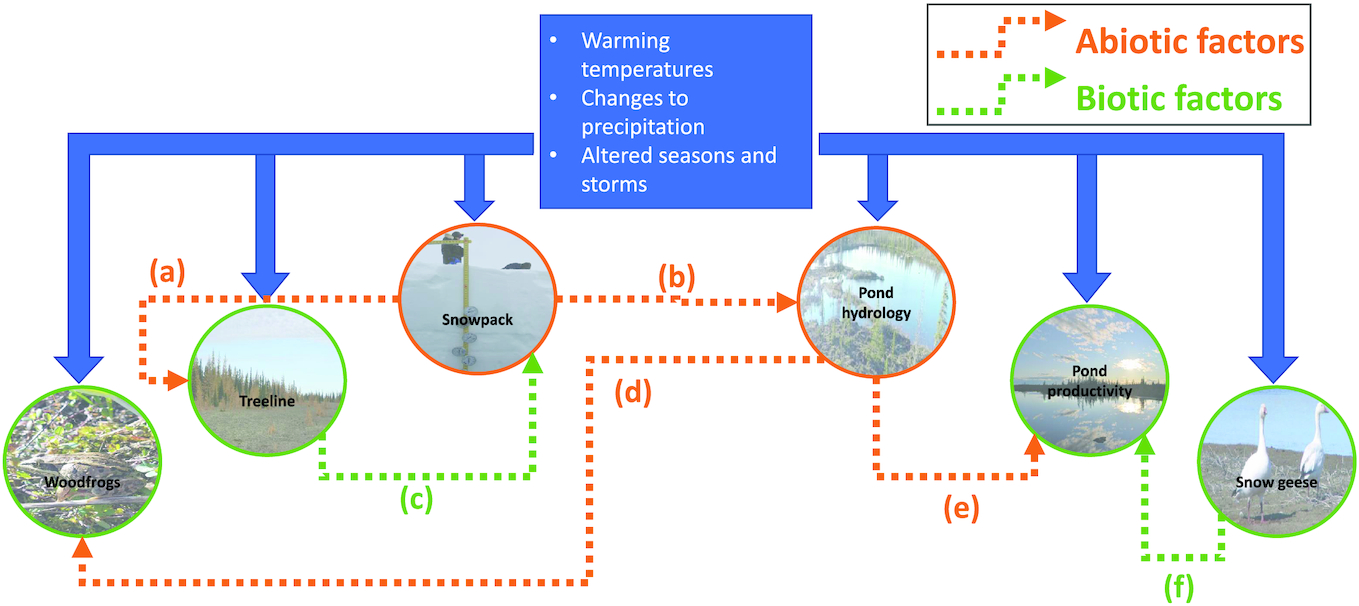
3. Warming temperatures
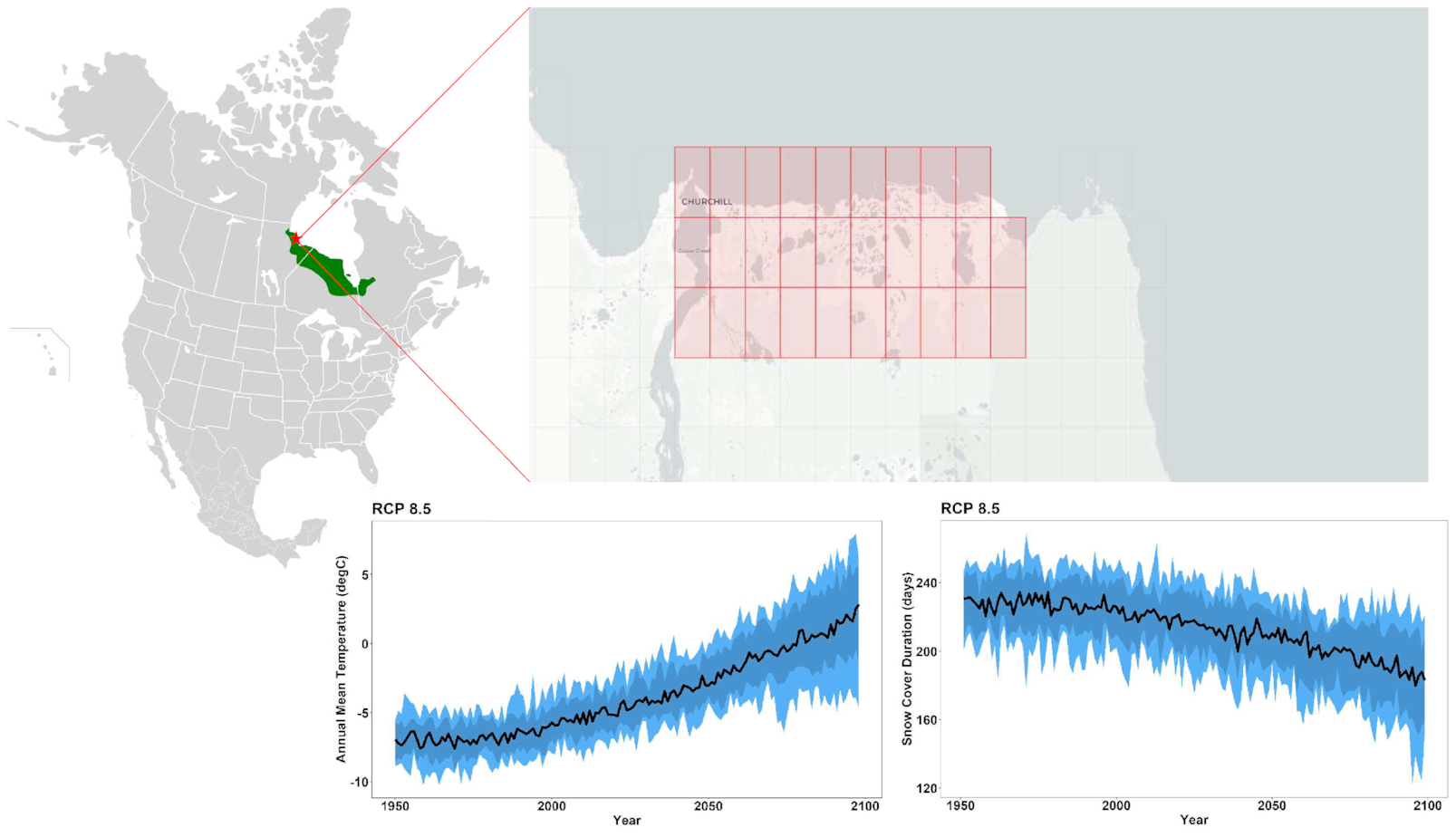
The trends in temperature in the HBL region are clear and consistent. Maximum, minimum, and average temperatures are projected to rise in all seasons, but the rate of increase depends on the indicator under consideration. Some of the most dramatic changes in temperature are projected in the winter season, where both minimum and maximum temperatures may be more than 10 °C warmer by the end of the century compared with the recent past (
Table 2). However, many ecological processes are sensitive to temperature changes at certain points in the year, given their particular annual patterns. As such, it is important to be specific when considering the impact of warming on terrestrial and aquatic ecosystems.
Canada's arctic treeline represents a critical biogeographic boundary, separating the boreal forest from the tundra. With climate warming, the treeline has been predicted to potentially move polewards by hundreds of kilometres and upslope by hundreds of metres (
MacDonald et al. 1998;
Leng et al. 2008). Studies in the Churchill region show a strong correlation in temperature and tree ring width during the early growing season, June–July (
Girardin et al. 2005;
Tardif et al. 2008;
Mamet and Kershaw 2013), where increasing maximum June (11.8–17.3 °C) and July (17.7–23.8 °C) daily maximum air temperatures are projected from the recent past to the end of this century (
Table 2). In particular, tamarack trees have experienced the greatest growth and at unprecedented rates in recent years (1980–2006) relative to other tree species. Annual growth has shown a strong correlation to climate, and in particular, autumn temperatures (
Girardin et al. 2005;
Mamet and Kershaw 2013), where the autumn maximum temperature is expected to continue to increase from 0.9 to 6.7 °C from the recent past to the end of this century in the HBL (
Table 2). Along with increased tree growth, studies have also found a threefold increase in tree density at three separate sites in the Churchill region, as a result of increased recruitment (
Mamet and Kershaw 2012).
