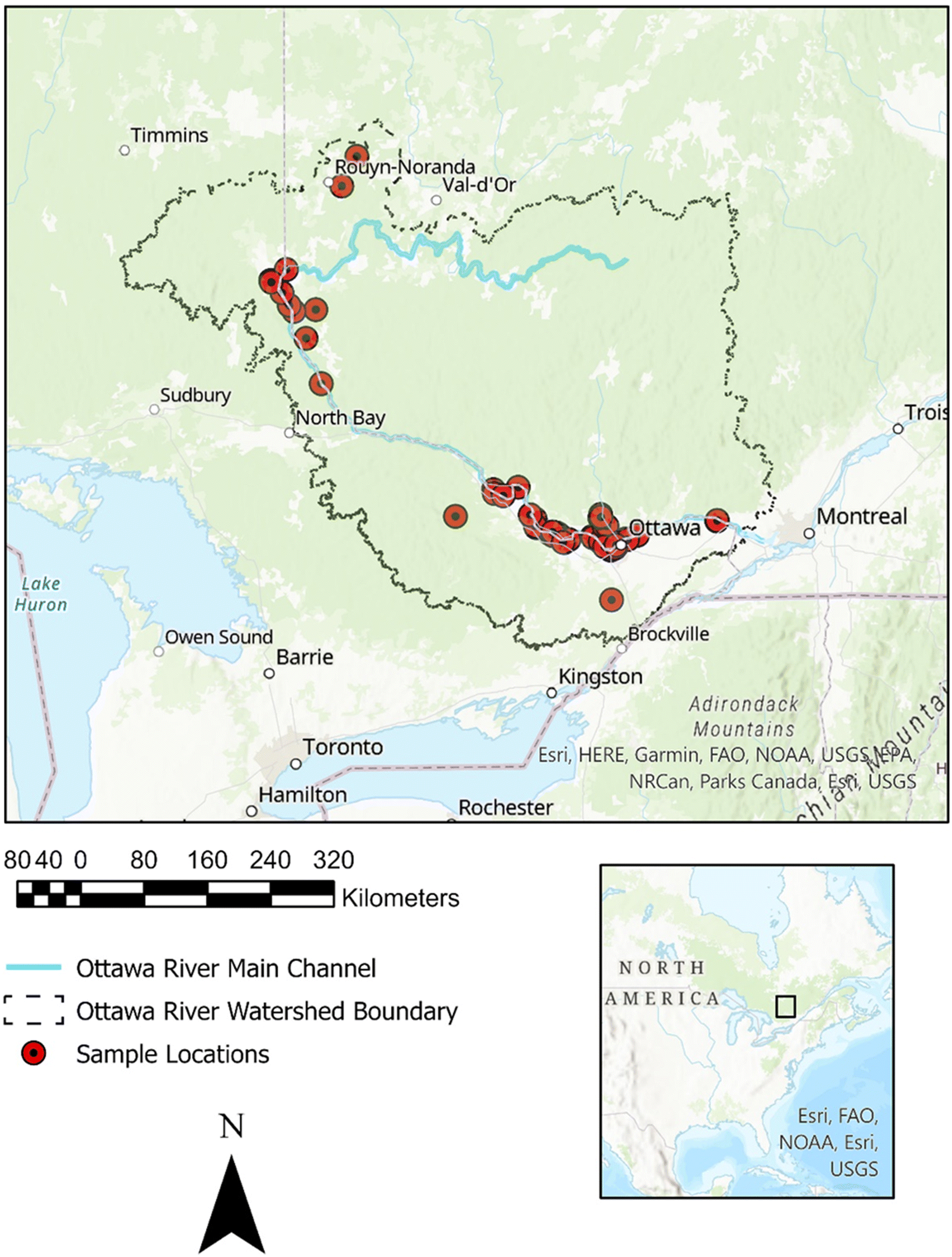
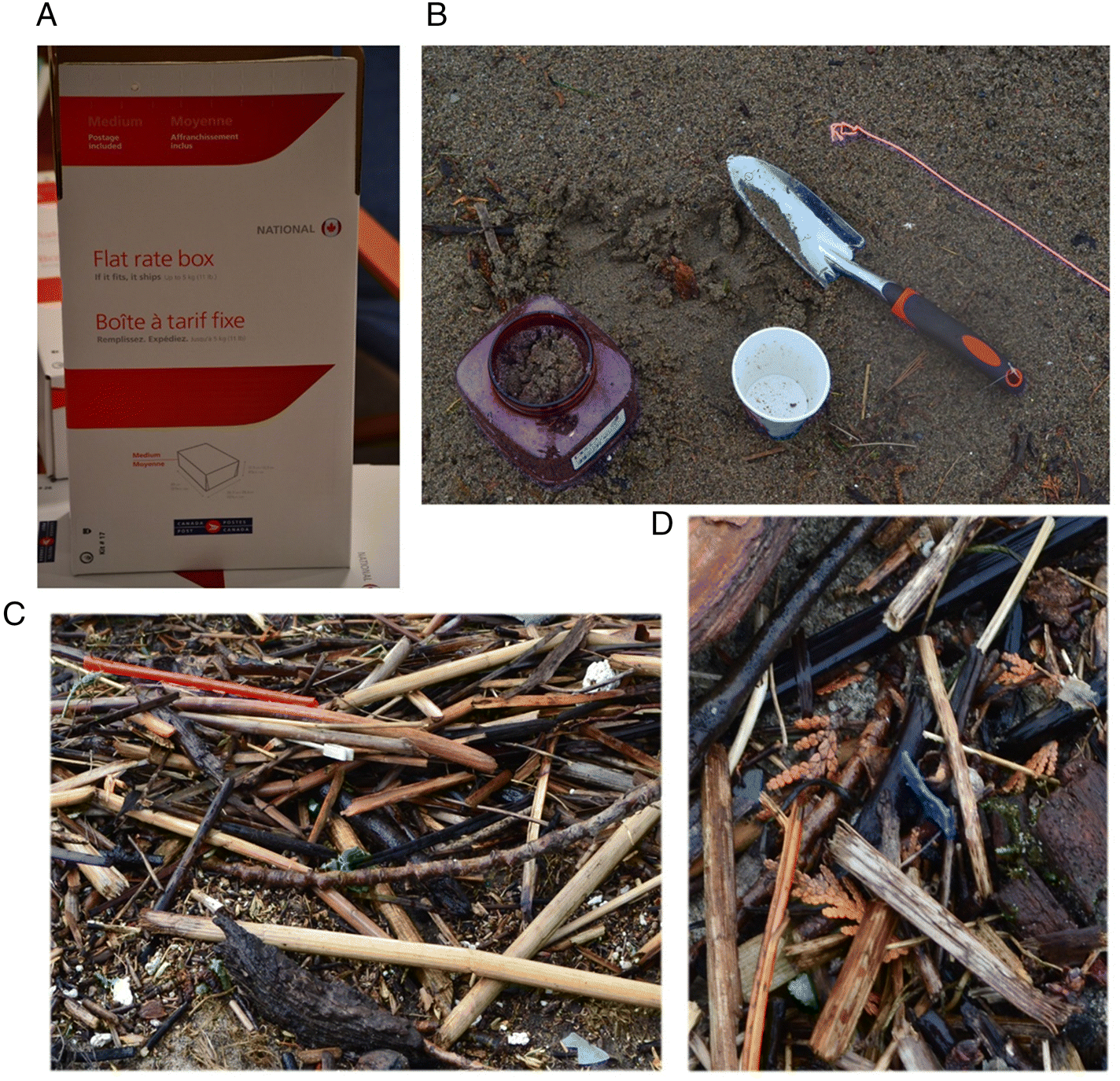
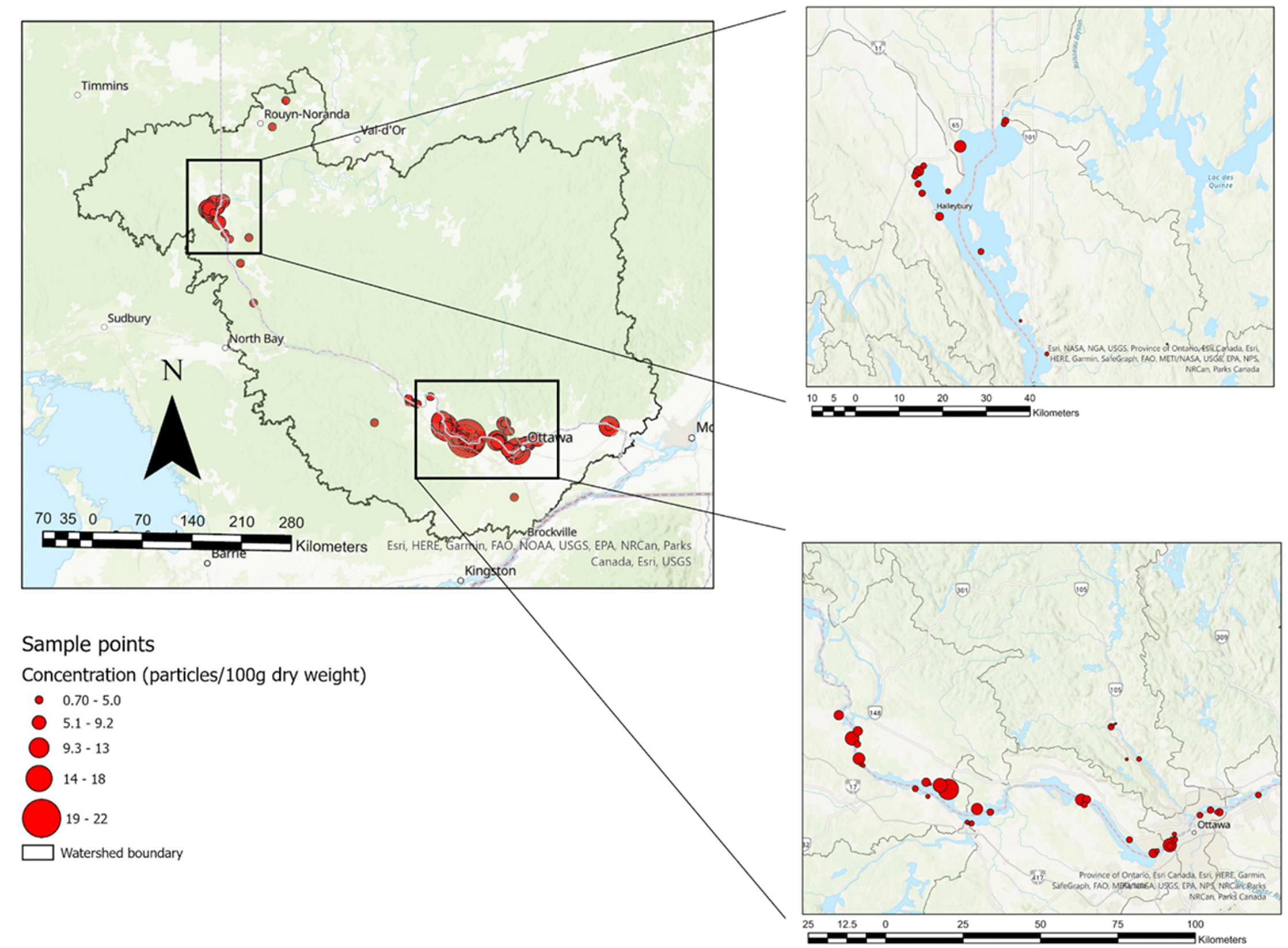
A total of 47 sediment sampling kits were distributed to Community Scientists. Sampling kits were handed to the Community Scientists in May 2019, after the Ottawa River freshet. Once the Community Scientists received the kits, they were free to sample during the late spring and summer period of 2019. Sample dates from the received sediment were between June and September (inclusive) of 2019. Each kit consisted of three containers for three bulk sediment samples, a paper 250 millilitre (mL) cup, and a bright pink string. Additionally, each kit contained an instruction sheet, and all materials were contained in a post-paid box addressed to the Aquatic Ecosystems and Environmental Change Laboratory address at Carleton University in Ottawa, Canada (
Fig. 2). The kits were handed out during an information session where the aims and desired protocols of the project were conveyed to the volunteers. Sampling procedures were based on the sampling protocol in
