Diversity at different scales
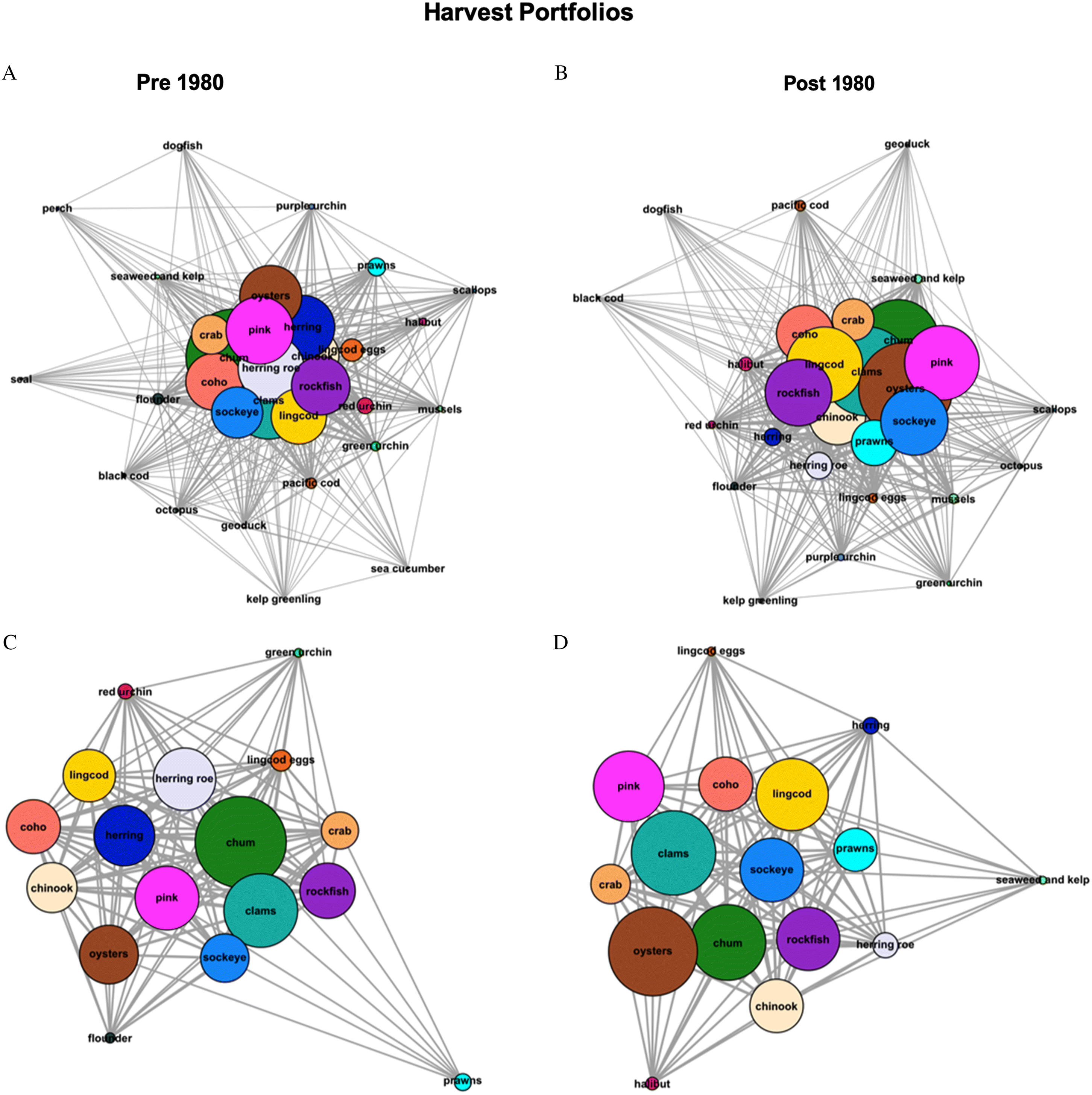
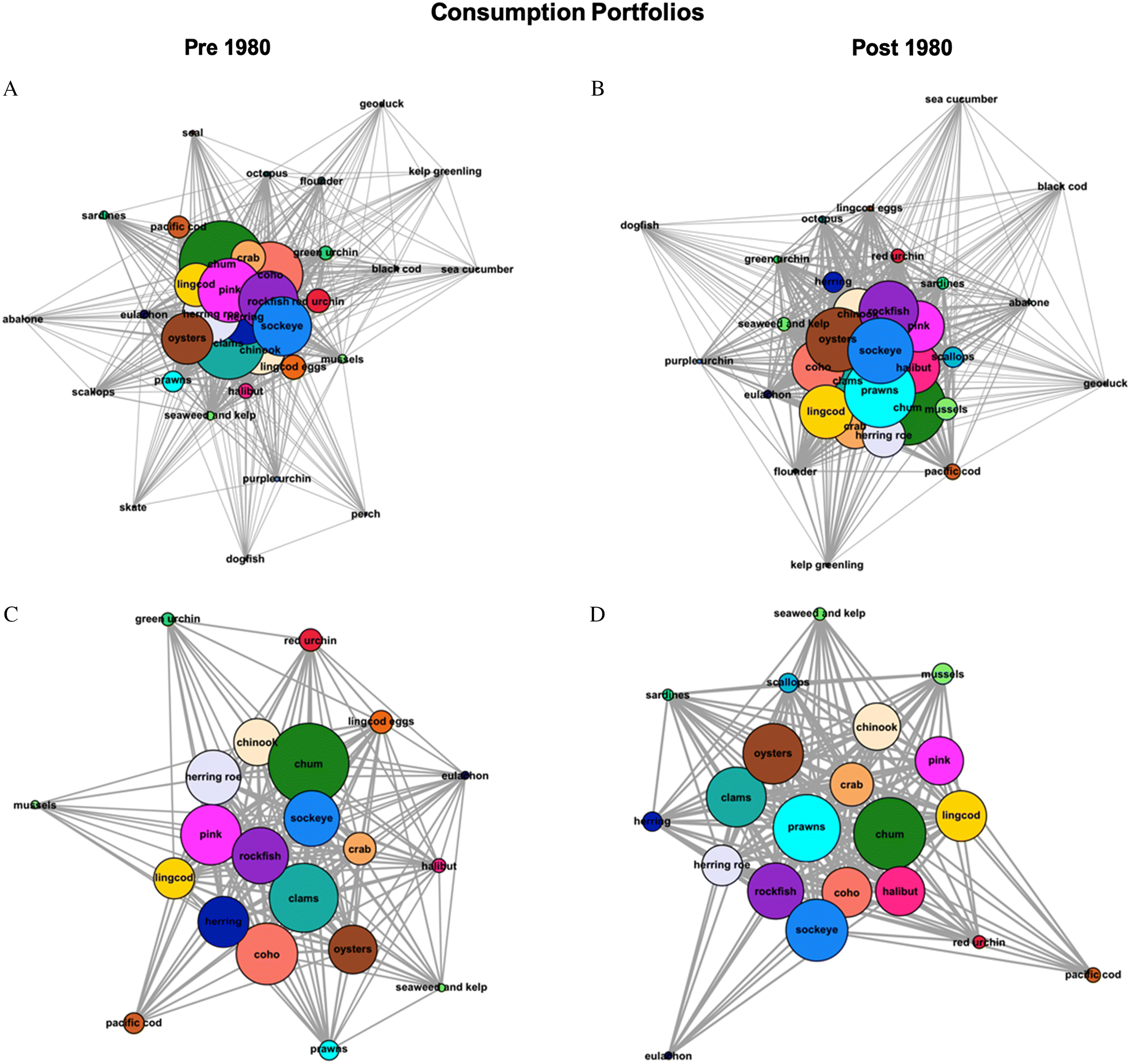
We found that the Nation’s harvest and consumption portfolios are more diversified at the community-level now, yet less diverse for individuals, compared to past decades. Specifically, we found that mean degree centrality scores of both harvest and consumption portfolios were higher post-1980 while the number of different seafoods was smaller for individuals, though not significantly so. Decreases in portfolio diversity among individual participants were driven, in large part, by declines in harvested and consumed traditional seafoods including Pacific herring, sea urchin, sea cucumber, lingcod eggs and seal (
Fig. 3A). This is also evident in shifting portfolio configurations, with seafood types shifting from the core of portfolios to the periphery (
Figs 1,
2). Concurrently, popular traditional seafood, such as salmon, rockfish, and crab, are being caught and consumed by more people, reflecting portfolio diversification at the community level. The emergence of new fisheries for deepwater benthic species, such as spot prawns and Pacific halibut, that are increasingly accessible to more harvesters, also drove increases in the community’s portfolio diversity. Because more people are harvesting and consuming these species, they moved from the periphery to the core of the Nation’s portfolios (
Figs 1,
2) without displacing other traditional seafood, which increased the number of connections within the entire portfolio. Less common seafood types such as seal, perch, and longnose skate were only mentioned by participants for the pre-1980 period, leading to a decrease in individual-level portfolio diversity but a more diversified community portfolio over time.
