Funding change: An environmental scan of research funders’ knowledge translation strategic plans and initiatives across 10 high-income countries/regions
Abstract
Introduction
Materials and methods
Data sources
Study selection
Data abstraction and analysis
Focus group
Results
Published and grey literature
| Published | Grey literature | Sources combined | ||||
|---|---|---|---|---|---|---|
| Country/Region | N | % of total sources | N | % of total sources | N | % of total sources |
| United States | 26 | 66.7% | 38 | 22.0% | 64 | 30.2% |
| Canada | 2 | 5.1% | 58 | 33.5% | 60 | 28.3% |
| United Kingdom | 3 | 7.7% | 34 | 20.0% | 37 | 17.5% |
| Australasia (Australia, New Zealand, and some neighboring Islands) | 2 | 5.1% | 14 | 8.1% | 16 | 7.5% |
| Denmark | 1 | 2.6% | 15 | 8.7% | 16 | 7.5% |
| Norway | 0 | 0% | 10 | 5.8% | 10 | 4.7% |
| Netherlands | 1 | 2.6% | 4 | 2.3% | 5 | 2.4% |
| Sweden | 2 | 5.1% | 0 | 0% | 2 | 0.9% |
| France | 1 | 2.6% | 0 | 0% | 1 | 0.5% |
| McLean et al. (2018)a | 1 | 2.6% | 0 | 0% | 1 | 0.5% |
| Total number of sources | 39 | 100.0% | 173 | 100.0% | 212 | 100.0% |
Published literature
Published literature: Organization and article characteristics
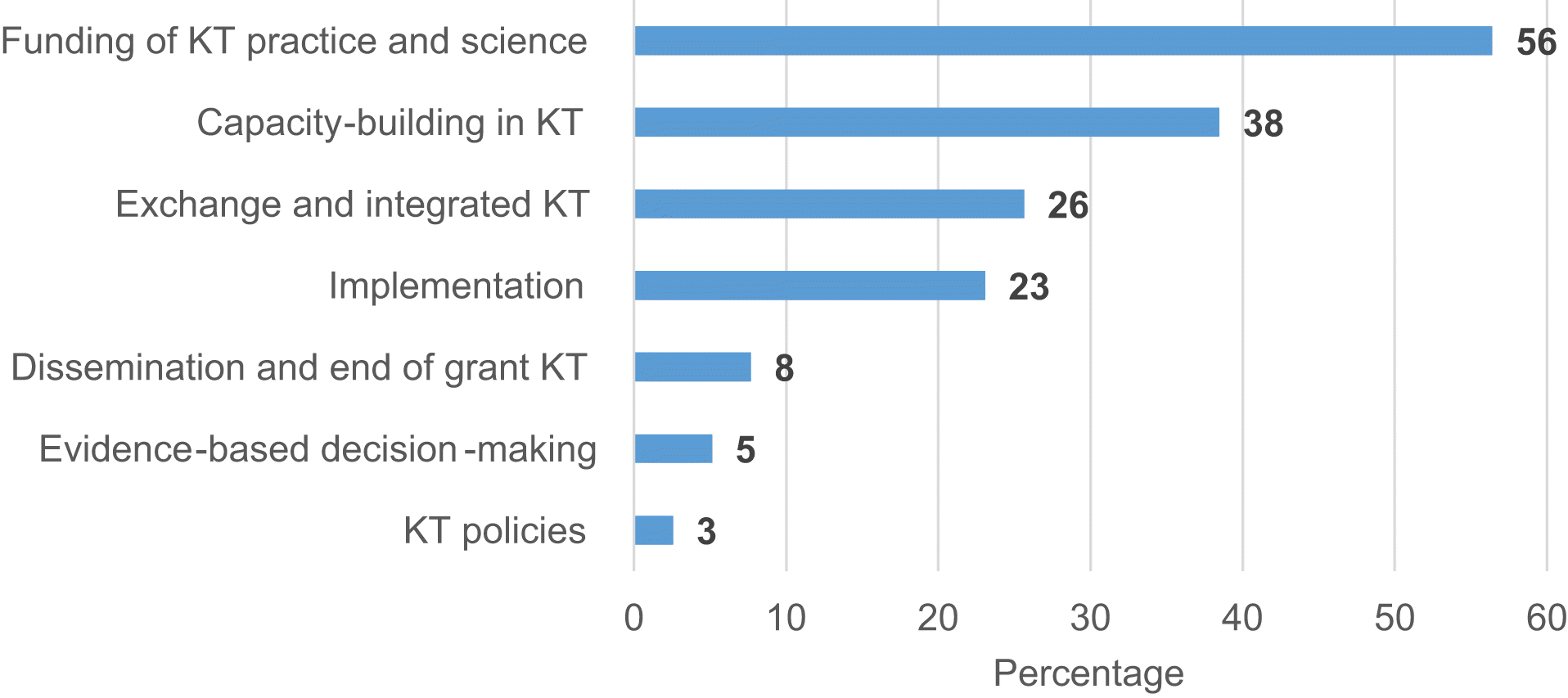
Published literature: KT initiatives
| KT sub-type | Country/Region | Themes | Example |
|---|---|---|---|
| Funding of KT practice and science | United States United Kingdom; Australasia | Programs to support the translation of basic science to clinical application Research grants to support dissemination and implementation research Funding to facilitate collaborative applied research between academics and health practitioners | The National Institutes of Health (NIH) have invested over $4 billion in nanotechnology research to move human disease conditions (most commonly cancer) from discovery to clinical application NIH Research Project Grant dissemination and implementation and translational research awards The National Institute for Health Research (NIHR) funds regional partnerships between National Health Service organizations and academic institutes to conduct research that addresses local needs, translates findings to practice, and builds organizational capacity for research |
| Capacity building in KT | United States | Funding and mentorship programs for underrepresented groups and early career researchers | The NIH Mentored Clinical Scientist Research Career Development includes awards for patient-facing research. These awards aim to support early career researchers to transition to independent researcher awards |
| Exchange and integrated KT | United States United Kingdom | Dedicated funding and guidance to support community engagement in research Mechanisms to support practitioner-driven research inquires | Patient-Centered Outcomes Research Institute’s (PCORI) Pipeline to proposal supports patient and stakeholder partnerships in conducting research and supports new stakeholders to develop such partnerships The UK Clinical Research Collaboration (UKCRC)-funded Centre for Translational Research in Public Health’s “AskFuse” service allows individuals in the health system to submit their questions to be paired with relevant researchers, to promote community-driven research |
