Introduction
Hydraulic fracturing of oil shale deposits (unconventional gas production) offers high potential for the production of natural gas (
Rivard et al. 2014), particularly with recent technological advances that have made the fracturing and extraction process more efficient (
Vengosh et al. 2013;
Vidic et al. 2013;
Soeder and Engle 2014). Hydraulic fracturing involves horizontal drilling at depths >1 km into the shale and injection of large volumes of fluids at high pressure (slickwater that is laced with viscosity gels and chemical additives including hydrocarbons) to assist in the recovery of gas (
Gregory et al. 2011;
Rivard et al. 2014;
Soeder and Engle 2014). Environmental concerns related to unconventional gas activities include the potential for groundwater contamination through stray gases or hydraulic connectivity from fracturing activities (
Vengosh et al. 2013,
2014;
Lavoie et al. 2014), the fragmentation and alteration of habitats as a result of water withdrawal and infrastructure development to support fracturing activities (
Entrekin et al. 2011;
Brittingham et al. 2014), and the need for safe disposal of the large volumes of recovered fracking fluids, which could cause groundwater and surface water contamination if insufficiently treated or if a spill occurs (
Entrekin et al. 2011;
Gregory et al. 2011;
Warner et al. 2013;
Lavoie et al. 2014;
Vengosh et al. 2014). Spills of untreated fracking fluids can release high concentrations of salts, organics, and metals into surrounding freshwaters (
Vengosh et al. 2014). Furthermore, an analysis of treated wastewater effluent from fracking activities in Pennsylvania indicated that some contamination of surface waters is possible even after treatment if fluids are discharged into nearby streams (
Warner et al. 2013). Despite the potential for environmental impacts, there remains a lack of information to support the development of diagnostic tools for environmental monitoring of these activities (
Vengosh et al. 2013,
2014;
Vidic et al. 2013;
Leger et al. 2016a,
2016b), and significant gaps exist in the monitoring of shale-gas production areas (
Entrekin et al. 2011;
Kinchy et al. 2016).
Shale-gas formations have particular geological characteristics (bedrock age, formation, organic content, and depth) that determine the accessibility of the shale gas and the potential yield (
Dyni 2003;
Rivard et al. 2014). The geological characteristics that determine suitability for shale-gas development may also play a role in determining physical and chemical characteristics of overlying freshwater habitats. For example, water chemistry composition and substrate bed material of rivers have been shown to vary across geology types (
Johnson et al. 1997;
Dow et al. 2006). Specifically,
Lento et al. (2019) found higher ion concentrations in streams underlain by Early Carboniferous bedrock (a formation with high shale-gas potential) than in surrounding streams. Several studies have noted that the variability in the chemical and physical habitat that is observed in freshwaters with different underlying geology is accompanied by strong differences in biotic communities across these systems, such as differences in species composition and dominant species (
Leland and Porter 2000;
Esselman et al. 2006;
Yates and Bailey 2010), even when geological shifts occur over small spatial areas (
Neff and Jackson 2011,
2012). Thus, it may be possible to predict the biota that would be present across natural differences in geology (including areas with and without shale-gas potential) based on known tolerances to the physical and chemical habitat characteristics associated with different types of bedrock geology. However, such prediction would require a better understanding of the characteristics of abiotic habitats and biotic communities found in freshwaters overlying geologic formations with high shale-gas potential. This information is necessary to describe the natural variation that may exist in these areas relative to other (potentially neighbouring) geological formations, but also to improve our ability to predict biotic and abiotic shifts within these systems as development and production increase.
