Introduction
In many species where males provide no parental care or other direct resources to their mates, females often exhibit preferences for mates that are “attractive” and (or) of superior intra-sexual competitive ability (see
Andersson 1994, pp. 124–142). Whether these preferences are maintained because females obtain indirect genetic benefits from being choosy is one of the most debated questions amongst evolutionary biologists (
Kirkpatrick and Ryan 1991;
Kokko et al. 2006;
Qvarnström et al. 2006).
Hamilton and Zuk (1982) hypothesized that female preferences evolve because elaborate traits in males reveal important information about the underlying genetic “quality” of their immune system; by being discriminating, females are able to produce offspring with greater potential for immunocompetence. This “good genes” model of sexual selection (
Andersson 1994) has been subsequently expanded and modified (
Folstad and Karter 1992;
Sheldon and Verhulst 1996;
Rolff 2002) to include a mechanistic explanation for why sexual signals act as “honest indicators” (sensu
Zahavi 1975): that there are unavoidable physiological conflicts that arise in the construction and maintenance of both ornaments and immune response mechanisms (due to the antagonistic action of hormones and (or) limits on energetic and nutritional resources).
If resources are the limiting factor, then both immune defense and sexual signals should be thought of as condition-dependent life history traits, the absolute and relative expression of which will depend on both the individual’s accumulation and allocation of resources (
Rowe and Houle 1996). If there is a high level of genetic variation for resource acquisition in a population, then individuals of high genetic “quality” (and their offspring) will be able to devote more resources to building and maintaining both immune systems and sexual advertisements than individuals of low “quality”. In contrast, if there is relatively more genetic variation for resource allocation, then trade-offs and negative correlations between these life history traits will be revealed (
van Noordwijk and de Jong 1986;
Houle 1991;
Westneat and Birkhead 1998). Genetic variation for allocation may be especially important if the sexes have different optimal levels of investment into immune function and other life history traits (due to sex-specific fitness-maximizing strategies). Males may benefit from greater investment into traits that increase attractiveness (and thus mating success), and be selected to invest less into immunocompetence than females (
Zuk 1990;
Rolff 2002). In cases of sexual conflict, alleles that may be beneficial when expressed in one sex may be maladaptive when expressed in members of the opposite sex (
Holland and Rice 1998;
Chippindale et al. 2001;
Pischedda and Chippindale 2006).
One way in which the relationships between life history traits can be explored is by comparing the phenotypes of offspring after one or multiple generations of divergent selection on one life history trait (
Zera and Harshman 2009). An early example of this approach was that of
Kurtz and Sauer (1999), who compared the immunocompetence of offspring sired by male scorpionflies (
Panorpa vulgaris) that differed in the degree of ornament elaboration and found a small immune benefit associated with females being choosy. More recently, a trio of experimental evolution studies, using the model species
Drosophila melanogaster, have examined life history trade-offs from different perspectives. In this species, males compete intra-sexually, as well as engage in elaborate inter-sexual courtships of females, using a wide range of chemical, visual, and acoustic signals (
Hall 1994;
Greenspan and Ferveur 2000). As in many insect species,
D. melanogaster does not possess an adaptive immune system and must rely on an innate system which confronts bacterial and fungal infections through the production of antimicrobial peptides, and a cellular system that primarily uses phagocytosis and encapsulation to combat parasitoids (
Broderick and Lemaitre 2009). The construction and maintenance of this immune system can be costly (
Kraaijeveld and Wertheim 2009), and studies of
D. melanogaster are proving to be central to understanding immune function in a wide variety of animal taxa (
Khush and Lemaitre 2000;
Hoffmann 2003). In the first study,