While strong correlations have been found with warmer temperatures and increased tree growth and density, other factors could alter the predicted growth and movement of the treeline. Fine-scale treeline and vegetation positions are dependent on local climatic (
Harsch et al. 2009;
Grafius et al. 2012), biotic, and topographic variables (
Cairns and Moen 2004;
Macias-Fauria and Johnson 2013;
Brown and Vellend 2014) as well as the actual treeline form (i.e., diffuse, abrupt, tree island, krummholz;
Mamet et al. 2015). In particular, tree islands, which act as key areas of overwinter snow water equivalent (SWE) accumulation, have been identified as a key hotspot for potential future recruitment with the potential to form a positive-feedback mechanism for future treeline range expansion (
Fitzpatrick et al. 2020), although some evidence suggests that changing temperature–snow depth interactions outside of optimal ranges may result in reduced seedling occurrence (
Hättenschwiler and Smith 1999). Therefore, understanding these local changes in treeline and tundra vegetation is crucial to forecast/understand how future warming trends will influence the terrestrial environment within the Churchill region (
Chapin et al. 2005;
Tinner et al. 2008;
Mamet et al. 2015).
Flat topography and flow restrictions in permafrost peatlands frequently result in small terminal ponds in patterned ground, ribbed fens, headwater lakes, and other lentic surface water bodies, in either closed basin or flow-through systems (
Duguay and Pietroniro 2005;
Boike et al. 2008;
Laurion et al. 2010). The majority of surface water features in permafrost landscapes are typically shallow and small (<1 ha;
Muster et al. 2013). These small water bodies have less total storage capacity than larger temperate systems and thus are sensitive to climatically driven changes in pond hydrology and nutrient cycling. In the HBL, 25%–40% of the land surface is covered by shallow water bodies (
Duguay and Lafleur 2003). These ponds range from 400 m
2 to 0.04 km
2 in surface area and from 0.1 to 1 m in depth (
Macrae et al. 2004). These ponds play important roles in surficial energy balance (
Eaton et al. 2001), water budget (
Boudreau and Rouse 1995), supporting diverse benthic communities (
Bonilla et al. 2005), and carbon cycling and storage (
Macrae et al. 2004). Increasing temperatures and nutrient input are some of the main drivers that ultimately result in alterations of the primary productivity of these pond systems (
Hobbie et al. 1999;
Flanagan et al. 2003). In general, northern aquatic ecosystems exhibit lower productivity as compared with their southern counterparts due to cooler temperatures. It is predicted that from the recent past to the end of this century, increased summer temperatures at the air–water interface (July maximum temperatures increasing from 17.7 to 23.8 °C;
Table 2) may result in an increase in arctic and subarctic freshwater productivity (
Rouse et al. 1997;
Hobbie et al. 1999;
Chapin et al. 2005;
Prowse et al. 2006;
Eichel et al. 2014;
Wrona et al. 2016).
These pond and wetland habitats of the HBL support the only two species of amphibians that can withstand the harsh northern climate of the region: the wood frog (
Lithobates sylvaticus) and the boreal chorus frog (
Pseudacris maculata). While there is currently no evidence to suggest a decline in the local populations of these two species, their subarctic location makes them susceptible to climatic influence, particularly given that they are poikilotherms and therefore strongly influenced by their external environment. An amphibian's basic physiological functions (i.e., growth, development, reproduction, and locomotion) are dependent on environmental temperature, specifically during the larval life stage (
Orizaola and Laurila 2009). As June maximum temperatures (as a proxy for when the animals are in their larval life stage period;
Davenport et al. 2017) in the HBL are projected to increase from 11.8 to 17.3 °C between 1976–2005 and 2070–2099, the effects could be beneficial to frog larvae through increased growth rates, and a reduction in time to metamorphosis resulting from increased wetland temperatures (
Blaustein et al. 2010). In the Churchill area, warmer water temperatures (increased by 1 °C) increased the survival and development rate of wood frog tadpoles by a factor of 1.8 and increased the overall size of the tadpoles by 16% at the end of 30 days (
Davenport et al. 2017). Given the strong coupling of water temperature to air temperature in the region in shallow water bodies (
Bello and Smith 1990), increases in the annual maximum air temperature (from 15.3 to 17.8 °C from the recent past to the end of this century;
Table 2) are likely to result in dampened but similar patterns to tadpole habitat. Other studies conducted within the wood frog's range determined that larger juveniles and individuals that metamorphosed early experienced higher survival rates, earlier ages at first reproduction, and had larger body sizes as adults (
Berven 1990). While these temperature increases remain beneath known thermal maximum for wood frog tadpole (
Castano et al. 2010;
Table 2), the capacity for temperatures to temporarily exceed these maxima could result in decreased survival during extreme temperature events.