| Implementation | United States Canada | Establishment of multidisciplinary networks to accelerate the implementation of medical technology Funding to build practitioner and institutional capacity for implementing research in patient care | Consortia for Improving Medicine with Innovation and Technology (CIMIT) is a network of academic medical centers and universities that have developed a model to accelerate implementation of research into devices, procedures, and technologies. The group collaborates with diverse stakeholders to implement such technologies Council of Academic Hospitals of Ontario’s (CAHO) Advancing Research to Improve Care (ARTIC) program supports academic hospitals in Ontario to implement research evidence to improve patient outcomes |
| Dissemination and end of grant KT | United States | Collaborations to identify challenges to, and opportunities for, conducting D&I research | The University of Southern California in partnership with the NIH and Kaiser Permanente held a symposium on conducting successful dissemination and implementation research |
| Evidence-based decision-making | France | Development of data and clinical networks to iteratively evaluate and improve clinical care | The France Ministry of Higher Education, Research and Innovation and Ministry of Health supported the development of a national network of schizophrenia expert centers that use an iterative data-driven approach to develop personalized care programs for patients |
| KT policies | United States | Mandating transparent and publically available dissemination as a requirement for funded teams | Various US-funding bodies (e.g., Agency for Healthcare Research and Quality (AHRQ), Centers for Disease Control and Prevention (CDC), Food and Drug Administration (FDA), NIH) mandate that manuscripts be stored in PubMed central while others require all trials to be registered on clinicaltrials.gov. PCORI has policies to target dissemination of findings to academic, lay and research audiences |
Published literature: KT initiative evaluation
| KT sub-type | Evaluation plans and (or) indicators described in published literaturea |
|---|---|
| Funding of KT practice and science | United States • National Institutes of Health (NIH)’s National Heart, Lung, and Blood Institute (NHLBI) Science Moving towArds Research Translation and Therapy program (SMARTT) program indicators (Ebert et al. 2016): ○ Number of Investigational New Drug (IND) applications supported by the program ○ Quality of interactions with the Food and Drug Administration (FDA) ○ Number of pre-IND meetings conducted ○ Number of Orphan Drug Applications submitted ○ Number of investigators supported through the planning process (even if they did not reach the stage of IND application) • The NIH nanotechnology portfolio indicators (Henderson and Shankar 2017): ○ Number of funded translational projects that resulted in a clinical trial • NIH’s National Center for Advancing Translational Sciences (NCATS) Clinical and Translational Science Awards (CTSA) program indicators (Hogle and Moberg 2014): ○ Success case studies (i.e., project-specific descriptions and researchers’ perceptions of program impact on researchers’ scientific achievement and career advancement) • NIH’s NCATS CTSA indicators of Emergency Department involvement (Meurer et al. 2016): ○ National survey to assess the degree of involvement of Emergency Care programs in CTSA institutions across the United States, as well as the programs’ use of CTSA resources and their degree of academic collaboration with