Horton et al. (2017). Four 250 mL grab samples were collected at one-metre intervals parallel to the shoreline. The research team indicated the optimal place to sample the shoreline was at a distinctive debris line on the shore, where the river level would have been before receding. The Community Scientists used the container, the length of rope (coloured bright pink to distinguish any possible contamination in the processing of samples) and a paper cup that held approximately 250 mL of sediment, for four collections pertaining to a 1 L bulk sample for one of the containers. The rope was pre-marked indicating one metre intervals, thus the volunteers collected one sample at the end of the rope, then at each of the two one-metre marks, and finally at the other end of the rope, representing the four 250 mL collections. Additionally, the rope had metal nails at each end to hold it in place during collection.
Community Scientists were asked to use their own trowel for the sediment collected and it was suggested that it was a metal trawl if possible. Each Community Scientist was provided with three containers and given the option to sample one location (utilising one to three of the containers), or at three different locations. The Community Scientists filled out the provided information sheet that included their name, GPS coordinates, location name, description of the sediments, and clothing colours worn during sampling. The Community Scientists then packed the bulk sample into the aforementioned pre-paid postal box and sent the sample to the laboratory where it was stored in a dark fridge for processing.
Additional analysis included exploring significance between urban and non-urban area concentrations, comparing lake areas to higher flow sections of the river, and comparing between the main channel and tributaries. Various t-tests were performed to establish any significance between these variables.