Rolff and Kraaijeveld (2003) selected for increased pathogen resistance in replicate experimental populations by exposing flies to the parasitic wasp
Asobara tabida. This selection resulted in the evolution of greater pathogen resistance, but at the cost of decreased larval competitiveness when food resources were limited (
Kraaijeveld and Godfray 1997;
Kraaijeveld et al. 2001). Despite no significant difference in body size between flies from unselected control populations and those from experimental populations, flies in the latter group had higher mating success (by achieving more matings in direct competition with males from a tester stock and by mating more rapidly) than did flies from the former group (
Rolff and Kraaijeveld 2003). The second relevant study by
McKean and Nunney (2008) took a different selective approach by creating experimental populations that experienced greater levels of sexual selection (by artificially creating male-biased sex ratios in every generation of culture). They found that relative to flies from the control populations, experimental flies were larger, developed more slowly, and males were more successful at obtaining matings. However, both male and female flies in the experimental populations were less resistant to immunological challenge from
Escherichia coli than those from the control populations. More recently,
Modak et al. (2009) compared the resistance to
E. coli in flies from populations that had been selected for faster development with those from corresponding control populations. They found that flies from the fast-selected lines exhibited higher rates of pathogen-induced mortality than did those from controls, suggestive of a genetic correlation between development rate and pathogen resistance. However, selection for faster development typically results in the evolution of smaller body size (e.g.,
Chippindale et al. 1997), which can also put male flies at a strong mating disadvantage (see
Partridge et al. 1987). Overall, these various experiments suggest that the components of life history (mating success, body size, immunocompetence, and development rate) are linked. However, the magnitude and sign of these relationships remain unclear, as together these studies indicate that selection for increased male attractiveness may cause decreased immunocompetence (
McKean and Nunney 2008), selection for increased immunocompetence may lead to increased male attractiveness (
Rolff and Kraaijeveld 2003), and that decreased immunocompetence is correlated with a increased development rate and smaller body size (
Modak et al. 2009).
Ultimately, support for a parasite-mediated sexual selection model to explain the evolution of some female preferences and elaborate male traits will emerge from a consensus of empirical tests of the genetic relationship between attractiveness, competitiveness, and immunocompetence. Here, we seek to add to this growing body of research by comparing the development, growth, and bacterial loads in D. melanogaster offspring that are sired by males differing in their mating success and comparing the performance of these offspring when experimentally exposed to the common insect pathogen Pseudomonas aeruginosa.
Results
The experimental addition of pathogen to the vial environment resulted in a significant decrease in the egg-to-adult survivorship rate of both “stud”- and “dud”-sired offspring compared to those control vials in which
P. aeruginosa had not been added (mean survivorship ± SE for pathogen positive vials: 0.710 ± 0.019; for controls: 0.811 ± 0.016). However, sire reproductive success had no statistically significant effect on offspring survivorship (mean survivorship ± SE for “stud” vials: 0.745 ± 0.016; for “dud” vials: 0.780 ± 0.020), and the interaction between sire reproductive success and pathogen exposure was also not statistically significant (
Table 1).
When we weighed the adult flies, we found that males from vials that had been exposed to the pathogen were marginally lighter (Cohen’s
d = 0.185) than those males developing in the non-pathogen vials (
P = 0.065), whereas neither sire attractiveness nor the interaction between sire and pathogen were significant (
Tables 2 and
3). For females, neither sire reproductive success, the presence/absence of pathogen, nor the interaction between these factors had a significant effect on their weight (
Tables 2 and
3).