the CTSA. • Conference on sustainability in KT activity indicators (Proctor et al. 2015): ○ Conference satisfaction survey at the end of the meeting • NIHR’s Collaboration for Leadership in Applied Health Research and Care, South West Peninsula (PenCLAHRC) indicators (Heaton et al. 2016): ○ Monitored the progress of the PenCLAHRC projects and used theory and observations to identify consistent characteristics that led to PenCLAHRC project success • New South Wales (NSW) Ministry of Health’s Population Health and Health Services Research Support (PHHSRS) indicators (Thackway, Campbell and Loppacher 2017): ○ The degree to which the funding contributed to relationships between researchers and policy-makers ○ The degree to which the funding increased researchers’ use of an “embedded approach” to research (i.e., building relationships in local settings and developing a deeper understanding of health system issues) ○ Policy stakeholders’ intended use of NSW Ministry of Health reviews |
| Capacity building in KT | United States • NIH’s National Institute on Drug Abuse (NIDA) “Graduate Certificate in Translational Research in Adolescent Behavioral Health Program” indicators (Baldwin et al. 2017): ○ Translational Research Impact Scale (TRIS) was used to evaluate. The scale includes three domains of potential impact: ▪ Research-related impact (e.g., increases in number of grant submissions and publications by translational researchers) ▪ Translational impacts (e.g., incorporation of clinical trial results into clinical guidelines) ▪ Societal impacts (e.g., strengthening and refining health-related policies, improvements in community health) • NIH’s NIDA, National Institute on Minority Health and Health Disparities (NIMHD), and National Cancer Institute (NCI)’s Clinical Research Education and Career Development (CRECD)-funded university program indicators (Estape et al. 2018): ○ Multidisciplinary career development, including: ▪ Programs’ achievement of diversity outcomes (i.e., composition of annual enrollment of students and diversity of disciplines represented in the composition of the students’ research committees) ▪ Amount of research being conducted on health disparities ○ Degree of multi-institutional participation (e.g., having partner schools’ faculty participate in the program’s admission committee) ○ Researcher career development metrics, including number of: ▪ Scientific presentations ▪ Peer-reviewed and public publications, honors, and awards ▪ Grant submissions ▪ Externally funded research projects ▪ Research and (or) academic appointments • NIH’s Translational Science Training Program (TSTP) indicators (Gilliland et al. 2017): ○ Participant self-reported questionnaire on knowledge and understanding of translational science, career development, exploration, and networking • Indicators of success rates of pediatric NIH career development awardees (i.e., K08 or K23 awards) transitioning to independent research (R01) awards (Good et al. 2018): ○ Number of researchers who accessed NIH RePORTER, an electronic repository of NIH-funded projects ○ Characteristics of successful career-development awardees • Indicators for NIH’s NCATS CTSA Mentored to Independent Investigator Working Group assessment of the “K2R” transition (Yin et al. 2015): ○ Number of K08 or K23 award winners that apply to, versus receive an R01 award ○ K2R acceptance timelines • NIH’s