We also detected differences in the relative abundance of various seafood types among the harvest and consumption portfolios. For example, spot prawns and Pacific halibut consumption has increased post-1980, while harvest of key traditional seafood, such as Pacific herring, lingcod eggs, and sea urchins, has significantly decreased (
Figs 1,
2;
Table S4,
S5). These patterns in individual and community portfolio diversity and relative abundance are driven by several key social-ecological mechanisms illuminated by experts in the community.
Mechanisms driving shifts in portfolios
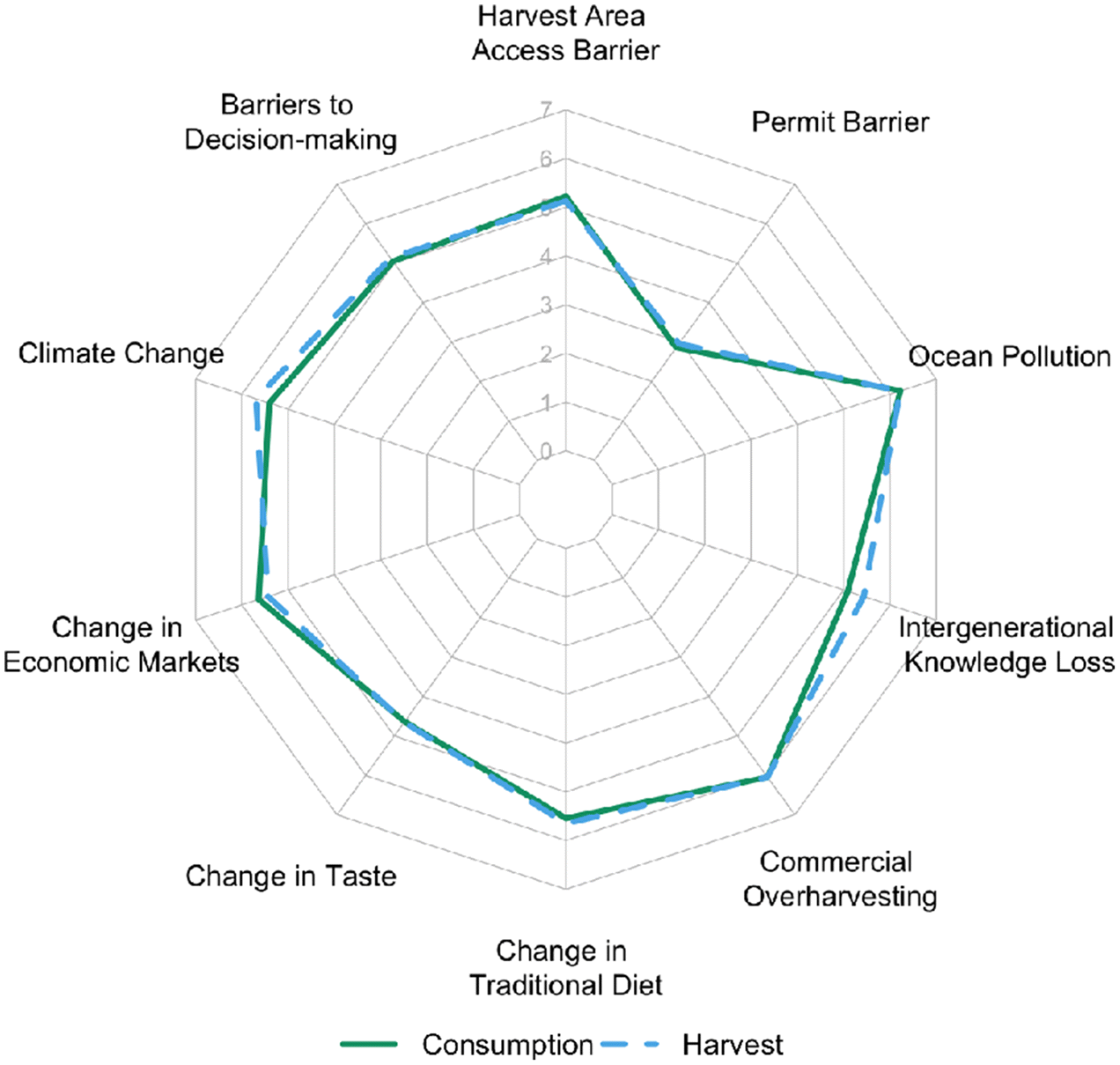
Four key social and ecological mechanisms were perceived by community experts as driving reduced diversity at the individual scale and increased diversification at the community scale among the Nation’s food fisheries portfolios. These included industrial and commercial activities under the authority of Canada’s centralized governance regime, loss in intergenerational knowledge transmission, learning and adapting to new ecological and economic opportunities, and the trade in seafood among coastal First Nations communities. In addition to these mechanisms, experts perceived commercial overharvesting and pollution as the most important factors driving shifts in fisheries portfolios, while issues surrounding fisheries access rights, now granted by the Nation’s government, were perceived as less important. These results reveal social-ecological factors affecting the Nation’s fisheries over the past eight decades and illuminate locally relevant adaptation strategies to bolster future resilience in this social-ecological system.
I. Industrial commercial activities and centralized colonial governance. Access to traditionally important seafood has been reduced by industrial-scale, commercial activities, such as fishing and logging, currently under the authority of a centralized governance regime. Although here we focus on FSC fisheries, many of the same species are also being exploited or indirectly impacted by commercial activities. In the Nation’s territory, this is exemplified by the decline in the harvest and consumption of Pacific herring and herring eggs (
