Overall weight of evidence across pathways
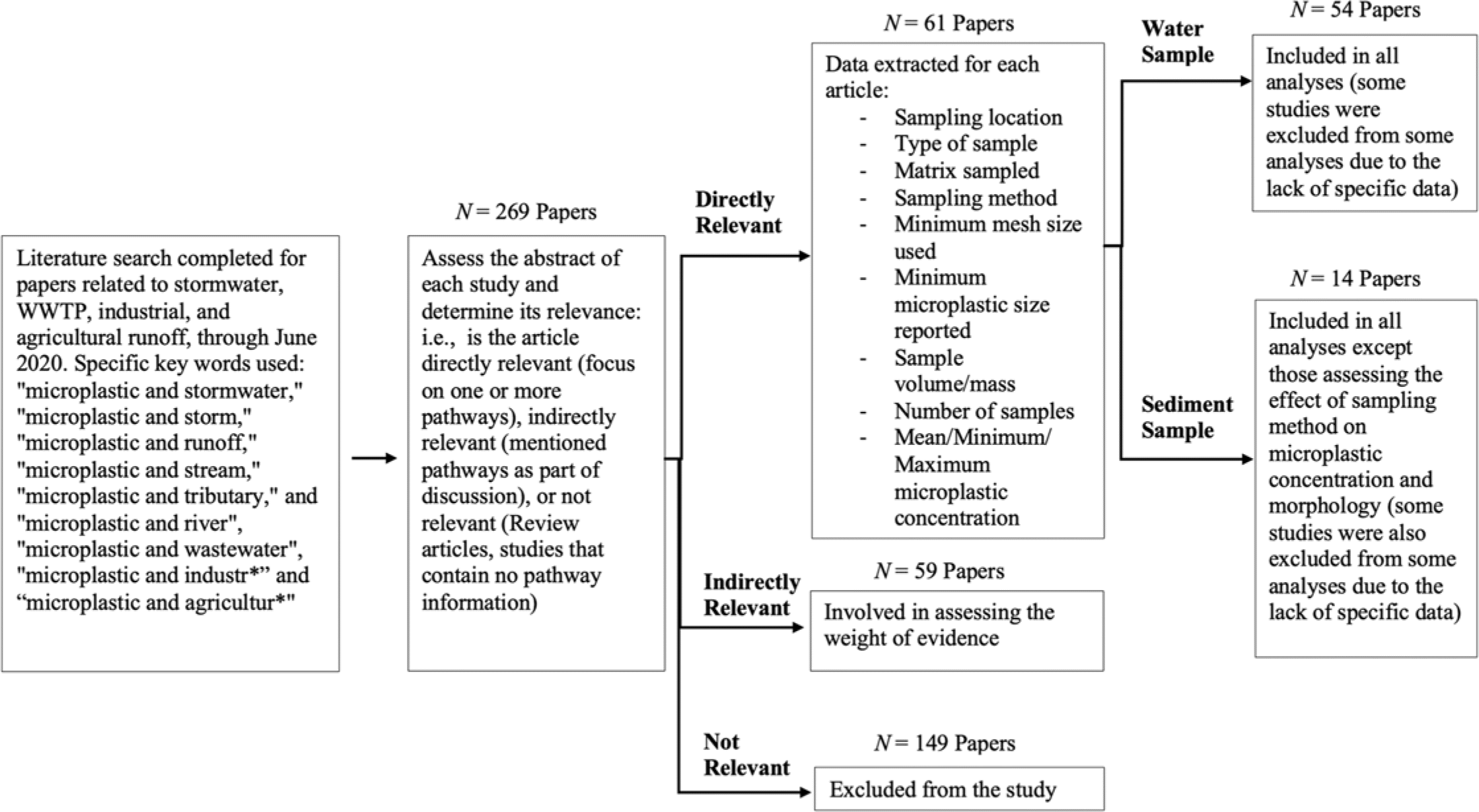
Our primary search resulted in a total of 269 articles containing keywords related to microplastics and the four pathways. Of these, 120 studies contained information that was either directly (n = 61) or indirectly (n = 59) relevant to microplastics in different pathways that contribute to plastic pollution in freshwater ecosystems (
Fig. 1A;
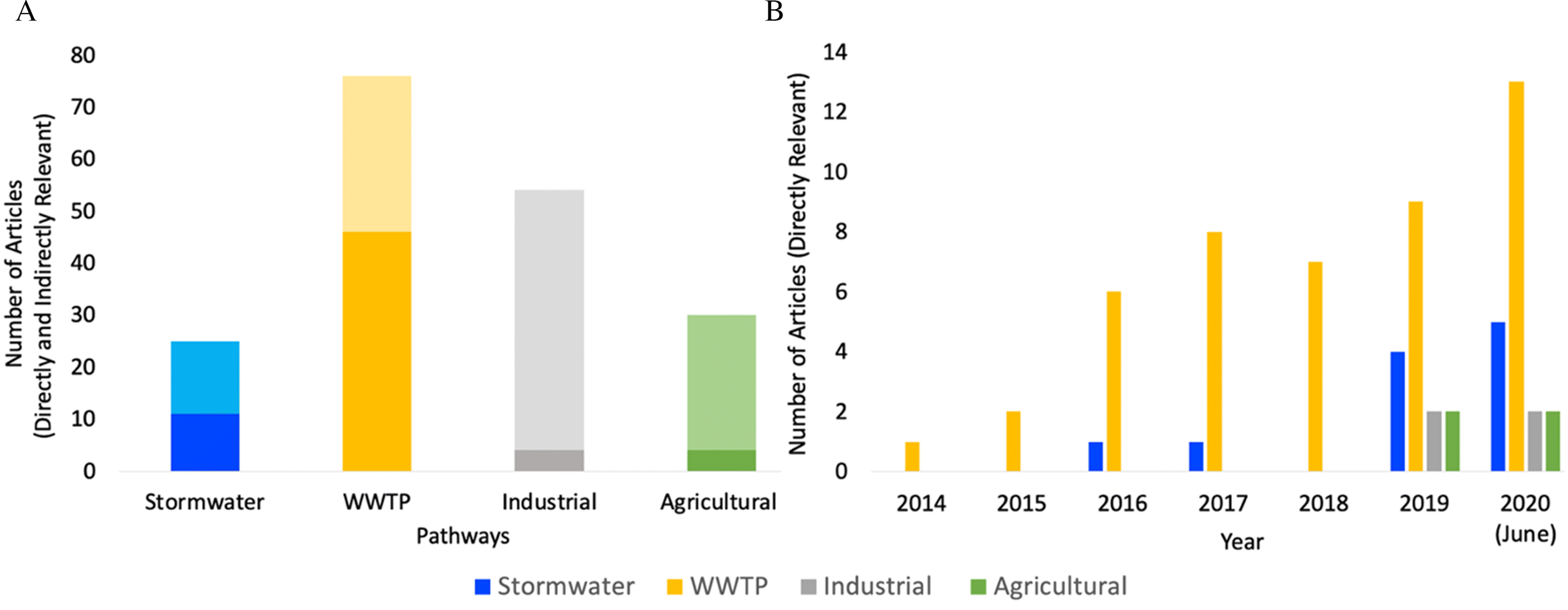
SI Data Table II). Of the 120 articles that were relevant to at least one pathway (directly or indirectly), 25 (21%) included stormwater runoff, 76 (63%) included WWTP effluent, 54 (45%) included industrial effluent, and 25 (21%) included agricultural runoff (
Fig. 2A). There were more indirectly relevant studies for all pathways except for WWTP effluent (
Fig. 2A), demonstrating the proportion of quantitative observations relative to those that discussed pathways as potentially important. In total, the number of directly relevant studies included 11 for stormwater, 46 for WWTP, 4 for industrial runoff, and 4 for agricultural runoff (for a list of the publications on each pathway informing this analysis, see
SI Data Table III, IV).
The list of assembled studies was examined for patterns in the publication record over time (
Fig. 2B). Although the first studies captured by our literature search date back to the 1980s, these were indirectly relevant, and the first directly relevant study was published in 2014 (
McCormick et al. 2014). This study focused on WWTP effluent, which reflects the early acknowledgement of WWTP effluent as a major pathway for microplastics to aquatic ecosystems (
Browne et al. 2011;
Eriksen et al. 2013). WWTP effluent remains the most studied pathway, incorporated in 50% of all directly relevant papers published in 2020.
Studies related to stormwater runoff appeared in the literature shortly after WWTP effluent, with the first directly relevant paper by