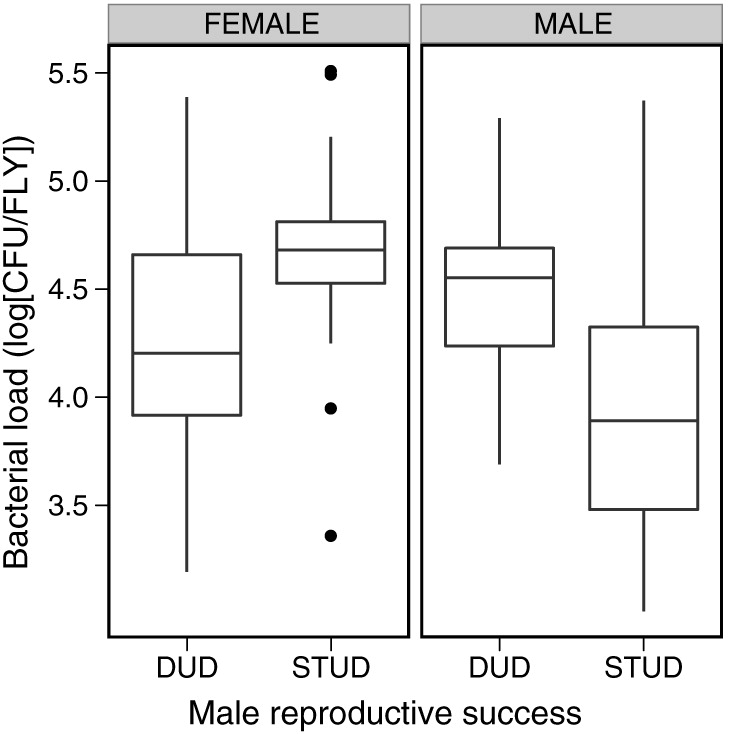
In our initial three-way analysis of the effect of sex, sire, and pathogen exposure, we observed that a fly’s bacterial load was strongly influenced by both their sex and the type of male that had sired them (
Tables 3 and
4). The nature of this interaction became evident when the sexes were analyzed separately: sire attractiveness had a significant effect on the size of the bacterial load carried in both sons (
P = 0.003) and daughters (
P = 0.036;
Tables 3 and
4), but not in the same manner. Sons sired by “stud” fathers carried a smaller bacterial load compared to sons sired by “dud” fathers (
Fig. 1; Cohen’s
d = 0.982). In contrast, the daughters sired by “stud” fathers carried a larger bacterial load then those daughters sired by “dud”-fathers (
Fig. 1; Cohen’s
d = 0.442).
In the first follow-up experiment (of male and female adult virgin offspring), neither of the main effects, nor their interaction, were statistically significant for either the females (sire;
F 1,41 = 0.01,
P = 0.91; pathogen exposure:
F 1,41 = 0.002,
P = 0.96; sire × pathogen exposure:
F 1,41 = 3.44,
P = 0.07;
Supplementary Material 2) or for males (sire;
F 1,25 = 1.77,
P = 0.20; pathogen exposure:
F 1,25 = 0.42,
P = 0.52; sire × pathogen exposure:
F 1,25 = 1.68,
P = 0.21;
Supplementary Material 3). In the second follow-up experiment (involving assays of females that differed only in the timing and (or) number of matings), there was no effect of treatment on the mean bacterial loads (
F 3,45 = 0.07,
P = 0.98,
Supplementary Material 4).
Discussion
Ever since
Darwin (1871) proposed the theory of sexual selection to explain the evolution of elaborate display traits in males, biologists have attempted to understand the extent to which the selective pressures produced from differential mating success complement, or conflict, with the pressures resulting from natural selection (
Andersson 1994;
Arnqvist and Rowe 2005). In this study, by experimentally mating females with males that had been previously assayed as being of relatively high or low sexual attractiveness and (or) competitiveness (i.e., “studs” or “duds”), we were able to measure the cross-generational effects associated with sexual selection. We found that differences in the attractiveness/competitiveness of potential males was related to observed differences in the bacterial loads carried by their offspring, but this effect ultimately depended on the sex of the individual fly in which these genes were expressed.
Overall, the addition of the bacteria to vials containing
D. melanogaster eggs resulted in an egg-to-adult survivorship rate that was lower than that observed in the control vials. Furthermore, males eclosing from the experimental vials were marginally smaller than those that had developed in the control vials. These results highlight the fact that
P. aeruginosa is a known pathogen of
Drosophila (
Boman et al. 1972;
D’Argenio et al. 2001), and is consistent with a previous study (
Young et al. 2009) that used the same strain of bacteria and means of infection that reported a similar negative effect on survivorship.