Evaluation of the biotic communities that are characteristic of the chemical and physical habitat conditions of shale-gas-producing geologic areas and monitoring of changes within those areas relative to surrounding geological formations is reliant on the selection of appropriate taxonomic groups, focusing on those that might be expected to respond to the anthropogenic stressors of interest (
Hering et al. 2006;
Goodsell et al. 2009;
Yates and Bailey 2010). In river systems, benthic macroinvertebrates (BMI) and fish are commonly used as bioindicators because they are widespread, easy to sample, and have well-documented tolerances to perturbation (
Bonada et al. 2006;
Resh 2008). In addition, each group has its own advantages: for example, BMI are relatively immobile and reflect local conditions, whereas fish are long-lived and have social and economic importance (
Bonada et al. 2006;
Resh 2008). However, each group of organisms also differs in its response to perturbation (
Hering et al. 2006;
Johnson et al. 2006), with a stronger response of BMI to local water chemistry and substrate size changes and a response of fish to physical habitat shifts, including temperature (
Hering et al. 2006;
Yates and Bailey 2011;
Bae et al. 2014). Selection of suitable indicator groups must involve a detailed evaluation of the response of target groups to relevant anthropogenic stressors (
Goodsell et al. 2009;
Yates and Bailey 2010). Where the full extent of potential stressors is unknown, the combined assessment of multiple groups may provide the best characterization of baseline conditions and biotic integrity in a system (
Bonada et al. 2006;
Hering et al. 2006). The use of multiple bioindicator groups in monitoring and assessment assumes that each biotic group has a complementary stressor response, i.e., that each group responds to different stressors, improving the diagnostic capacity of the suite of bioindicators and increasing the likelihood of detecting impacts (
Hering et al. 2006).
A measure of similarity might be expected in the characterization of rivers or lakes by different organism groups where there are large-scale environmental drivers acting on those freshwater systems, if all organisms respond in a similar manner (
Paavola et al. 2006;
Johnson et al. 2007). One way to assess the differences and similarities in the responses of groups of organisms is to evaluate community concordance, which measures the extent to which there is coherence or consistency among biotic assemblages, i.e., similarity in the characterization of stations based on the community structure of different groups of organisms (
Jackson and Harvey 1993). Significant levels of community concordance have been found in assessments of BMI, fish, and other freshwater organism groups (e.g., diatoms, bryophytes, plankton, macrophytes) in both lakes and rivers (
Kilgour and Barton 1999;
Johnson and Hering 2010;
Neff and Jackson 2013), though in some cases the strength of concordance was dependent on spatial or temporal scale of assessment (
Allen et al. 1999;
Heino 2002;
Paavola et al. 2006;
Bowman et al. 2008). At small spatial scales, researchers have noted a lack of concordance among communities where local-scale drivers dominate and have suggested that each organism group responded to different environmental and anthropogenic pressures (
Paavola et al. 2003;