Baldwin et al. 2016. Recent increases in stormwater runoff studies reflect a growing focus on this pathway (
Fig. 2B), especially since urban and stormwater runoff carry tire wear particles among other contaminants (
Sutton et al. 2019;
Tian et al. 2021). Stormwater retention ponds serve as microplastic retention sites on the landscape, and as conduits for microplastics to receiving waters (
Liu et al. 2019a;
Moruzzi et al. 2020). Stormwater ponds in industrial/commercial areas have higher microplastic concentrations than those receiving highway and residential runoff (
Liu et al. 2019a). More research on microplastic abundance, composition, upstream sources, and transport is needed to assess the role of stormwater in microplastic pollution in aquatic ecosystems, and the capacity for existing storm water management infrastructure to capture microplastic pollution.
The recognition of agricultural activities as a source of microplastics to aquatic ecosystems is relatively recent (
Nizzetto et al. 2016;
Liu et al. 2018;
Lv et al. 2019). Plastics are used in agricultural applications, and can degrade into microplastics during use. Like industrial runoff, microplastics from agricultural drainage has few direct measurements (
Fig. 2A). Microplastics have been reported in rice fields (
Lv et al. 2019) and in biosolids applied to agricultural fields (
Crossman et al. 2020;
Nizzetto et al. 2016). Biosolids contain concentrated microplastics removed during wastewater treatment (
Lusher et al. 2019). In addition to biosolids, plasticulture such as plastic mulching materials, contributes to microplastics in agricultural fields (
Steinmetz et al. 2016). Recently, concerns about agriculture-derived microplastics were cited as a top priority in the state of California (
California Ocean Science Trust 2021). Understanding agricultural sources of microplastics to aquatic ecosystems also requires more research on microplastic movement within soils, drainage networks (e.g., tile drains and retention ponds), and interactions with plants (
Rillig et al. 2017).