While the direct effects of warming temperatures on organisms are evident in the examples described above, in the complex ecosystem of the HBL, there are several factors that mediate these impacts. These mediating factors mean that the expected increases in growth, range expansion, or evapotranspiration may not be observed directly or as quickly as expected if the processes were solely driven by changes in climate. One well-studied example of this is the relationship between climate change projections and net changes in biomass and productivity. While some projections suggest net greening in the region (i.e., net increases in biomass;
Sturm et al. 2001), others suggest net browning (
Walker and Johnstone 2014;
Phoenix and Bjerke 2016), or complex interactions between the two (
Myers-Smith et al. 2020). If tundra productivity rises, an increase in the abundance of shrubs is a likely outcome (
Myers-Smith et al. 2011). Over the last 50 years, shrub expansion has been observed throughout the arctic tundra. Already there is evidence of shrub-tundra replacing tussock-tundra along the southern limit of the Alaskan tundra (
Sturm et al. 2001;
Myers-Smith et al. 2011). The characteristics and spatial arrangement of vegetation are an important influence in the formation and evolution of snow depth in the tundra (
Fig. 3). As shrubs increase the snow-holding capacity of the arctic landscape, an increase in shrub abundance would have important implications for the snowpack in a subarctic environment. Where the landscape transitions from willow-dominant, to shrubby tundra, to tussock-tundra,
Sturm et al. (2001) observed a corresponding decline in snow depth, in conjunction with a decline in the overall height and abundance of shrubs. Permafrost thaw, both gradual (active layer thickening) and rapid (loss of periglacial features such as palsas and pingos), could result in changes in snowpack distribution, such as a reduction in snow drifting (
Seppälä 2011). From such observations, it can be surmised that the tundra could experience deeper snowpacks in the future, as shrubs increase in density and extent.
In the windswept areas near treeline, where arboreal vegetation is sparse, cool soil temperatures are common. Soils under closed forests are also cooler due to shade (
Mamet and Kershaw 2013;
Mamet et al. 2015). Increasing June (2.6–8.4 °C, from the recent past to the end of this century) and July (7.7–3.8 °C) daily minimum air temperatures as well as changes to shallow active layer moisture regimes altering the thermal conductivity of shallow peat soils may alter the present cool soil temperature regime. Currently, this soil temperature can limit the production of dry matter, restrict root growth, and delay photosynthetic reactivation in the spring (
Tranquillini 1979;
Mamet et al. 2015). In addition to the direct impacts of wildfire on slowing treeline advance (
Timoney et al. 2019), postfire soil thermal regimes may also affect recovery trajectories (
Morison et al. 2020).
For amphibian populations, there is a trade-off between the rate of development and growth, which could be exacerbated by climate change (
Blaustein et al. 2010). In general, the rate of development increases as temperatures rise, often by shortening the larval period (
Balustein et al. 2010).
Davenport et al. (2017) found that the size at which
L. sylvaticus individuals metamorphosed decreased slightly by approximately 5.5% with 1 °C of warming. This minor cost suggests that a response to environmental change that produces rapid development, and allows for an earlier departure from winter ponds, is not likely a primary threat to the fitness of frogs at northern latitudes (
Davenport et al. 2017). However, if increased climate warming shortens the wetland hydroperiod, then future individuals might experience a further reduction in size at metamorphosis, thus impacting their probability to survive their first winter, to survive to their first breeding, and for female frogs, impacts on clutch size (
Bishir et al. 2018). Alternatively, earlier emergence and continuing warmer temperatures may allow for longer foraging periods, where the HBL is projected to experience an increase in July maximum daily temperatures from 17.7 to 23.8 °C from the recent past to the end of this century (
Table 2), which could compensate for smaller sizes at emergence (
Benard 2015), although some limits to the plasticity of development may be near physiological capacity (
Davenport et al. 2017). Both scenarios, changes in metamorphosis date and foraging activity during the active season, will influence time to sexual maturity, movement, and overwintering potential, which has not been directly observed in the HBL but other northern populations appear to demonstrate this mechanism (
Amburgey et al. 2018;
Fitzpatrick et al. 2020).