Kraaijeveld et al. (2001) hypothesized that the common embryonic origin of the head muscles (used in feeding) and the hemopoietic organ (that produces the hemocytes that are responsible for the cellular immune response) may provide a proximate explanation for any developmental trade-offs in
Drosophila. Thus, the relatively smaller size of males in the presence of pathogens is consistent with previous work that has found that flies experimentally evolved in an immunologically challenging environment also exhibited lower feeding rates (
Fellowes et al. 1999), and that there are developmental trade-offs between growth and immunocompetence (
Modak et al. 2009). The fact that this phenomenon was only observed in male offspring may reflect the effects of sex-specific selective pressures. It has been repeatedly suggested (
Trivers 1972;
Zuk 1990;
Rolff 2002) that a male’s lifetime reproductive success may depend more on the attraction of mates than it does for females, where variation in fitness is often strongly correlated with body size, as larger females have more resources to invest in fecundity (
Lefranc and Bundgaard 2000;
Pitnick and García-González 2002;
Long et al. 2009). Thus, the sexes may be under divergent selection over the allocation of limited resources; this is a promising avenue for future research.
Sex-specific responses to pathogen exposure were also observed in our assay of bacterial loads. While the sons of “stud” males carried fewer bacteria than did those sired by “dud” fathers, the opposite pattern was observed in daughters, where those sired by “duds” had smaller bacterial loads. This pattern of sire attractiveness-linked immunocompetence observed in sons is partially consistent with the theoretical predictions initially made by
Hamilton and Zuk (1982), as well as the empirical observations of
Rolff and Kraaijeveld (2003): that females are selecting to mate with males for their superior immune response alleles. These effects may be further exaggerated if the magnitude of maternal effects (or paternity-induced effects) differed between the mothers mated to “studs” or “duds” (e.g.,
Kotiaho et al. 2003). However, the gains experienced by the sons sired by “stud” males are not shared with their sisters, who carried relatively high bacterial loads. Such a pattern is suggestive of the presence of sexually antagonistic allelic variation, whereby the effect of an allele on Darwinian fitness ultimately depends on the sex in which it is expressed (
Chippindale et al. 2001;
Arnqvist and Rowe 2005;
Pischedda and Chippindale 2006). This type of genetic conflict arises because, while most of the genome is shared between the sexes, the phenotypes and life-histories associated with fitness-maximization are often quite different between males and females (
Holland and Rice 1998). As the development, maintenance, and utilization of an immune system are costly (
Rolff and Siva-Jothy 2003;
Kraajijeveld and Wertheim 2009), and an individual’s available resources are finite, it is likely that conflicts over investment should arise between the sexes (
Rolff 2002). Specifically, it has been hypothesized that females will be selected to invest more heavily into immune function, as a prolonged lifespan will increase the opportunity to produce offspring, whereas in males, variance in fitness is not associated with longevity, but instead with mating rate, and consequently may be selected to bias resource investment away from immune function and toward other fitness-maximizing traits (
Rolff 2002;
Rolff et al. 2005). This hypothesis is supported by evidence of systematic female biases in the expression of some immune function traits in insects, indicative of a history of divergent selection on the sexes (
Nunn et al. 2009). It is our conjecture that our observations are consistent with the effects of sexually antagonistic genetic variation acting on both individual physiological “condition” (sensu