Despite the historic and ongoing dominance of studies focusing on WWTP effluent as a pathway delivering microplastics to aquatic ecosystems, our meta-analysis highlights the importance of stormwater and other pathways. We also illustrate a clear need for more studies that measure the abundance and diversity of microplastics in more diverse pathways. Because our search stopped in July 2020, we quickly surveyed the literature in Summer 2021 to see if more studies have been published on any of these pathways. We found six directly relevant papers on stormwater, seven on WWTP, and four each on industrial and agricultural. While more research has started to focus on each individual pathway, WWTP effluent is still the dominant pathway studied, and thus, more research is needed to expand our understanding of the relative contributions (e.g., relative loads to the environment) from all of these pathways.
Microplastic concentrations across pathways
From the 54 directly relevant studies included in the analysis, most quantified and characterized microplastics in water (n = 49), and fewer completed analyses in sediments (n = 11). The extracted data from each study can be found in the Supporting Information (
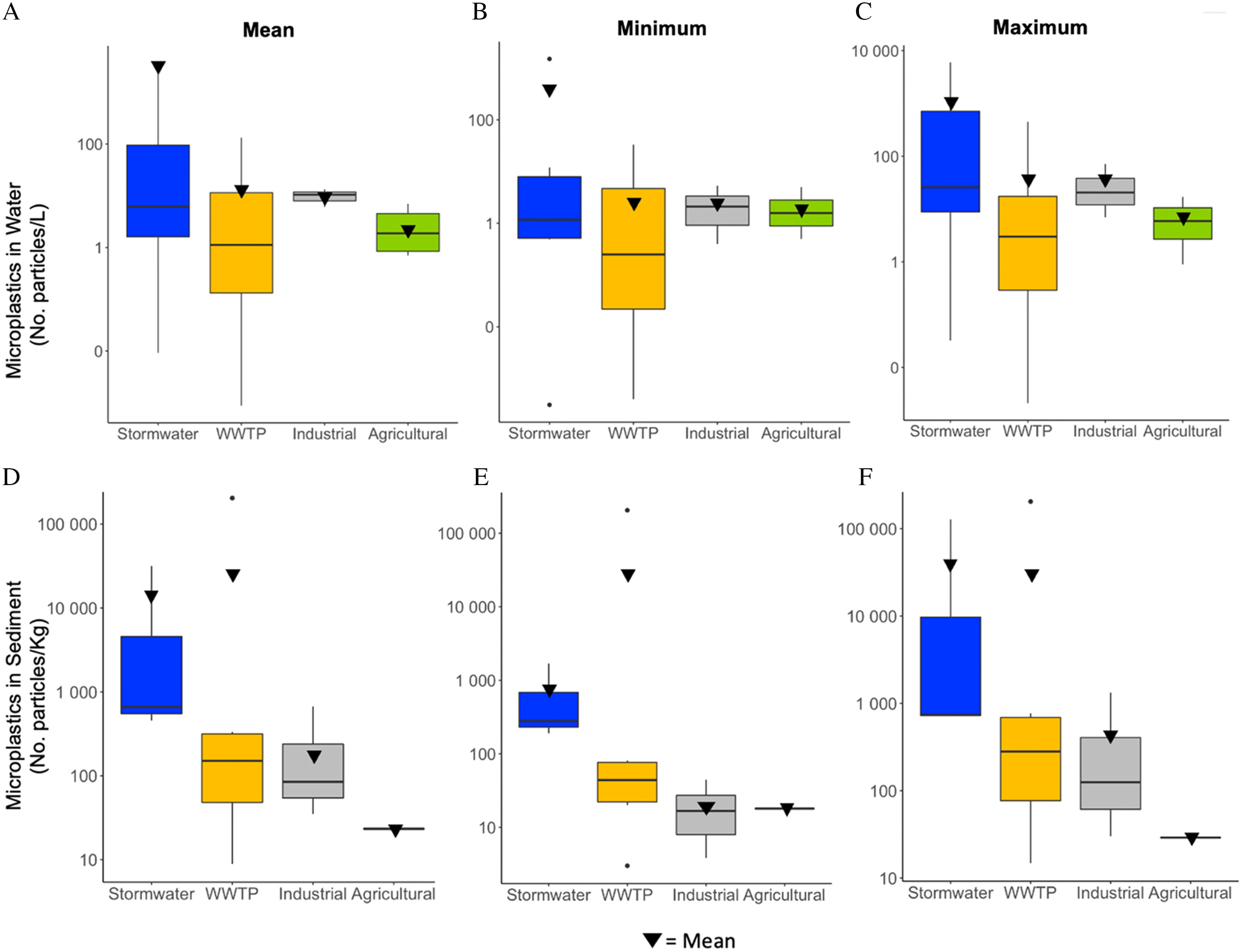
SI Data Tables III, IV). We first investigated the patterns of microplastic concentrations reported in water among pathways. Despite the dominance of research efforts on WWTP effluent across the literature, our results show that stormwater runoff had the highest overall mean concentration (676 microplastic particles/L; n = 6) compared to the other three pathways (
Fig. 3A–C). Stormwater also had the greatest variability in concentrations, ranging from 0.009 (
Baldwin et al. 2016) to 3862 particles/L (
Järkskog et al. 2020). We note some of the highest concentrations of microplastics in stormwater runoff samples were from stormwater retention ponds where microplastics accumulate. Still, studies that sampled several pathways in one region observed a similar pattern, where microplastic concentrations in stormwater runoff were higher than WWTP effluent (Stormwater = 15.4 particles/L, WWTP effluent = 13.3 particles/L (
Grbic et al. 2020); Stormwater = 8.1 particles/L, WWTP effluent = 0.058 particles/L (
Zhu et al. 2021)) The second highest mean concentrations were found in WWTP effluent (mean = 12.6 microplastic particles/L; min = 0.0088 particles/L, max = 132 microplastic particles/L; n = 39) (
Carr et al. 2016;
Hidayaturrahman and Lee 2019). In industrial effluent/runoff, the mean concentration of microplastics was 9.9 microplastic particles/L (n = 3), ranging from 6.1 microplastic particles/L (
Alam et al. 2019) to 13.3 particles/L (
Deng et al. 2020). Concentrations were the lowest in agricultural runoff, with a mean of 3.1 microplastic particles/L (n = 4), ranging from 0.7 (
Lv et al. 2019) to 7 microplastic particles/L (
Alam et al. 2019).
While the trends in microplastic concentrations observed among pathways is a valuable start to understanding their relative role in microplastic pollution, differences in