Table S4,
S5). While herring remain relatively central to harvest and consumption portfolios (
Figs. 1,
2), experts reported that herring was “fished out” of the Nation’s waters in 1983 and that commercial fisheries targeting adult females for their roe for export to international markets were the main driver of this change (pers. comm. by eleven experts;
Paul et al. 2014). Such reports are consistent with the literature assessing the magnitude of the Pacific herring collapse (
Cleary et al. 2010;
Essington et al. 2015).
Given the large ecological, economic and cultural consequences for the Nation and community, commercial overharvesting was perceived by experts as one of the most important drivers of change in harvest and consumption portfolios (
Fig. 4). In the past, decisions to open herring fisheries were solely in the hands of Canada’s federal fisheries agency, Department of Fisheries and Oceans (DFO). Today, the fisheries Minister still retains the discretion to open the fishery regardless of recommendations based on available science and other knowledge sources (
Klain et al. 2014). Moreover, Indigenous livelihood objectives, self-determination, and ways of life are yet to be incorporated into herring management (
von der Porten et al. 2016). For example, even though Strait of Georgia herring stocks are reported as having “spawning biomass […] at a historic high” (
DFO 2019), the Nation considers abundances to be low compared to their historical baseline and wish to conserve stocks such that they might return to the territory (pers. comm. Clint Williams). A similar tension has emerged in Sitka, Alaska, where divergent views of herring population status by the state management agency and Indigenous harvesters (
Thornton and Kitka 2015) drove a lawsuit by the Sitka Tribe of Alaska seeking protection of the subsistence herring fishery. The Nation is a party to a Joint Fisheries Committee with DFO to facilitate learning and shared decision making. As a Modern Treaty Nation with rights recognized by the Government of Canada and BC, it explains why fisheries access granted by the Nation was not perceived as an important barrier by experts in the community (
Fig. 4).