Rowe and Houle 1996) and the allocation of resources toward immune defence. When “studs” sire sons, they contribute alleles that may produce phenotypes of superior (male-benefit) physiological condition, and relatively lower allocation toward immune investment. However, because these individuals are in a relatively good physiological state, they have more resources to allocate to growth, development, and maintenance needs, allowing them to withstand bacterial challenges. Meanwhile, the sons of “duds” end up in worse physiological state, with fewer resources to allocate to all traits (including immunity). As such, they are less able to mount a defence against pathogens. The patterns of bacterial loads observed in females are also consistent with the effects of sexually antagonistic alleles. Females sired by “studs” inherit many “male-benefit” alleles from their fathers, which are deleterious when expressed in a female genetic background. Daughters of “studs” may be expressing alleles that divert resources
away from immune function, as well as other alleles (e.g., for locomotory behaviour, as in
Long and Rice 2007) that result in a relatively inferior physiological condition. Consequently, these females may be less capable of mounting an immune response, manifested as higher bacterial loads. In the case of daughters of “duds”, these females have inherited alleles that—while at a selective disadvantage when expressed in males, are beneficial when expressed in females. These females may be in a better physiological state and able to allocate more resources to their immune system, resulting in relatively lower bacterial counts. Ultimately, such sexually antagonistic alleles contribute to the so-called “gender load” (
Rice and Chippindale 2002) in sexually reproducing species, as their presence interferes with the action of selection to remove deleterious alleles from the gene pool, and ultimately depresses the fitness of the population.
Our results are consistent with the recent findings of
Vincent and Sharp (2014), who assayed the immunity of 50 homozygous lines of
D. melanogaster exposed to
P. aeruginosa and also found evidence of sexual antagonism in the patterns of resistance and tolerance to the bacterial pathogen expressed by male and female genotypes. More broadly, our study joins a growing body of research on the link between immune and reproductive traits, and how selection may shape their expression in both males and females. While the specific environment, selective pressures, and underlying genetic architecture of traits in our lab-reared IV population of
D. melanogaster are unlikely to be directly comparable to other study systems, our results highlight the potential for sex-specific selection to shape evolution of immunocompetence in many other species. For instance, in the beetle,
Callosobruchus maculatus, both males and females raised under male-biased sex ratios exhibited a decrease in the investment in antibacterial immune function (measured as lytic activity) compared to individuals from populations evolving under female-biased sex ratios (
van Lieshout et al. 2014). Increased sexual selection acting on males resulting from heightened competition/conflict may have led to the fixation of male-benefit alleles (and by corollary the loss of female-benefit alleles) from the gene pool, and subsequently resulted in an evolutionary a shift toward phenotypes in which fewer resources are allocated toward immune function. It is possible that this may have also occurred in
McKean and Nunney’s (2008) study of
D. melanogaster, where they observed decreases immunological resistance in flies from populations experimentally evolving under male-biased sex ratios. In the yellow dung fly,
Scathophaga stercoraria, the experimental removal of sexual conflict (via enforced monogamy) for multiple generations led to the evolution of females capable of exhibiting a greater immune response compared to those from polyandrous lines (
Hosken 2001). Thus, depending on the magnitude of the selective pressures acting on males and females, different levels (and dimorphisms) of investment into immunocompetence can evolve (see
Vincent and Gwynne 2014). Furthermore, our study complements previous studies (e.g.,
Fedorka and Mousseau 2004;
Oneal et al. 2007) that have reported distinct sex-specific benefits/costs associated with female mating biases, by highlighting an important fitness-related trait (immunocompetence) that may be associated with differences in offspring competitive success. Integrating information on sex-specific fitness-maximizing strategies, pathogenic risks, developmental costs, and resource availabilities in both males and females should lead to better understanding inter- and intra-specific diversity in immune and reproductive traits.