NCATS CTSA KL2 career development program indicators (Schneider et al. 2015): ○ Survey to CTSA education core program administrators on the institutional environment where each KL2 program was implemented (e.g., KL2 program funding, number of KL2 positions available, required coursework) ○ Application and progress reports as well as institutional data were used to assess KL2 awardees’ early career outcomes (e.g., demographic information, department, professional status, number of grants and publications before and after KL2, career outcomes after KL2 program) |
| Exchange and integrated KT | United States • PCORI’s National Patient-Centered Clinical Research Network (PCORnet) evaluation approach (Terry 2017): ○ Three committees (data, engagement, and research) iteratively assess if the program is achieving “authentic engagement” ○ Program is committed to being transparent about their lessons learned |
| Implementation | United States • Consortia for Improving Medicine with Innovation and Technology (CIMIT) indicators (Parrish et al. 2015): ○ Evaluated clinical, academic, and commercial outcomes to assess the success of their program (e.g., return on investment) • Council of Academic Hospitals of Ontario (CAHO)’s Adopting Research to Improve Care (ARTIC) program conducted both a project-level and program-level evaluation (Moore et al. 2016). No specific indicators were listed for the project-level evaluation. Program-level indicators: ○ Reach of the ARTIC program ○ Sustainability of the ARTIC program ○ Spread of the ARTIC program ○ Data collection measures: interviews with program stakeholders and local teams, surveys with all participating sites, document review from the project team leads and the ARTIC program office (each team was required to submit a monitoring and evaluation component) |
| Dissemination and end of grant KT | N/A |
| Evidence-based decision-making | N/A |
| KT policies | N/A |
Published literature: KT initiative challenges and opportunities
| KT sub-type | Audience | Key challenges to KT initiative implementation/sustainability | Key facilitators/opportunities for KT initiative implementation/sustainability by research funders |
|---|---|---|---|
| Funding of KT practice and science | For funders | Limited available funding for KT research With increased focus on translation science, there are fears about the impact on basic science research | Fund research on sustainability and capacity building Use “dispersed” leaderships models to increase buy-in and success of funded teams (e.g., involve multiple stakeholders, develop shared accountability for project success) Make continued funding contingent on meeting benchmarks (e.g., Science Moving towArds Research Translation and Therapy program (SMARTT) program) |
| Capacity building in KT | For funders | Invest in resources to support career development for early career researchers (particularly as related to translational research and relationship building) | |
| Exchange and integrated KT | For funders | Communities (e.g., patients, public) are often not meaningfully engaged in research processes Budgets to support community-partnered research are not adequate for meaningful, continued engagement Research and public health processes may not be compatible (e.g., public stakeholders require answers on a short timeline) | Allocate dedicated funds to support community engagement and the use of integrated KT Provide resources and mentorship to funded research teams to promote uptake of meaningful integrated KT (e.g., Patient-Centered Outcomes Research Institute (PCORI)’s Pipeline to Proposal mechanism) Ongoing forums and conferences can promote community partnership in dissemination and implementation |