Additionally, logging related activities under the authority of a centralized governance regime have decreased the harvest and consumption of clams (pers. comm. Eugene Louie). Although clams are still centrally important traditional seafood in the Nation’s harvest and consumption portfolios (
Figs. 1,
2), their relative abundance has decreased due to beach closures associated with pulp mill contamination and high fecal coliform levels. Pollution was also perceived as one of the most important drivers of change amongst experts. Although some beaches might be safe to harvest, ultimately, beach closure decisions reside with federal governing authorities that often operate with limited resources and available data (
Flemming 2019). Consequently, clam harvest closures are often due to an absence of evidence rather than evidence of contamination.
Finally, predation by marine mammals, such as seals and sea lions, and a lack of participation in decisions surrounding these predator populations were identified as an important driver of change among portfolios (
Table 1). Traditionally, seals were hunted and managed by the Nation (
Paul et al. 2014) but are not represented in harvest or consumption portfolios post-1980 (
Fig. 1,
2). Seals and sea lions consume a diversity of benthic and pelagic fish, such as salmon and Pacific herring (
Nelson et al. 2019). Therefore, they potentially compete with humans and other culturally important species, such as killer whales (
Chasco et al., 2017), for shared prey. Currently, Canada’s federal fisheries agency, DFO, has the authority to manage these marine mammals, constraining opportunities for the potential revitalization of traditional hunting and management systems (
Reidy 2019).
II. Changes to Intergenerational knowledge transmission. Reduced diversity among the Nation’s fisheries portfolios at the individual-level also was driven in part by the disruption of knowledge transmission of specific harvesting practices across generations. For example, the loss of seal from harvest and consumption portfolios is primarily due to loss of hunting knowledge associated with the legal restrictions on seal harvest in 1970. Furthermore, the substantial decreases in abundance of chum salmon, lingcod eggs, and red and green sea urchins, were attributed to a lack of knowledge among younger generations on how to collect and process these traditionally important seafood (
Figs. 1,
2). While there are “still many sea urchins out there [in the marine environment], […] no one wants them. I will only go get them if an elder is wishing for them” (pers. comm. Lee George). As intergenerational knowledge loss increases, participation declines, reducing the likelihood of cultural preservation (
Turner et al. 2008), in turn contributing to the erosion of SES resilience by hindering learning opportunities and impeding collective action required to respond to disturbance and changes (
Biggs et al. 2012).
III. Adapting to new ecological and economic opportunities. While opportunities to transfer traditional knowledge might be declining, learning and adapting to new ecological and economic opportunities has been a key mechanism driving changes in the Nation’s harvest and consumption portfolios. For instance, spot prawns have become a more central component of both the community’s harvest and consumption portfolios over time, reportedly due to increased market demand (
British Columbia Seafood Industry: Year in Review 2016) and modern fishing gear that facilitates access to deepwater fisheries. Spot prawns were not a traditionally important food fishery. However, community harvesters have learned to adapt to this new opportunity and harvest them for food in association with the commercial fishery. Moreover, as a result of Pacific halibut “moving into the area” (pers. comm. Lee George), the harvest and consumption of this species has increased. Halibut is now offered as part of the Nation’s community food fish program in place of previously abundant seafood types like Fraser River sockeye salmon. Both learning and adapting to new opportunities are key characteristics of resilience (
Biggs et al. 2012). However, human health could be negatively impacted if current trends continue since salmon species are nutritionally different from halibut, especially in terms of fatty acids, a required nutrient that salmon provide 70% of in First Nations’ diets (
Marushka et al. 2019). Moreover, continuously shifting to new fishing opportunities as previously abundant seafood types become less abundant could lead to the serial depletion of species, as has been shown in both commercial (
Armstrong et al. 1998;
Karpov et al. 2000) and subsistence fisheries (
Salomon et al. 2007).