It is worth noting at this juncture that our initial estimates of bacterial load were based on counts made from adult flies that were ∼14 days old (∼4–5 days post-eclosion), collected from their natal vial. As such, the adults that we assayed had been living in vials containing waste products, a rich microbial community and were likely to have been (multiply-) mated. To better understand the differences in the bacterial loads of “stud”- and “dud”-sired male and female offspring, and the role of mating, we subsequently conducted two complementary follow-up experiments designed to shed light on potential factors that might have given rise to our observed patterns. In the first of these assays, we set out to determine if the differences in bacterial loads seen in mated, adult flies assayed 4–5 days after their eclosion were also apparent earlier in their life cycle. As such, we measured bacterial loads in newly eclosed (virgin) flies, and found no differences between those sired by “studs” and those sired by “duds”. This suggests that the effects of immunity-related genes associated with differential bacterial load that are linked to sire mating success may not be manifested phenotypically until later in their adulthood. This hypothesis is consistent with previous work by
Chippindale et al. (2001) and
Gibson et al. (2002) in which it is suggested that sexually antagonistic alleles in
Drosophila are not expressed during the juvenile part of the life cycle, in which the sex roles are most similar (but see
Perry et al. 2014). It remains to be seen how (or if) the differences in immunocompetence phenotypes associated with paternal reproductive success in the presence or absence of a pathogen translate into differences in individual reproductive success in males and (or) females. Empirical measurements of offspring lifetime reproductive success will help shed light on the role that sexual selection plays in the evolution of immunocompetence, and the likelihood of resolution of any underlying conflict over this trait.
In addition to experiencing immunological challenges from the bacteria-laden media of their natal vial environment, it has been suggested that males transmit bacteria to their mates during mating (
Miest and Bloch-Qazi 2008). Furthermore, the physical damage incurred by females during copulation (
Kamimura 2007) might provide additional avenues for infection. If so, the differences seen between “stud”- and “dud”-sired daughters may reflect differences in the number and (or) types of males mated. If “stud” males do possess alleles favouring the high mating rate phenotype, and there is both sexual conflict and an intersexual correlation between the sexes for this trait (as suggested by
Holland and Rice 1998), then it may be that daughters of “studs” end up with greater bacterial loads due to a relatively higher mating rate or differences in the types of males with which they meet. To begin to test this hypothesis, we experimentally mated females to differing numbers of males and (or) at different times and compared their bacterial loads to virgin females of the same age. Our test failed to detect any significant differences between these groups. While
Miest and Bloch-Qazi (2008) did find that males could transfer bacteria to their mates during mating, their protocol involved dipping a male’s abdomen into a bacterial broth before mating, which may have increased the likelihood of detecting a successful transfer. Other studies (reviewed in
Chapman 2001;
Lawniczak et al. 2007) have found that that several of the compounds that make up the seminal fluid of
D. melanogaster males have protective antimicrobial properties (also see
Mueller et al. 2007). Furthermore,
Peng et al. (2005) report that the innate immune system of females is stimulated by the presence of the seminal peptide,
Sex-peptide, which might have mediated any potential change in bacterial loads in the mated flies. It should be noted that this assay was conducted in vials containing fresh media, which was more sterile than typical culture conditions, and that we did not measure the mating success of the fathers of our target females. Future studies of bacterial loads on flies in which age, developmental environment, and (or) the number of mating partners as well as parental mating success may provide a better understanding of the nature of this sex-linked variation in immunocompetence.
In summary, we provide evidence that there are important immunological consequences associated with mate-choice outcomes in the model species, Drosophila melanogaster. In our study system, offspring produced by females who mate with reproductively successful males display greater immunocompetence, but this pattern is confined to sons, and is offset by immunological disadvantages experienced in their daughters. This pattern may reflect the operation of sexually antagonistic alleles over optimal allocation to the immune system. Our post hoc experiments suggest that the differences in bacterial loads observed between “stud” and “dud”-sired offspring are not evident upon their eclosion as adults, but become more apparent over time, and that the differences in daughters may not be due to differences in their mating rates. Ultimately, the extent to which these differences contribute to variation in female lifetime reproductive success will determine the strength and direction of sexual selection, and shape our understanding of the evolution of life history traits in both sexes.