| Implementation | For funders For research teams | Lack of implementation knowledge/capacity among funding organizations Lack of appropriate funding/clear funding requirements can limit number/impact of implementation activities Evaluation of implementation efforts can be resource intensive | Build capacity among research funders so they perceive success of implementation as part of their role Provide appropriate resources to research teams to facilitate successful implementation Aim to secure resources needed to execute KT work (e.g., personnel with required skills, dedicated research time, funds) Use multidisciplinary teams with strong facilitators and a focus on end-users to increase success of KT projects |
| Dissemination and end of grant KT | For funders and research teams | Research priorities, processes, and outcomes do not always fit the needs of the healthcare system. Methods to combine effectiveness and improvement science may address these challenges | The use of multidisciplinary teams can promote dissemination of research findings |
| Evidence-based decision-making | For funders and research teams For research teams | Resource limitations (including time, personnel, and funds) and negative attitudes can restrict evidence-based decision-making | Leadership (at the funding or health system level) can promote uptake of evidence-based decision-making |
| KT policies | For funders | There is a general lack of dissemination requirements for funded research projects; few organizations have policies to promote dissemination and uptake of funded research | Consider outlining minimum dissemination requirements for funded research projects. Dissemination should ideally be accessible to the public and provide capacity building tools to improve research uptake |
Grey literature
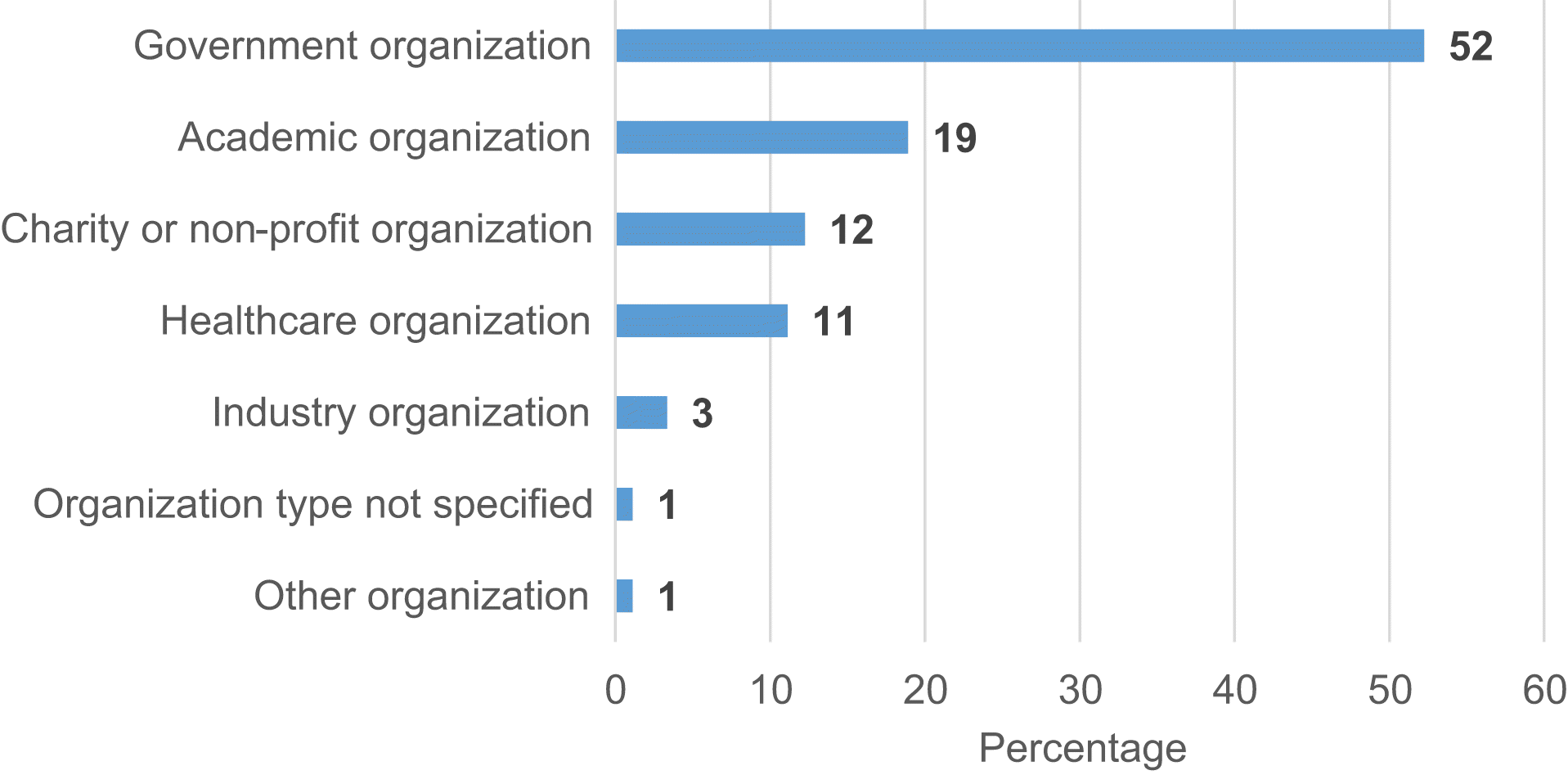
Grey literature: organization and source characteristics
| Organization (n = 21) | Country | Type of organization | Number of sources included in analysis |
|---|---|---|---|
| Social Sciences and Humanities Research Council (SSHRC) | Canada | Government organization | 6 |
| Natural Sciences and Engineering Research Council of Canada (NSERC) | Canada | Government organization | 7 |
| Canadian Institute for Advanced Research (CIFAR) | Canada | Charity or non-profit organization | 4 |
| Health Quality Ontario (HQO) | Canada | Government organization | 8 |
| Council of Academic Hospitals of Ontario (CAHO) | Canada | Government organization | 5 |
| Saskatchewan Health Research Foundation (SHRF) | Canada | Government organization | 4 |
| Michael Smith Foundation for Health Research (MSFHR) | Canada | Government organization | 13 |
| Research Nova Scotia (RNS)a | Canada | Charity or non-profit organization | 3 |
| Alberta Innovates (AI) | Canada | Government organization | 8 |
| U.S. Department of Veterans Affairs (VA) | United States | Government organization | 10 |
| Patient-Centered Outcomes Research Institute (PCORI) | United States | Charity or non-profit organization | 15 |
