regions using 95% confidence intervals around the medians (
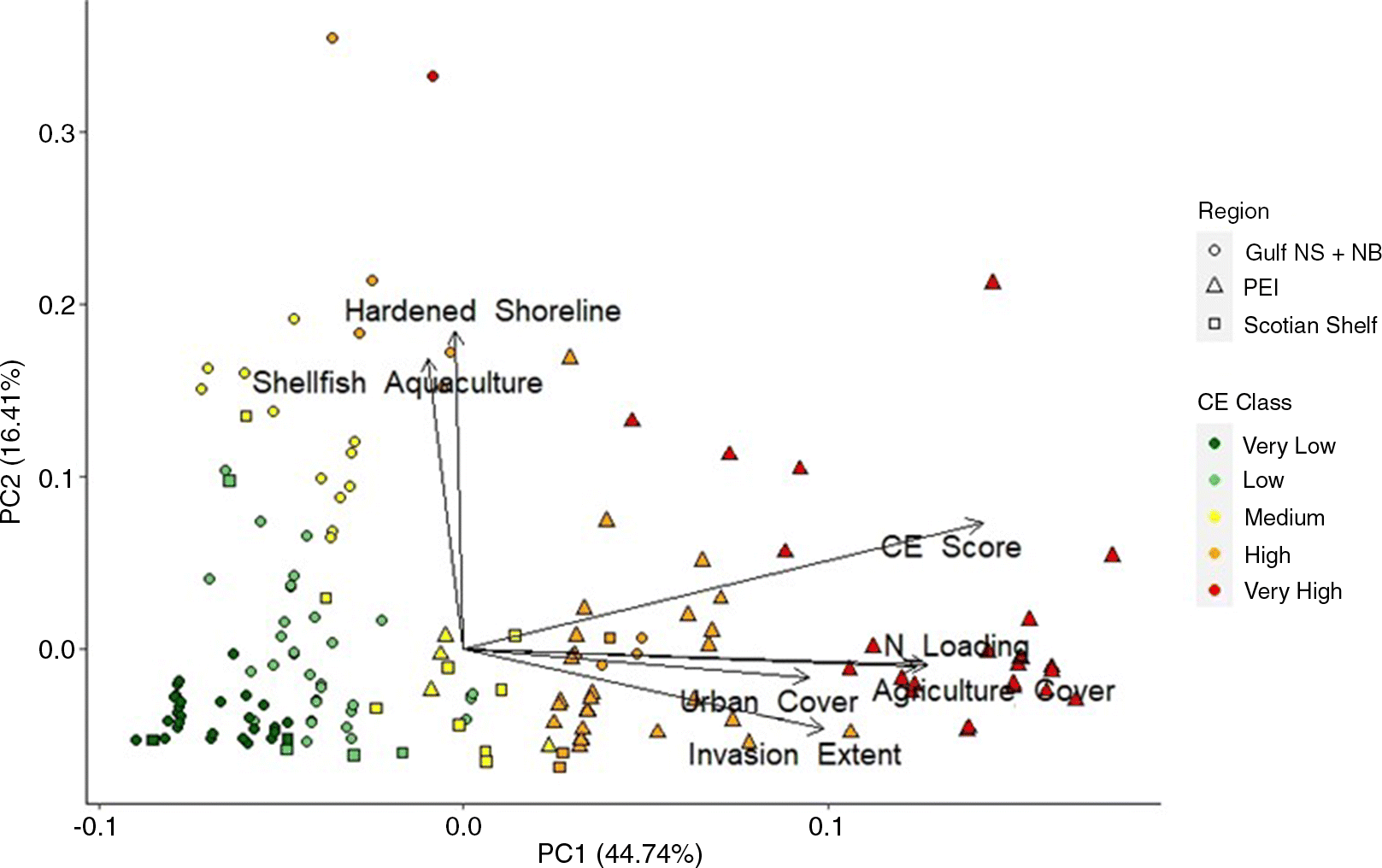
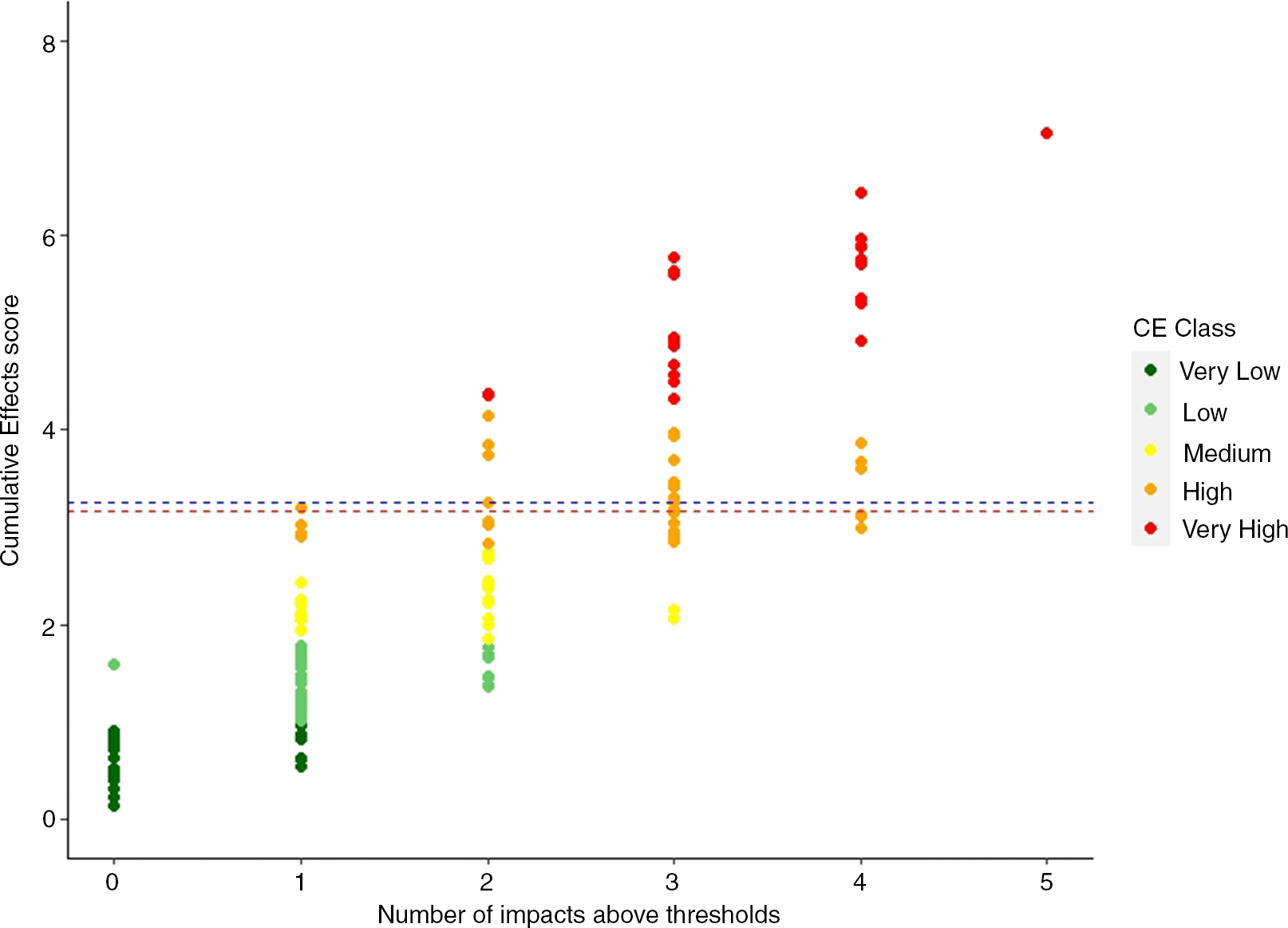
Chambers et al. 1983). For regional comparisons, we split the Gulf of St. Lawrence into the mainland coast (Gulf NS + NB) and PEI. We grouped seagrass beds into five CE classes (very low to very high impact) based on the Jenks natural breaks classification method, which groups CE scores into classes by minimizing within-class variability and maximizing between-class variability. We conducted a Principal Components Analysis (PCA) using the six individual stressor scores and the CE score for each seagrass bed to identify the stressors that explained the most variation in the magnitude of human activities across seagrass beds. We centered and scaled variables before analysis. We considered any variable that had a loading that contributed more than one variable worth of information (>0.378) as an important contributor to the principal component. The PCA was conducted using the vegan package in R (
Oksanen et al. 2021).