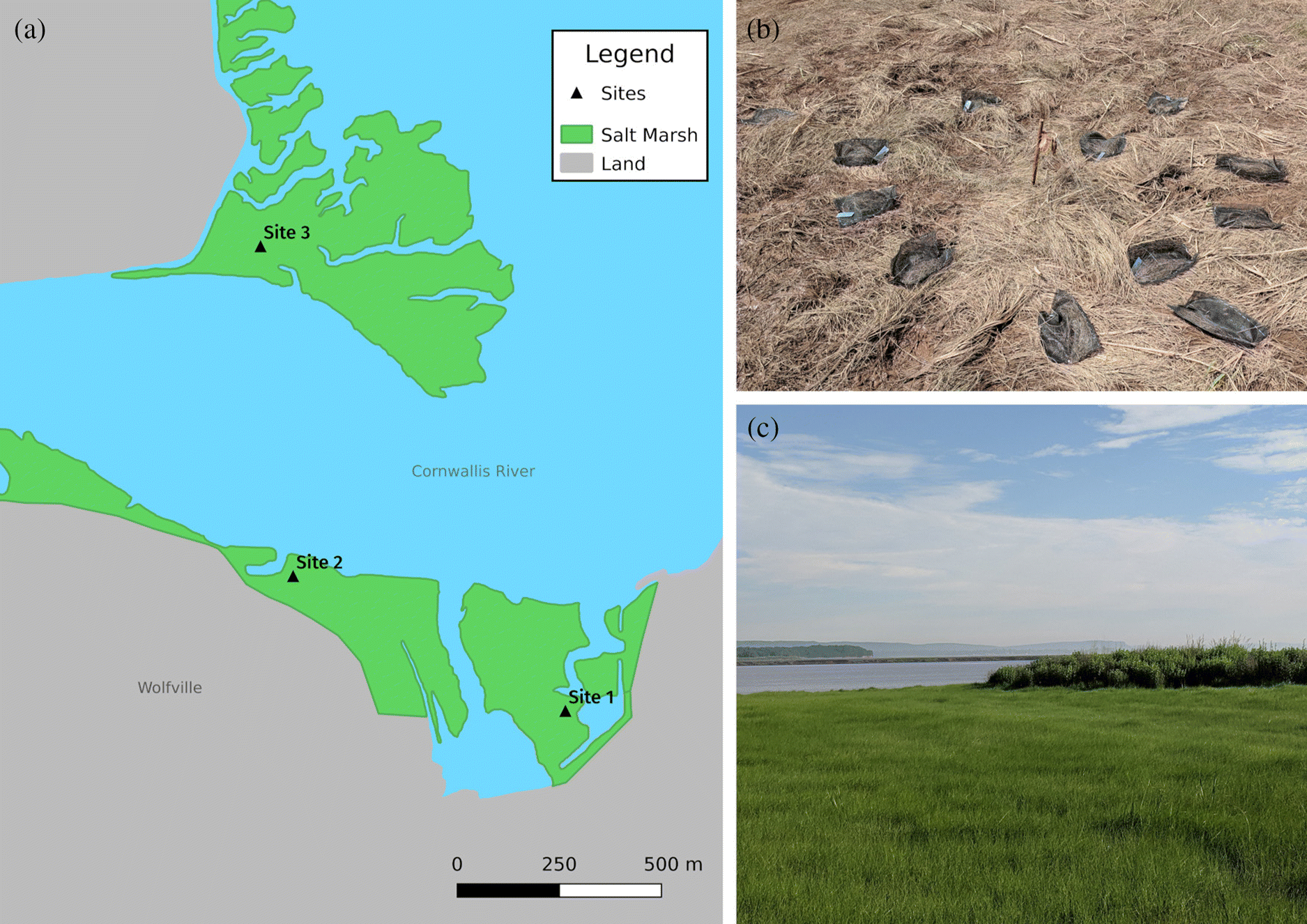
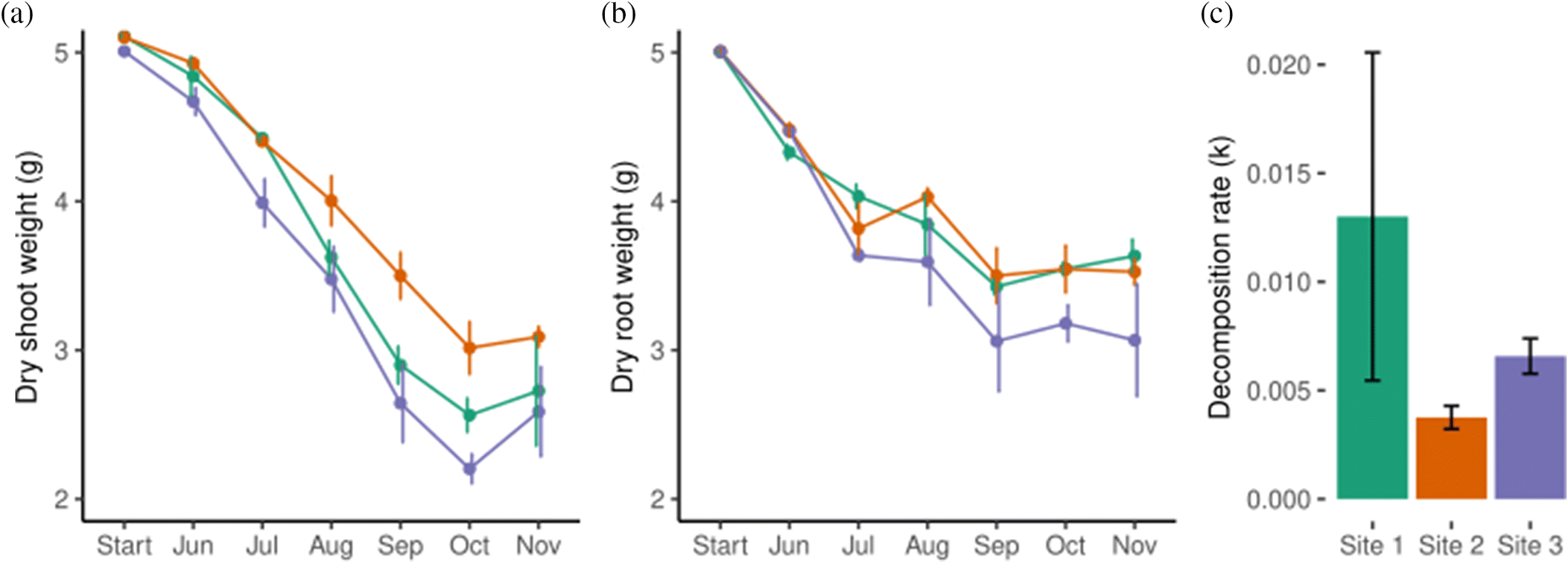
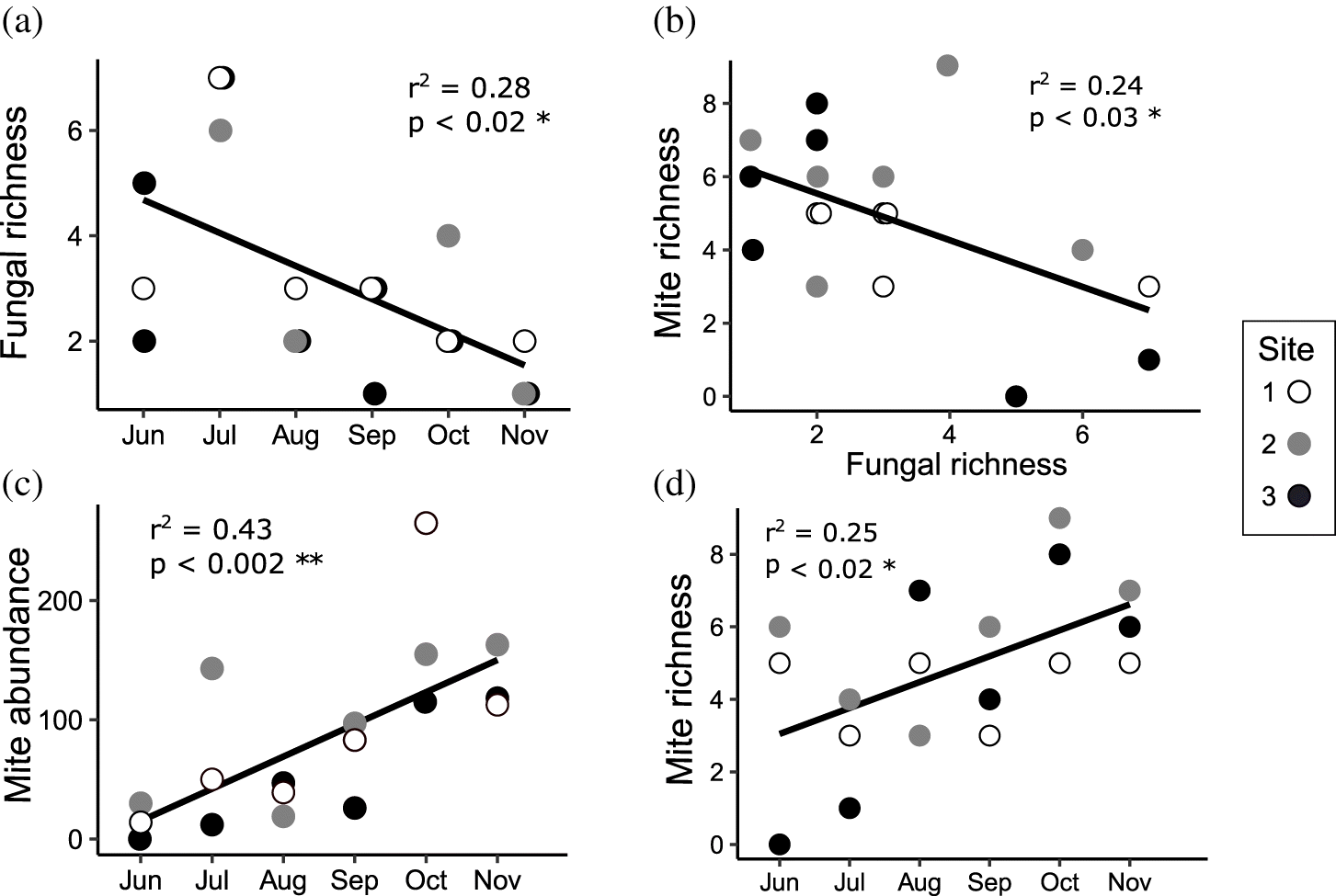
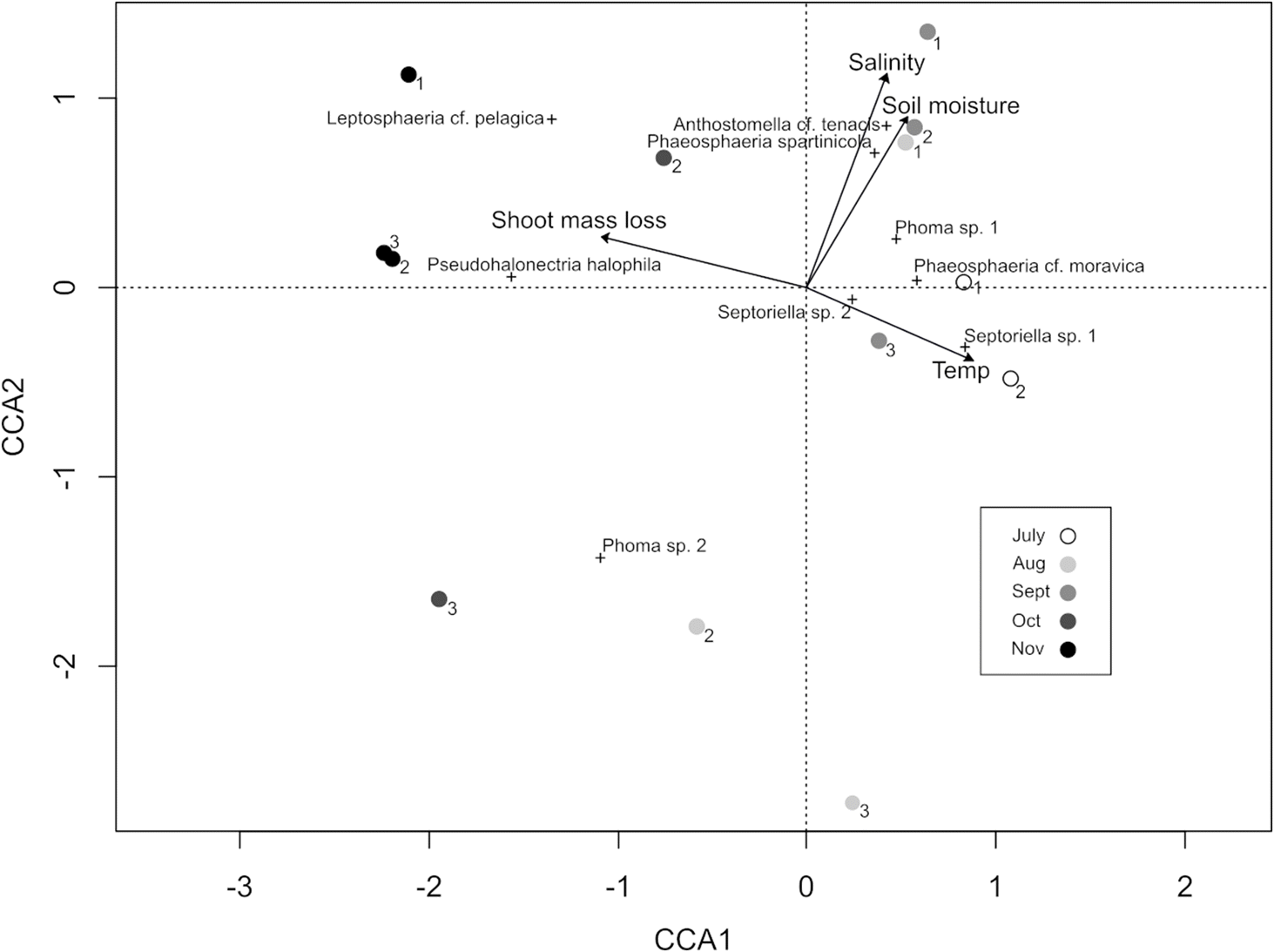
This study was conducted in the Wolfville salt marsh in Wolfville, Nova Scotia, Canada, on the megatidal Minas Basin, which experiences diurnal tides. The marsh faces northward onto the Cornwallis River, with a second fragment on the north bank. Three sites were chosen, with two situated in the western and eastern parts of the southern portion of the marsh, divided through the middle by Wolfville Harbour. The third site is located in the northern portion of the marsh, separated from the other two sites by the Cornwallis River (