4. Changes to precipitation and the water balance
Trends in precipitation in the HBL are less uniform than trends in temperature. There are few, if any, substantive changes in precipitation metrics projected (
Table 2). There is a clearer increase in potential evapotranspiration, from 360 mm in the recent past to 453 mm by the end of the century. Similarly, the fraction of annual precipitation as snow is projected to decrease, consistent with shorter winters and later onset of freezing temperatures (
Table 2). These changes in precipitation have important implications for both terrestrial and aquatic ecosystems across the HBL.
Condensed snow drifts or snowpacks are a critical part of the HBL, providing physical protection to vegetation from the abrasion of blowing snow, and acting as insulative layers, reducing soil desiccation during winter months (
Essery and Pomeroy 2004). Specific characteristics of a snowpack can influence the surrounding landscape on both a physical (e.g., meltwater production and heat flow) and a biological (e.g., plant phenology and invertebrate populations) level (
Kershaw and McCulloch 2007). Warming arctic temperatures will likely change snow cover characteristics, however, recent work suggests that this relationship may not be consistent across all regions of the subarctic. Some studies have found long-term significant increases in the seasonal snow depth in Europe and Asia (
Kohler et al. 2006;
Bulygina et al. 2009), whereas other studies have found significant snow depth decreases over parts of northern Canada (
Atkinson et al. 2006). Globally, trends in SWE have also varied regionally, where SWE has increased over Eurasia and northern Russia, but decreased over northern Canada (
Bulygina et al. 2010;
Kong and Wang 2017).
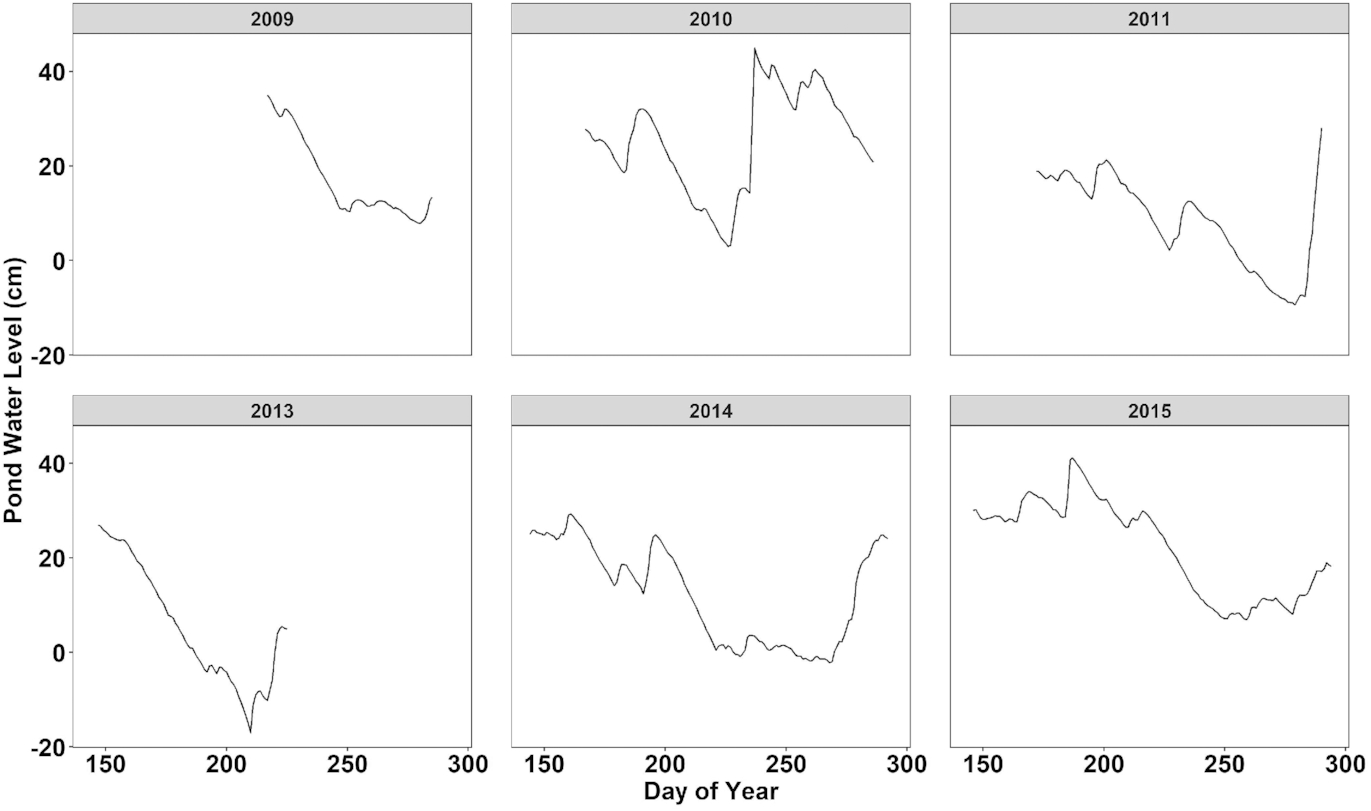
The small and shallow nature of ponds in the HBL renders them vulnerable to hydroclimatic stresses. Two separate hydrologic regimes annually control the water balance of these ponds. First is the accumulation of snow over the winter period before a relatively rapid melt and initial recharge of pond storage. Second, a gradual decline in water levels over the summer is primarily controlled by the difference between liquid-phase precipitation and evapotranspiration (
Fig. 4). Currently, of these two components, winter SWE accumulation remains an important part of the annual water budget for ponds (