concentrations do not equate to their relative
load into freshwater ecosystems. Understanding the overall contribution of each pathway to an aquatic ecosystem requires measuring the discharge as well (volume/time). For example, discharge of wastewater effluent typically occurs as a relatively constant volume over time (although it can vary with time of day and in systems with combined sewers). Discharge from stormwater and agricultural inputs, however, are more variable in duration and magnitude than WWTP effluent. Previous studies that have attempted to estimate microplastic loads, such as
Boucher et al. (2019),
Zhu et al. (2021), and
Werbowski et al. (2021), suggest a dominance of stormwater as a pathway to local aquatic ecosystems over WWTP effluent. To the best of our knowledge, no study has measured all four pathways in a single ecosystem, which is needed to generate a comparison of total microplastic load across pathways.
Microplastic concentrations in sediments downstream of potential inputs followed a similar overall pattern to water, where the mean concentrations and variability among studies was greatest in sediments receiving stormwater (collected from ponds, wetlands) compared to other pathways (
Fig. 3D–F). Across all studies, however, sediments impacted by WWTP effluent had the highest mean concentration at 34,300 microplastic particles/kg (n = 6). Values ranged from 8.9 microplastic particles/kg (
Talvitie et al. 2015) to 205,000 microplastic particles/kg (
Haave et al. 2019). The extremely high concentration reported in
Haave et al (2019) skews the mean, affecting the pattern observed here. Sediments impacted by stormwater had the second highest mean microplastic concentration at 10 900 microplastic particles/kg (n = 3), ranging from 468 (
Ziajahromi et al. 2020) to 31 700 microplastic particles/kg (
Liu et al. 2019a,
2019b). Sediments impacted by industrial effluent/runoff had the third largest mean concentration at 263 microplastic particles/kg (n = 3), ranging 35 microplastic particles/kg (
Alam et al. 2019) to 670 microplastic particles/kg (
Deng et al. 2020). Only one study focused on agricultural runoff, which had the lowest mean concentration among the pathways at 23.3 microplastic particles/kg (n = 1;
Alam et al. 2019).
Overall, these results provide some insight into the relative concentrations of microplastics within each of the four transport pathways explored here, but there are some important caveats for the inferences generated from this analysis. This dataset reflects a modest number of studies and samples, which use a range of sampling approaches and parameters (e.g., particle size), and show high variability and skewed distributions. We stress these comparisons are preliminary and illustrate the need for more research which includes multiple microplastic pathways using consistent protocols applied across each. Furthermore, measurements of microplastic should be paired with discharge and conducted with sufficient frequency to quantify particle loads to assess the relative contributions of each pathway to a water body (e.g., lakes or rivers).