Heino et al. 2005;
Infante et al. 2009;
Larsen et al. 2012). In contrast, the higher concordance among communities at regional scales has been suggested to result from large-scale environmental drivers acting in a similar fashion across broad spatial scales (e.g.,
Heino 2002;
Paavola et al. 2006). If this is the case, then the unique physical and chemical habitat of stream systems in geological areas with shale-gas potential has the potential to drive concordance among stream assemblages, even if there are differing responses of organism groups to localized environmental drivers.
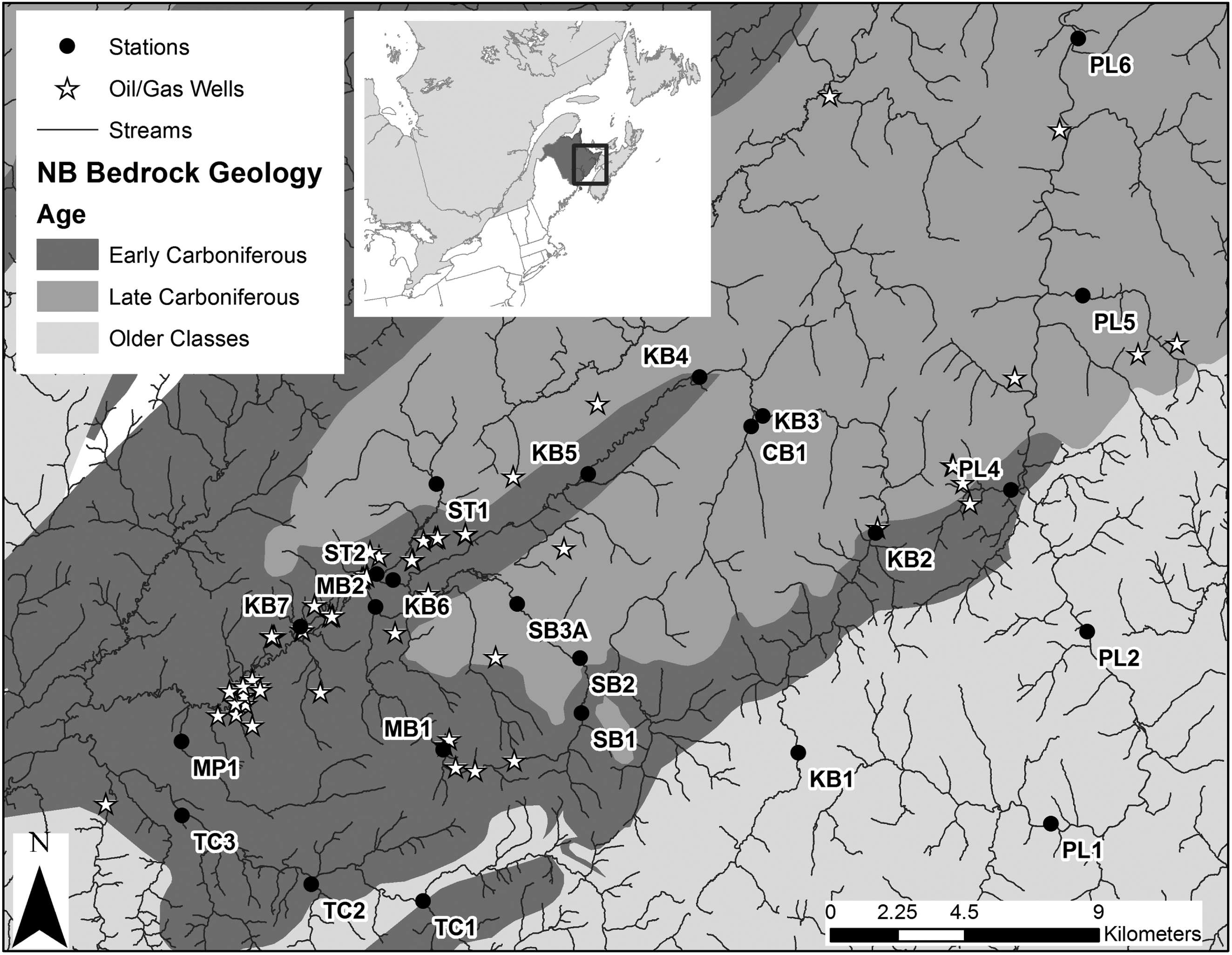
The purpose of this study was to characterize stream fish and BMI assemblage structure in southeastern New Brunswick, Canada, an area underlain by different ages of bedrock (Early-Late Carboniferous and older-age sedimentary and nonsedimentary bedrock), identify dominant drivers for fish and BMI, and assess the level of community concordance among fish and BMI in these systems. Areas underlain by Early Carboniferous bedrock have a high potential for shale-gas extraction, yet characterization of the physical and chemical habitat and biotic communities of freshwaters in these areas has been limited (
Kinchy et al. 2016). Therefore, the assessment of biotic and abiotic patterns within these systems and in relation to surrounding geological age classes has the potential to inform management and biomonitoring of freshwater systems in shale-gas development areas. Community concordance among fish and BMI assemblages was predicted to be high if (
i) BMI and fish displayed similar patterns because they responded to the same environmental drivers or (
ii) geological age class acted as a large-scale driver of community structure, leading to increased concordance among organism groups.
Discussion
Monitoring and assessment to detect the potential impacts of shale-gas activities relies on the identification of appropriate response variables, including the selection of monitoring endpoints that will respond to relevant anthropogenic stressors. In our study, BMI assemblage structure was strongly related to water chemistry variables such as conductivity, which characterized areas with shale-gas potential, and the normal range of conductivity as well as several BMI metrics in Early Carboniferous stations were distinct from stations without shale-gas potential. Conductivity, in particular, would be expected to change in response to spills or other potential impacts of unconventional gas production, such as groundwater contamination, overland runoff, or release of treated effluents, which implies a strong potential for BMI abundance and composition to be used as a diagnostic tool to detect shale-gas impacts. In contrast, fish assemblage composition had a stronger association with a gradient in nutrients that was independent of geologic age, and with turbidity, which was highest in stations with high shale-gas potential. Despite the difference in driving variables, BMI and fish provided similar characterization of study sites, as evidenced by significant concordance. The response of BMI and fish taxa to differing environmental drivers suggests that these two taxonomic groups could be utilized in concert to detect and identify community shifts in response to a number of different environmental and anthropogenic pressures.