Schindler and Smol 2006), although high shrub growth (
Robinson et al. 2021) and relief allow for the trapping and redistribution of snow in low-SWE years (
Bouchard et al. 2013).
Following the snowmelt period, within the summer water balance, potential evapotranspiration is expected to increase (360–454 mm from the present to the end of the century), with an approximately equal increase in annual precipitation. Previous work using historical imagery analysis and modelling of summer water balance in the HBL concluded that ponds in this region are not vulnerable to summer drying and may instead be at risk for great expansion due to a lack of evaporation increase coincident with increases in precipitation in the future (
Macrae et al. 2014). This is consistent with future water balance projections that show no substantive changes in the difference between annual precipitation and potential evaporation between the recent past (80.1 mm) and the end for this century (76.0 mm), although mid-summer drying events may still be possible. As a result of warming, a shorter larval period for tadpoles increases the likelihood that individuals will successfully metamorphose before pond desiccation, and consequently increases the chance of overall survival and future reproductive success (
Berven 1990;
Blaustein et al. 2010). This is increasingly important as warmer subarctic temperatures also threaten the hydroperiod of the ponds and wetlands (
Davenport et al. 2017).
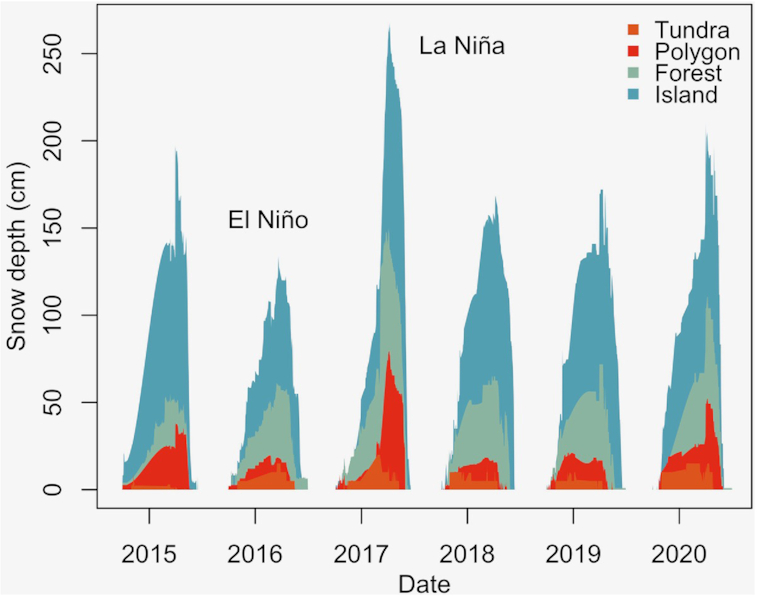
In the Churchill area, the organization of the vegetation significantly impacts the characteristics of the wind, and in turn, causes variation in the snowpack (
Scott et al. 1993;
Kershaw and McCulloch 2007). During the winter months, this vegetation interacts in several ways with the snowpack. In general, portions of the landscape with little to no plant presence experience wind erosion and redistribution of snow to the forested and shrubby areas. Conversely, a larger volume of plant cover leads to little erosion and redistribution, and thus results in a high accumulation of snow (
Essery and Pomeroy 2004;
Kershaw and McCulloch 2007). In their study on midwinter snowpacks,
Kershaw and McCulloch (2007) observed that the surface roughness of the forests and tree islands accumulated up to 18× the mean snow-water-equivalent, in comparison to the open tundra.