Microplastic morphologies across pathways
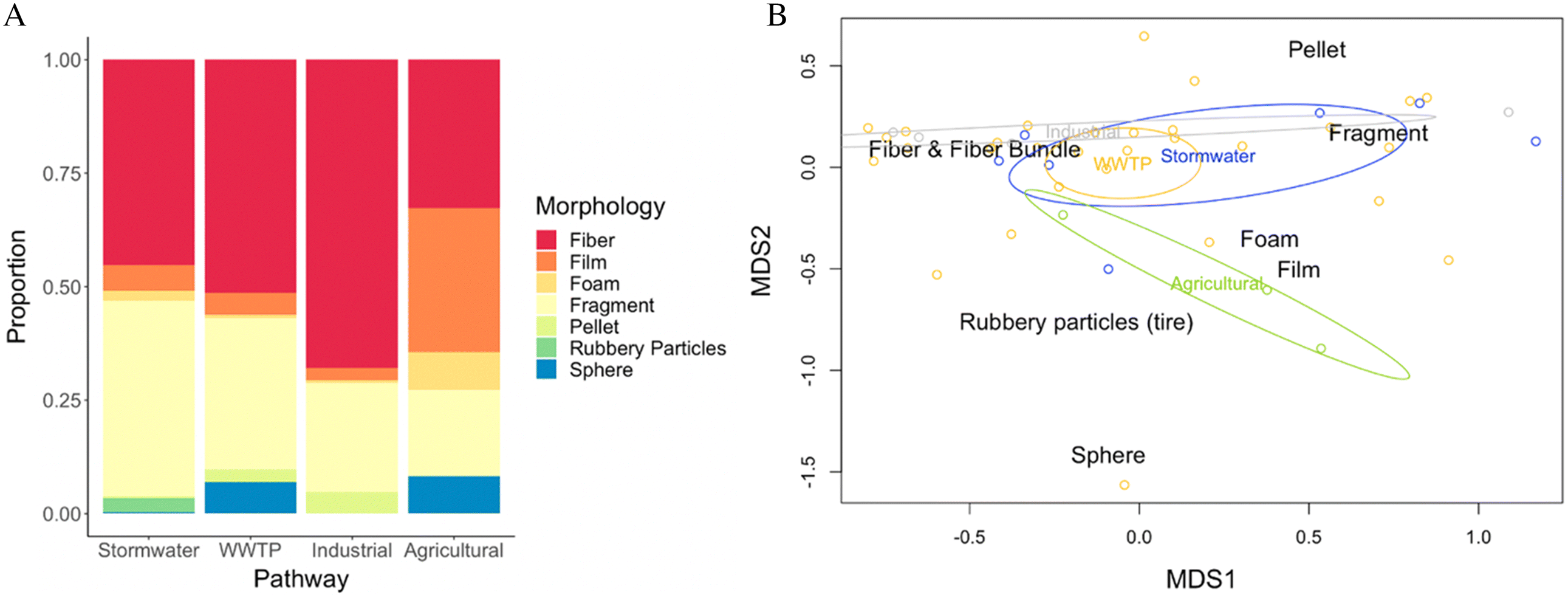
Material shape offered some insight into particle composition across pathways. All four pathways were dominated by fibers and fragments, with a mean abundance of 45.7% and 43.1% in stormwater, 54.6% and 28.4% in WWTP effluent, 67.9% and 24% in industrial effluent/runoff, and 32.7% and 19% in agricultural runoff, respectively (
Fig. 4A). Still, there were some unique morphologies among pathways. Rubbery particles were only found in stormwater runoff. Spheres were found only in WWTP effluent and agricultural runoff. In industrial effluent/runoff the relative abundance of pellets was higher than other pathways, and in agricultural runoff film was more common than other pathways. The morphology distributions were not significantly different among pathways (PERMANOVA,
p = 0.15) and overlap of datapoints on the NMDS occurred among all pathways, likely as a result of fibers and fragments (
Fig. 4B). The NMDS showed the largest overlap among WWTP effluent and stormwater. Industrial effluent/runoff also had some overlap with each of stormwater and WWTP effluent. Agricultural runoff had the least overlap, suggesting that agricultural runoff has the most unique morphology distributions.
Although the assemblages of microplastic morphologies did not show a significant difference among the four pathways, the data can still facilitate inferences about potential microplastic sources. For instance, stormwater runoff was the only pathway with rubbery particles reported. These particles were likely tire and roadwear particles (tires and crack sealant), which have been reported in stormwater (
Grbic et al. 2020;
Järlskog et al. 2020;
Werbowski et al. 2021). The spheres in WWTP effluent and agricultural runoff are likely from personal care products flowing through the plants or captured in sludge and applied to land (
Hidayaturrahman and Lee 2019;
Lv et al. 2019). Other studies, such as
McCormick et al. (2014), also identified spheres, attributed to personal care products as a common morphology in WWTP effluent. Pre-production plastic pellets were relatively unique to industrial effluent/runoff. This may be expected if plastic industry is upstream. Similarly, a high proportion of fibers was observed in studies that sampled near textile industries (
Deng et al. 2020). Finally, a large percentage of films were reported in agricultural runoff, consistent with the use of plastic mulch (
Lv et al. 2019a,
2019b;
Zhang et al. 2019). These unique morphologies could potentially act as signatures of dominant sources for a particular pathway, which would be useful in monitoring to inform source reduction (
Helm 2017).