Fig. 1). Site 1 (45.094672°, −64.355659°) was situated in the southeast of the marsh. The site was lower than the other two, permitting more frequent tidal inundation. Wetter sediments allowed for greater cover by
Sporobolus alterniflorus (Loisel.) P.M. Peterson & Saarela (formerly
Spartina alterniflora), although the site is still dominated by
Sporobolus pumilus. Nevertheless, the relative abundance of
S. alterniflorus compared with sites 2 and 3 is the most obvious distinguishing feature among the three sites. Site 2 (45.097542°, −64.364201°) was in the southwest of the marsh, close to the bank of a creek, 0.75 km northwest of site 1. This site was higher than site 1 resulting in drier sediments and a virtual monoculture of
S. pumilus. Plant cover was very dense at this site, lacking any bare sediment. Site 3 (45.104801, −64.365388°) was in the northern part of the marsh, 0.85 km north of site 2 and 1.35 km northwest of site 1, only a few meters away from the northern bank of the Cornwallis River, which was rapidly eroding the marsh edge. This site was also composed entirely of
S. pumilus; however, plant cover was thinner than at site 2, with prominent patches of bare sediment between the plants. Individual plants tended to grow shorter here than at sites 1 and 2. Additional discussion of the marsh plant composition of the Wolfville salt marsh can be found in
Chapman (1937), dating back to a period when the marsh was highly disturbed due to agricultural activity, where the author notes
S. pumilus (as
Spartina patens) formed isolated clumps rather than dominating the high marsh as it does now, except for an area in the northern portion of the marsh where
S. pumilus was not collected for hay, and in this portion the grass was the dominant species.