| National Institutes of Health (NIH) | United States | Government organization | 13 |
| Medical Research Council (MRC)b | United Kingdom | Government organization | 11 |
| Wellcome Trust (WT) | United Kingdom | Charity or non-profit organization | 4 |
| National Institute for Health Research (NIHR) | United Kingdom | Government organization | 15 |
| Health Foundation (HF) | United Kingdom | Charity or non-profit organization | 4 |
| National Health and Medical Research Council (NHMRC) | Australia | Government organization | 7 |
| Health Research Council of New Zealand (HRC) | New Zealand | Government organization | 7 |
| Norwegian Medical Research Council (NMRC) | Norway | Government organization | 4 |
| Danish Agency for Science, Technology and Innovation (DASHE) | Denmark | Government organization | 10 |
| Netherlands Organization for Health Research and Development (ZonMw) | The Netherlands | Government organization | 15 |
| Total number of URLs reviewed | 173 | ||
Grey literature: KT goals and initiatives
Grey literature: stakeholder groups
Focus group
Focus group: Participant characteristics
Focus group: KT initiatives
Focus group: KT “blue sky” ideas
| “Blue sky” idea themes | Illustrative quotation |
|---|---|
| Research funding system be re-engineered to emphasize “pull innovation” | “Go upstream and identify what the problems are… the way research has been funded, it seems to be designed for the scholarly circuit of the research and the goal of the circuit is to get another trip around the circuit, and it’s not to actually influence practice or actually change the design on the backend. So I think I would do a methodological reengineering of the system to make sure we had all those things, and I think pull innovation is really important.” – Participant 15 |
| Funders appoint a team within the funding organization to support KT and communications | “I would create a dedicated team inside my organization completely dedicated to KT being able to promote some different actions in terms of funding, but also in terms of I don’t like to say communication because it’s KT is more than communication but that is the point.” – Participant 85 |
| Funders create a mechanism for research results to flow into a relevant database | “It’ll be really good to have an ability to go in there and then utilize the funding that we do with our researchers and have that key aspect at the end of the research that the data that’s generated can actually flow into, can be put in a certain manner that can flow into this large database. So it could be utilized, so connectivity.” – Participant 67 |
| Funders increase engagement of community organizations to enable evidence implementation | “I would also try to find a way to engage more strongly municipalities and community organizations. Part of evidence should be translated in interventions led by them.” – Participant 85 |
| Funders embed KT experts within decision-making bodies | “I’d love to be able to embed both senior level in the health authorities with someone who understands knowledge translation. I know the people that we work with who are at the mid-level are really striking their heads against a wall, trying to get senior management to understand the benefits around knowledge translation and what needs to be done.” – Participant 43 |