While the patterns observed here are suggestive, more research is necessary to link microplastic morphologies to input pathways. A more standard classification of microplastic morphologies is necessary to allow harmonized monitoring to improve source apportionment and inform policies. For instance, some studies classified rubber as fragments (
Zhu et al. 2021), and spheres as pellets (
Eriksen et al. 2013;
Mason et al. 2018). This confounds synthesis across studies. For example, the pellets contributing to wastewater in
Fig. 4 are most likely spheres, based on inferences of them as spherical microbeads from personal care products in the publications. However, we had to list them as pellets because it is how they were categorized in their datasets. Moreover, the small sample sizes for many pathways in the literature, especially agricultural and industrial effluent/runoff, may preclude accurate estimation of unique morphologies. This field of study is quickly growing, and newly published articles on related industrial and agricultural runoff pathways that occurred since our data synthesis date (June 2020) will increase available morphological data. For example,
Werbowski et al. (2021) sampled stormwater and similarly reported a high proportion of rubbery fragments.
Sang et al. (2021) also found a high percentage of fragments in their stormwater sample.
Zhu et al. (2021) sampled wastewater and similarly reported spheres as a signature of WWTP effluent. On the other hand,
Treilles et al. (2021) and
Lutz et al. (2021) reported higher microfiber concentrations in stormwater, followed by fragments. Thus, further studies with greater consistency in morphological classification of microplastic particles in these pathways will help to elucidate these patterns.
Implications to inform monitoring strategies
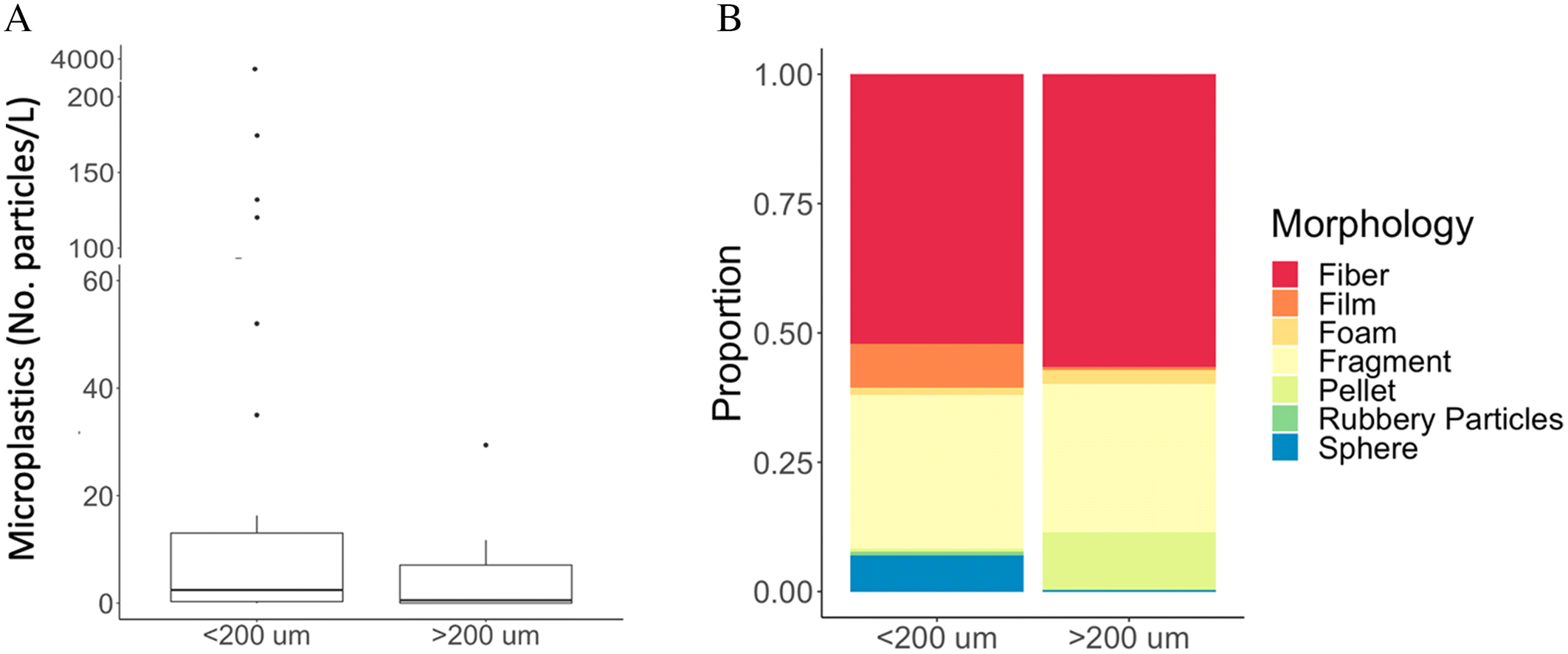
In addition to analyzing the concentrations and morphologies of microplastics in each pathway, we examined the data generated by different sampling methodologies. Due to the small number of studies that focused on microplastics in sediments, we only included studies that sampled microplastics in water (n = 49). First, studies were separated by sampling mesh size (which is an indicator of the smallest particle size captured): less than 200 μm and greater than 200 μm (See
SI Data Tables XIII, XIV). Among all studies included in this analysis, 41 (84%) sampled microplastics <200 μm, and eight (16%) sampled microplastics >200 μm. We noted a trend that researchers taking samples in a relatively smaller water body, such as stormwater retention ponds (
Liu et al. 2019a) and WWTP effluent tanks, favored water collection with pumps, bottles, or buckets, followed by sample filtration in the lab. Studies sampling in larger bodies of water, such as rivers and lakes, tended to use net sampling methods with a larger mesh size.