Essery and Pomeroy (2004) simulated a 1 m threshold, at which shrub density could hold the maximum amount of snow. This suggests that dense areas of shrubs could have as comparable an impact on snowpack as forested areas. In many studies, the deepest snow has been associated with areas of tall, dense shrubs, frequently near riverbanks, resulting in approximately tripled snow accumulation (
Scott et al. 1993;
Sturm et al. 2001). Moreover, El Niño and La Niña teleconnections may have a considerable influence on fine-scale patterns of snow accumulation (
McClung 2013;
Fig. 3).
The summer pond water balance is controlled by the differences in precipitation minus evapotranspiration, particularly in ombrogenic peatland catchments in the HBL and other subarctic environments (
Rouse et al. 1997). The frequency and intensity of storms relative to the timing and amount of potential evapotranspiration and other water losses will determine the magnitude of water table variability. At the landscape scale, encroachment of trees into the tundra could facilitate dispersal and colonization of new or additional ponds in the future (
Bishir et al. 2018). This could mediate the effects of ponds drying or unsuitable habitat and allowing for additional population recruitment. However, decreases in snowpack during the winter could have negative effects on the body condition of wood frogs, which could reduce reproductive output but allow for earlier foraging (
Benard 2015;
O'Connor and Rittenhouse 2016).
5. Seasonality and storms
Boreal ecology is dominated by role of cold temperatures and snowy conditions on boreal ecology, in which “any ecological study in northern environments is a study of winter impacts” (
Pruitt 1978). Changes to the shifting seasonality and relative length and strength of winter will likely play a large role in the HBL. Changes to winter precipitation could be as important than rising temperatures to snowmelt phenology in this region. Despite warmer temperatures, increased winter snowfall has led to delayed snowmelt (up to 0.2 days/year in the last 21 years) in the Canadian Arctic (
Bjorkman et al. 2015), However, in the HBL, the initiation of snowmelt is projected to move earlier from an average of May 4th for the period 1976–2005 to an average of April 23rd for the period 2070–2099, and a decrease in annual snow cover duration from 225 to 189 days for the same period. In this condensed winter season, the proportion of annual precipitation as snow will decrease from 41.6% in 1976–2005 to 36.1% in 2070–2099, as well as the initiation of snowmelt is anticipated to occur earlier under a changing climate (from May 4th in the recent past to April 23rd at the end of this century).
The length of the growing season is important for plant communities, but studies across the subarctic have shown that the direction and magnitude of change are very species specific (
Richardson et al. 2013). Tree ring studies in Churchill suggest that both late spring warmth and late onset of snow in the fall were correlated with biomass accumulation in white spruce (
Tardif et al. 2008). Of the three main species of trees found in Churchill (tamarack, white spruce, and black spruce), tamarack has experienced the greatest growth at the treeline and has shown an increasing sensitivity to the lengthening growing season (
Mamet and Kershaw 2013;
Mamet et al. 2019). Given projections that the length of the frost-free season will increase from 96 to 145 days from the recent past to the end of this century, unravelling these species-specific responses is key to understanding trajectories of tree community dynamics.
Snowmelt is often the greatest single annual hydrologic input in subarctic regions and may represent up to one half of annual water budget inputs within a period of a month or less (
Carey and Woo 2001), although snowmelt hydrographs are dominated by near-surface old water chemical signatures (
Hayashi et al. 2004;
Carey et al. 2013). Thus, summer season water budgets are sensitive to the magnitude and timing of the winter snowpack and vulnerable to drying with decreases in snowfall.