| Funders evaluate the quality and impact of KT initiatives conducted by funders and funded teams | “Co-determine who needs to be at the table and they would have the time and money to be there. You co-design solutions with them at the table and then you would co-implement those solutions and evaluate at the back end.” – Participant 15 |
Discussion
Limitations
Conclusion
Acknowledgementsc
Footnote
Abbreviations
- AHRQ
- Agency for Healthcare Research and Quality
- AI
- Alberta Innovates
- ARTIC
- Adopting Research to Improve Care
- CAHO
- Council of Academic Hospitals of Ontario
- CDC
- Centers for Disease Control and Prevention
- CHeReL
- Centre for Health Record Linkage
- CIFAR
- Canadian Institute for Advanced Research
- CIHR
- Canadian Institutes of Health Research
- CIMIT
- Consortia for Improving Medicine with Innovation and Technology
- CMS
- Centers for Medicare and Medicaid Services
- CRECD
- Clinical Research Education and Career Development
- CTSA
- Clinical and Translational Science Awards
- DASHE
- Danish Agency for Science, Technology and Innovation
- DOD
- US Department of Defense
- FDA
- Food and Drug Administration
- HF
- Health Foundation
- HQO
- Health Quality Ontario
- HRC
- Health Research Council of New Zealand
- HRSA
- Health Resources and Services Administration
- IND
- Investigational New Drug
- KT
- Knowledge Translation
- MRC
- Medical Research Council
- MSFHR
- Michael Smith Foundation for Health Research
- NCATS
- National Center for Advancing Translational Sciences
- NCI
- National Cancer Institute
- NHLBI
- National Heart, Lung, and Blood Institute
- NHMRC
- National Health and Medical Research Council
- NIDA
- National Institute on Drug Abuse
- NIGMS
- National Institute of General Medical Sciences
- NIH
- National Institutes of Health
- NIHR
- National Institute for Health Research
- NIHR SPHR
- NIHR School for Public Health Research
- NIMH
- National Institute of Mental Health
- NIMHD
- National Institute on Minority Health and Health Disparities
- NINDS
- National Institute of Neurological Disorders and Stroke
- NMRC
- Norwegian Medical Research Council
- NSERC
- Natural Sciences and Engineering Research Council of Canada
- NSF
- National Science Foundation
- NSHRF
- Nova Scotia Health Research Foundation
- NSW
- New South Wales
- ORA
- Outcome Reporting Accelerator
- PenCLAHRC
- Collaboration for Leadership in Applied Health Research and Care, South West Peninsula
- PCORI
- Patient-Centered Outcomes Research Institute
- PCORnet
- Patient-Centered Clinical Research Network
- PHHSRS
- Population Health and Health Services Research Support
- RF
- ResearchFish
- RNS
- Research Nova Scotia
- SMARTT
- Science Moving towArds Research Translation and Therapy program
- SHRF
- Saskatchewan Health Research Foundation
- SSHRC
- Social Sciences and Humanities Research Council
- TRIS
- Translational Research Impact Scale
- TSTP
- Translational Science Training Program
- UKCRC
- UK Clinical Research Collaboration
- USDA
- U.S. Department of Agriculture
- VA
- U.S. Department of Veterans Affairs
- WT
- Wellcome Trust
- ZonMw
- Netherlands Organization for Health Research and Development
References
Supplementary material
- Download
- 95.49 KB
Information & Authors
Information
Published In

History
Copyright
Data Availability Statement
Key Words
Sections
Subjects
Plain Language Summary
Authors
Author Contributions
Competing Interests
Funding Information
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
There are no citations for this item