Concentrations of microplastics varied between sampling mesh sizes (
Fig. 5A). From the 44 studies using <200 μm sampling, mean and median concentrations were 105 and 2.45 microplastic particles/L, respectively. Mean concentrations ranged from 0.001 to 3862 microplastic particles/L. From the studies using >200 μm sampling, concentrations were lower with mean and median concentration of 5.97 and 0.53 microplastics particles/L, respectively. Samples collected with larger mesh also showed less variability among studies, with mean concentrations ranging from 0.001 to 29.4 microplastic particles/L. We observed a very similar pattern relative to sample volume, whereby smaller volume samples had higher concentrations and greater variability (
Fig. S1).
We compared morphological diversity captured using each method. Morphological diversity collected by both methods was dominated by fibers and fragments, with 54.5% fiber and 26.4% fragment reported in samples collected with a smaller mesh and 57.7% fiber and 26.1% fragment reported in samples collected with a large mesh (
Fig. 5B). Samples collected with a smaller mesh size reported a greater proportion of spheres (6%), and samples collected with a larger mesh size reported a greater proportion of pellets (11%). Studies using a smaller mesh size also reported a slightly greater diversity of shapes, including all seven morphologies discussed in this review. Samples collected with a larger mesh size only included six morphologies, which did not include rubbery particles. Again, patterns documented according to mesh size were very similar relative to sample volume, with all samples being dominated by fibers and fragments, and smaller volumes capturing greater morphological diversity (
Fig. S1).
These results suggest that studies sampling with a smaller mesh size capture higher concentrations than samples taken with large mesh sizes. This is most likely because these methods capture smaller particles, and a broader spectrum of size classes. The result regarding morphologies is not consistent with
Hung et al. (2021) who report more diversity of microplastic size and morphologies in trawl samples (with larger mesh size) compared to water samples (filtered through a finer mesh) sampled from the same sites in San Francisco Bay. The greater diversity of morphologies observed in smaller mesh sampling here is likely due to where in the water column and how samples were taken in the different pathways – typically from smaller water bodies or directly from wastewater effluent tanks and stormwater retention ponds. Other factors, such as the location, could also play a role in the different microplastic concentrations and morphologies reported. In this study, sampling nets with larger mesh size were mainly used to sample microplastics in the river, typically downstream of an effluent pipe.
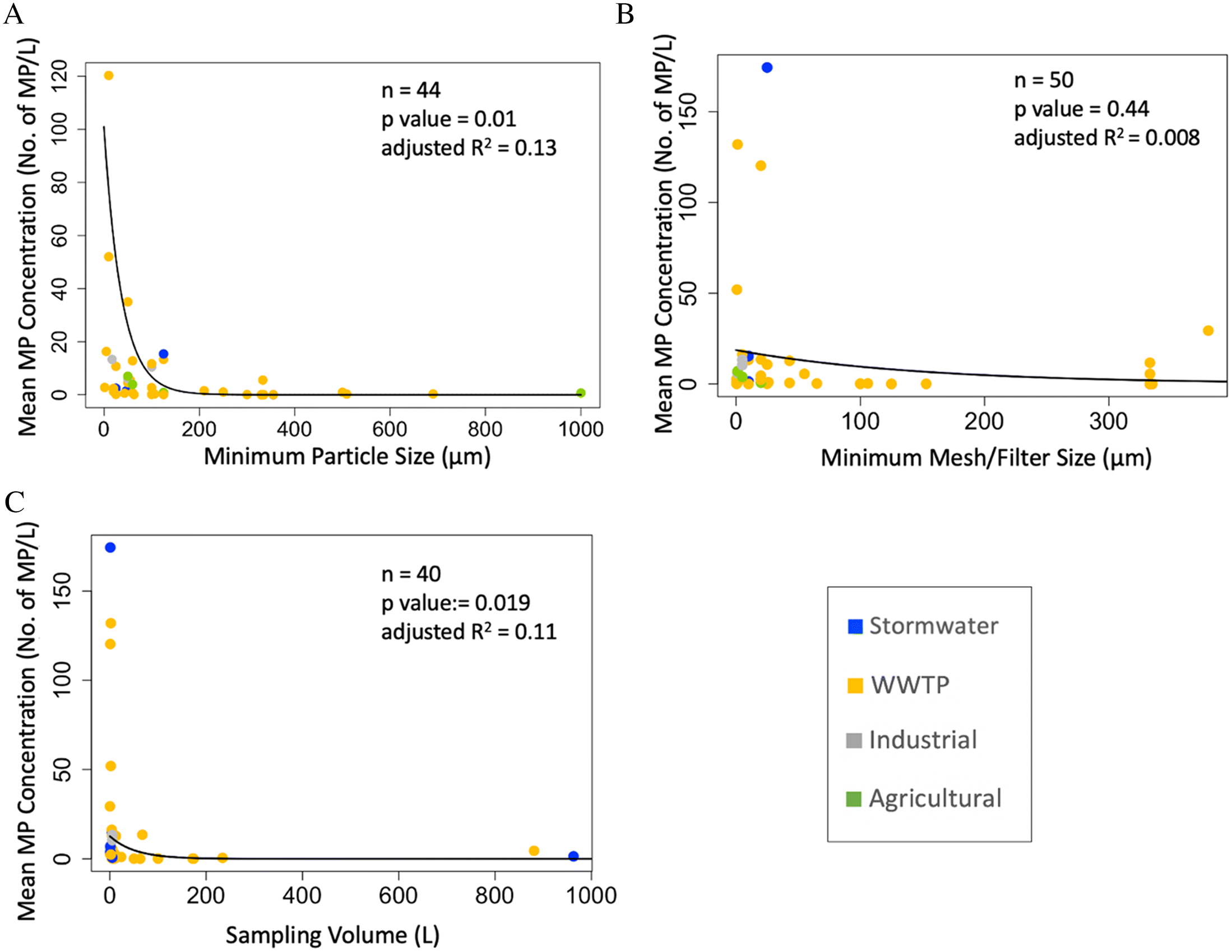
Finally, we explored whether the minimum microplastic size analyzed (explained by minimum microplastic size reported because studies did not report a method detection limit), the minimum microplastic size sampled (explained by mesh size or minimum filter size for bulk water), and the volume of water sampled affects concentrations reported (expressed as the mean microplastic concentration reported in each study). We found a significant negative relationship between the minimum microplastic size reported and the mean microplastic concentrations observed (
Fig. 6A). We did not find a significant relationship between minimum mesh size and the mean microplastic concentrations observed (
Fig. 6B). Similar to minimum size reported, we found a significant negative relationship between the volume of water sampled and the mean microplastic concentrations observed (
Fig. 6C). In other words, concentrations of microplastics were higher when smaller particles were reported, and lower volumes of water were sampled.
Similar patterns and results have also been reported in