Laboratory analysis
Sediment preparation and density separation were based on the oil extraction protocol (OEP) (
Crichton et al. 2017), with some modifications to adjust for processing times due to the large amount of sediment. The OEP was chosen as it uses canola oil as reagent, whereas other density separation techniques use harsher chemicals. For example, though zinc bromide (ZnBr
2) has demonstrated the ability to extract higher density polymers, up to 1.7g cm
−3 (
Prata et al. 2019), canola oil’s lower toxicity was a factor in selection as ZnBr
2 may pose a threat to health or the environment if mismanaged (
Mani et al. 2019). Additionally, canola oil does not typically react with the fibres, thus there should be no changes to the structure and (or) the colour of fibres during density separation. Furthermore, an additional advantage of canola oil is cost, especially for larger projects, where it can become an important factor. For example, sodium iodide (Nal) can be an effective additive for density separation, separating plastics up to 1.6g cm
−3, however, where canola oil may cost approximately $0.96 Canadian dollars (CND) per sample (
Crichton et al. 2017), Nal can cost approximately $90.00 CND per sample and ZnBr
2 approximately $922 CDN a sample (
Crichton et al. 2017). Another commonly used reagent for density separation is sodium chloride (NaCl), which can be as cost effective, approximately $10.00 CDN a sample (
Crichton et al. 2017). However, NaCl typically only recovers lower density plastics, up to 1.2 g cm
−3 and could lower recovery rates significantly for environmental samples (
Prata et al. 2019). Furthermore, some of the brine solutions for density separation are based on the specific density of the solution, therefore, exclude certain polymer types with higher densities such as Polytetrafluoroethylene (PTFE) (
Lechthaler et al. 2020). Recent testing on canola oil has highlighted the capability of extracting microplastics in the density range from 11–1760 kg m
−3 and in the size range from 0.02–4.4 mm (
Lechthaler et al. 2020).
An additional factor to consider for density separation for larger projects is processing time. Comparatively, the OEP has a relatively short processing time when compared to other extraction procedures. For example, the OEP does not retain high amounts of organic material, whereas other reagents such as calcium chloride (CaCl
2) requires overnight settling due to higher amounts of organic material that requires longer settling times (
Stolte et al. 2015). Laboratory tests do confirm good recovery for the OEP, with an average recovery rate of 96.2% (± 2.2%) (
Crichton et al. 2017). Additionally, other oil extraction protocols, for example castor oil (
Mani et al. 2019), also exhibit good recovery rates in laboratory tests (99% ± 4%). However, when oil extraction protocols are applied to some environmental matrices, recovery rates may decline. For example, the castor oil protocol recovery rates drop to approximately 75% (± 13%) when used on riverine suspended solids (
Mani et al. 2019). Nonetheless,
Crichton et al. (2017) did apply the OEP validation by spiking beach samples of various grain sizes to try and represent real word conditions for extraction, rather than examining efficiency rates purely on laboratory testing.
Each sample was prepared under a laminar flow hood, transferred to a metal tray, and covered with foil before being transported to an oven for drying. Each sediment sample was dried in an oven at 70 °C (
Corcoran et al. 2015), which is considered to be below the melting point of all common polymers. Furthermore, 70 °C would not provide conditions that could alter the inherent shape of polymers that may be in the sample (
Kalpakjian and Schmid 2008). Drying times were between 6 and 72 h, dependent on the moisture levels of each received sample. Once a sample was dried, a dry weight of the sample was calculated and transferred to a laminar flow hood for density separation.
The samples received were a variety of grain sizes (it was stipulated in the presentation to the Community Scientists, an ideal sediment size range), thus, the first step after drying and before density separation was to put the sample through a 2 mm sieve and 1 mm sieve, with visual inspection for particles after each sieving. The canola oil was prepped for analysis by filtering it through a 100-micron mesh, to remove possible contamination within the canola oil vessel. For density separation, 100 g of the (dry) sediment sample was placed in a 500 mL beaker. Two hundred millilitres of deionized water were then added to the beaker. A clean glass stirring rod was used to swirl the water to create a vortex, so the entire sediment fraction was submerged in the water. After a short settling period, 10 mL of filtered canola oil were added to the beaker and the sediment stirred vigorously for 30 s so the canola oil would come into contact with the entire sediment fraction. The mixture was then left to settle until the oil layer separated fully to the top of the water. Once separation was completed, the oil and water were decanted from the sediment into a separatory funnel. The beaker sidewalls were then rinsed to dislodge any particles and the water and oil layer decanted again into the separatory funnel. The above process was repeated until the separatory funnel was three quarters full, then shaken vigorously for 30 s to ensure any plastics dislodged during decanting were re-introduced back into the oil layer. The mixture was then left to settle until the oil and water layers separated. Once settling was complete, the oil layer with plastic particles was retained and the water and sediment layers were released and discarded. The oil layer was then emptied into a vacuum filtration system with an 80-micron pre-inspected mesh. The filters were then backwashed into a petri dish and the filter and petri dish contents examined for microplastics under a stereomicroscope. Identified particles were then separated into fragments and fibres, and the colours of each fragment and fibre were noted.