IV. Increased connectivity among communities via trade of seafood. While consumption and harvest portfolios decreased in diversity at the individual level, they also exhibited clear changes in diversification at the community-level, in part due to the seafood trade within the community itself and with other coastal communities. For example, while Pacific herring is no longer as abundant in the Nation’s waters as prior to 1980 (pers. comm. by eleven experts;
Paul et al. 2014), many people obtain herring eggs to consume from other coastal Indigenous communities, primarily the Heiltsuk Nation who have an active herring spawn-on-kelp food fishery (pers. comm. Paul August). Eulachon is another seafood type that is traded for with other Nations since it is consumed but not harvested by community in their traditional territory. Trade of traditional seafood with other Nations supports coast-wide connectivity, which facilitates continued familial, cultural, and economic relationships, thereby enhancing resilience to local disturbance (
Biggs et al. 2012). However, the use of herring eggs and eulachon has decreased over time due to changes in traditional diet and people’s taste, suggesting that decreased participation in these harvest and (or) consumption practices could reduce SES resilience. This also has implications for Indigenous health as these forage fish provide essential nutrients, such as vitamin A and essential fatty acids, in traditional diets (
Marushka et al. 2019).
Adaptation opportunities in our partner Nation context
“In the summertime I would always go out on the canoe with my mother to get salmon and even then, my mother told me that it won’t always be like this” (pers. comm. Doreen Point). Resilient fishing communities are better able to adapt to future disturbances, maintain their food and nutritional security and their cultural well-being. As such, our results help inform future alternative adaptation strategies that have both local and global relevance. Because we found no evidence that the perceived importance of drivers differed among individual participants, regardless of their age, gender, occupation or boat ownership, potential adaptation initiatives are likely to be perceived as relevant across the entire community. The social-ecological and political context however of coastal First Nations along Canada’s Pacific coast are unique to each Nation, consequently our specific findings here may be only broadly relevant to other coastal Indigenous communities in BC.
Adaptation strategies should be tailored to specific SESs and reflect local characteristics, such as cultural norms and values (
Andrachuk and Armitage 2015;
Rotarangi and Stephenson 2014), social networks (
Barnes et al. 2017;
Bodin 2017), politics (
Gelcich et al. 2010) and place-specific environmental characteristics (
Olsson et al. 2006): “We always fished and there has always been fish, we traditionally managed our own [fisheries]” (pers. comm. Scott Galligos). As such, given knowledge from community experts and drawing on resilience theory, our results suggest that adaptation strategies fostering knowledge transfer to younger generations and decentralizing natural resource management authority to local scales would support resilience in this system.
While climate change was perceived as an important driver of change, it was not ranked as most important, in contrast to the majority of academic literature (
Barange et al. 2014;
Ford et al. 2020;
Rudd 2014;
Savo et al. 2017;
Weatherdon et al. 2016). This is likely due to the magnitude and occurrence of other local stressors that impact the community’s daily lives, including clam contamination closures. Furthermore, it is difficult to observe long term, incremental climate trends, especially if experts are not old enough to benefit from a longer time horizon. However, climate change is projected to reduce potential catches of species targeted by the community (
Weatherdon et al. 2016). Therefore, changes in traditional seafood harvest and consumption portfolios could reflect, in part, species-specific changes driven by climate change, including extreme events like marine heatwaves and heat domes. When planning for the future, understanding novel drivers of change identified by Indigenous communities will be key to developing successful adaptation strategies (
Cisneros-Montemayor et al. 2016;
Donatuto et al. 2016;
Eckert et al. 2018;
Terbasket and Shields 2019). In the case of the Nation, reinvigorating traditional marine mammal hunting and stewardship may have diverse health and well-being benefits.