Lorenz et al. (2019), which illustrate an exponential increase in microplastic abundance as the detectable particle size decreases. This is consistent with our results above regarding <200 μm and >200 μm sampling methods. Capturing smaller microplastics is not easy, but needed to estimate total concentration of microplastics across wide size gradients. Overall, we recognize the complementarity in taking samples with different size meshes. The mesh size used should be chosen based on the objective of the research and capabilities of the laboratory. The trend we observed in
Fig. 6B may have been due to the method detection limit in the laboratory not being comparable to the size of the mesh used in the field (i.e., sampling smaller microplastics than can be measured in the lab).
Fig. 6C suggests that a smaller sampling volume is also associated with an increase in the concentration of microplastic, consistent with studies elsewhere (
Covernton et al. 2019), suggesting the increase of sampling volume is associated with the decrease in sampled microplastics concentrations. However, this result is probably confounded by the use of grab sampling, which has a lower volume and lower mesh size than other sampling approaches. We do not suggest taking a smaller volume, as taking larger volumes reduces the variability and more accurately represents the environment (
Hung et al. 2021).
While sampling method may impact microplastic concentrations, the relationship revealed by our analyses explained a modest amount of variation (i.e., 11%–13%). Many other factors drive variation in microplastic concentrations, including season, location, and matrix (i.e., stormwater retention ponds and wastewater effluent tanks). For example, we may expect the stormwater retention ponds to show higher concentration of microplastics than rivers receiving urban runoff due to dilution in the river. Furthermore, results may be affected by laboratory methods for extraction and analysis which were not explored here. Finally, our regression results were skewed by extreme values, so more research which spans a wider gradient of methods and particles will resolve relationships between volume, mesh size, and particle concentrations.
Conclusions and implications for management
Overall, more studies are needed to expand data collection to a greater diversity of microplastic pathways into freshwater ecosystems. Stormwater runoff, industrial effluent/runoff, and agricultural runoff showed microplastic concentrations that were in the same range or higher than those recorded in WWTPs effluent. Our results also suggest the potential use of unique morphologies in each pathway to act as a signature for some particle types (e.g., rubbery particles in stormwater runoff), which can help inform monitoring and mitigation strategies. The morphologies detected can also indicate sources that might be key contributors to a particular pathway, informing upstream solutions. Finally, our study assessed sampling methodologies and how mesh size, volume, and detection limits for size impacted concentration and diversity of microplastics. We did not find any clear trend suggesting how samples should be taken and processed. Instead, we recommend consistent sampling protocols (to facilitate meta-analysis and synthesis) that align with the objective of the research. For example, research directed at measuring microplastic toxicity may want to sample smaller particles via bulk water samples filtered through a fine mesh. Research targeting pellets in industrial runoff would benefit from using nets with larger mesh to sample more volume to capture a representative sample of 3 mm pellets. Multiple pathways contribute microplastics to aquatic ecosystems and further research should consider a broader spectrum of transport pathways, including stormwater runoff, industrial effluent/runoff and agricultural runoff, in addition to WWTP effluent. Taking a more holistic approach will improve our understanding of plastic pollution dynamics and inform more effective policies for monitoring and mitigation.