Bouchard et al. (2013) demonstrated that a peatland pond in the HBL was susceptible to unprecedented summer desiccation following a low melt runoff spring. In addition to atmospheric components of the water balance, pond and wetland hydrologic storage and transport processes in the HBL and other permafrost regions are frequently governed by “fill-and-spill” dynamics (
Spence and Woo 2002,
2003). Fill-and-spill systems are characterized by a series of storage units divided by topographic features, each serving a storage function until its storage capacity is exceeded and excess water spills into the next storage unit in the elevational sequence, demonstrated for a pair of instrumented pond catchments in the HBL wherein runoff generation was not initiated until static storage thresholds were exceeded (
Morison et al. 2017a). This complex hydrology means that water budget responses to increases in temperature and changes to precipitation will be heterogeneous across the landscape.
Most ponds in this region are oligotrophic based on low concentrations of available nutrients and low algal biomass in the water column (
Bos and Pellatt 2012). Generally, additional nutrient inputs to ponds incite phytoplankton growth, leading to a darkening of the water column and decreased light availability for phytobenthos from both organism growth and dissolved/suspended nutrient loadings (
Rautio et al. 2011). However,
Eichel et al. (2014) found through controlled nutrient amendments to microcosms that the shallow subarctic freshwater ecosystems are able to respond rapidly to an increased supply of inorganic nutrients from an external source that is likely to occur if climate warming continues. The uptake of these nutrients is also reliant on lake sediments and their associated benthic communities to process the nutrient inputs. Additionally, ponds in the HBL are extremely sensitive to evaporative desiccation, especially in years of low snowmelt (
Bouchard et al. 2013;
MacDonald et al. 2017). Increases in open-water evaporation (projected to increase by nearly 25% from the recent past to the end of this century) could increase evapoconcentration (
Morison et al. 2017b) and potentially lead to a further increase in productivity within HBL freshwater ecosystems. Changes to in-pond primary production could have implications for higher trophic levels within the food web, including wood frog and boreal chorus frog food availability (
Stephens et al. 2015), although these increases could be curbed by pond temperatures approaching algal thermal maximum tolerance values (
Chen 2015).
The magnitude of rainstorms is expected to increase, from an annual single-day maximum precipitation of 27–34 mm from the recent past (1976–2005) to the end of the century (
Table 2), although projected changes to summer precipitation are extremely varied across space, and generalizations are therefore more difficult (
Shook and Pomeroy 2012;
Harder and Pomeroy 2013). Across multiple years, short-term (i.e., hour-to-hour) variations in pond chemistry exceeded seasonal-scale variation in pond chemistry (
Morison et al. 2017a,
2017b), illustrating the potential of individual precipitation events to dictate factors mediating pond hydrochemical responses to climatic change. The relative vulnerability of these shallow pond and wetland systems to short-term, event-based hydrochemical changes may be controlled hydrologically, particularly for conservative chemical species, in which the relative proportion/contribution of new and old water will dictate the extent of these short-term responses. For more bioavailable chemical species such as mineral nutrient forms, the arrival of nutrients to ponds in short pulses may be rapidly taken up, on a timescale of hours to days (
Eichel et al. 2014). The resulting productivity and increased phytoplankton biomass (
Symons et al. 2012) could serve a function of additional aquatic carbon uptake, potentially forming a negative feedback with climate change, and maybe act as an additional food source for wood frogs and boreal chorus frogs.
In addition to stressors of direct climate forcing, there has been a dramatic increase in features indicative of permafrost decay in a subarctic peatlands in the HBL (
Payette 2004), including thermokarst ponds and plateau collapse scars, which can act as a vector of nutrient mineralization (
Morison et al. 2018) and redistribution into ponds (
Morison et al. 2019). Permafrost is particularly vulnerable to changing seasonality. The loss of a steeper thermal gradient in winter (with winter daily maximum temperatures warming from −20.4 to −9.9 °C and minimum temperatures warming from −28.4 to −16.9 °C in the period from 1976–2005 to 2070–2099) may result in gradual or rapid permafrost thaw. Modelling work suggests that while permafrost may persist across much of the HBL region to the end of the 21st century, the spatial extent of continuity, active layer depth, and the duration of frozen and thawed conditions may show extreme fluctuations with climatic variability, with corresponding impacts on the distribution of surface water and pond chemistry (
Zhang 2013). Permafrost thaw interactions with other forms of disturbance, such as increased frequency and severity of wildfires, are known to impact pond hydrology and chemistry (
Gibson et al. 2018;
Granath et al. 2021).