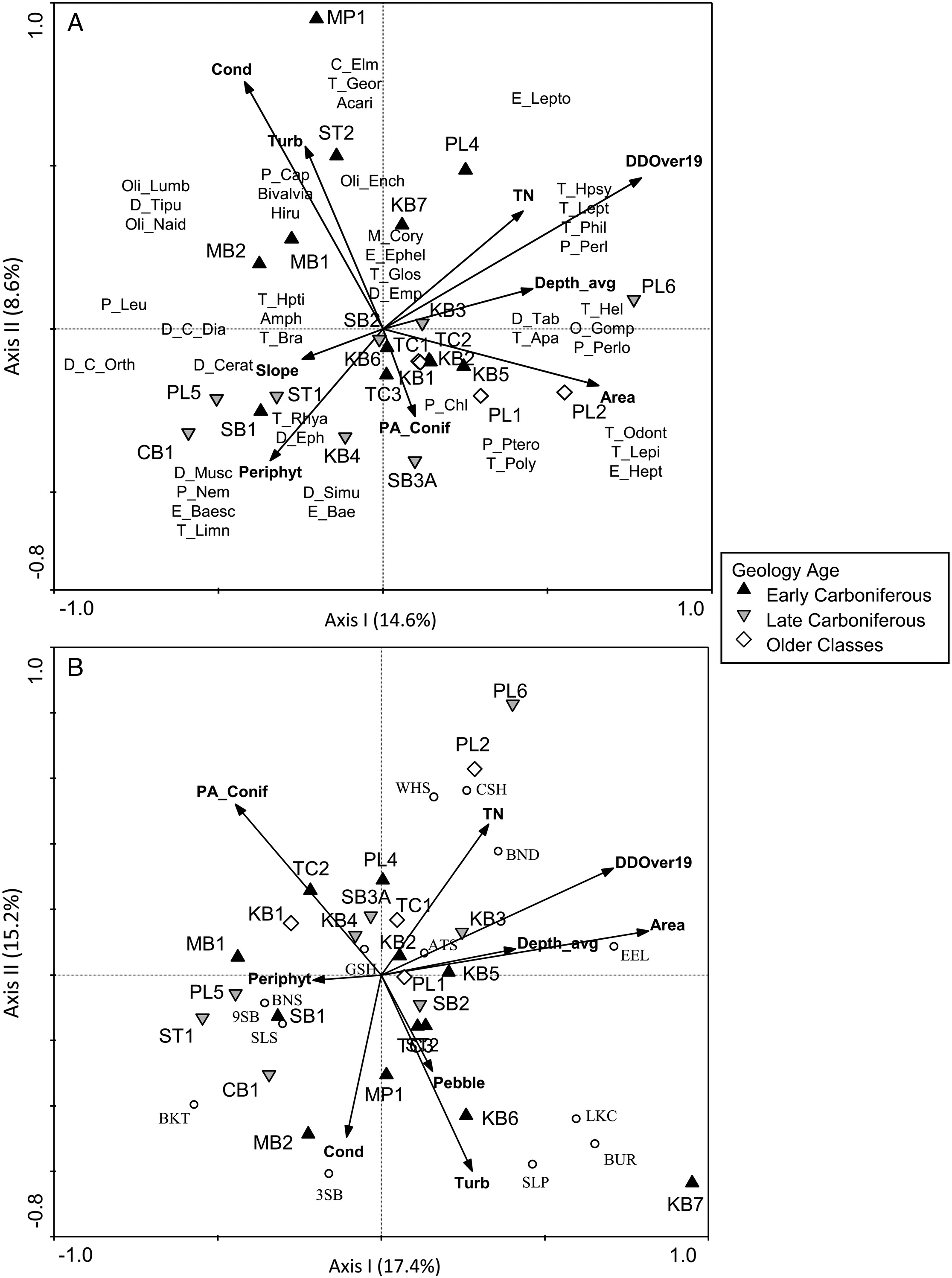
Biotic associations across environmental gradients
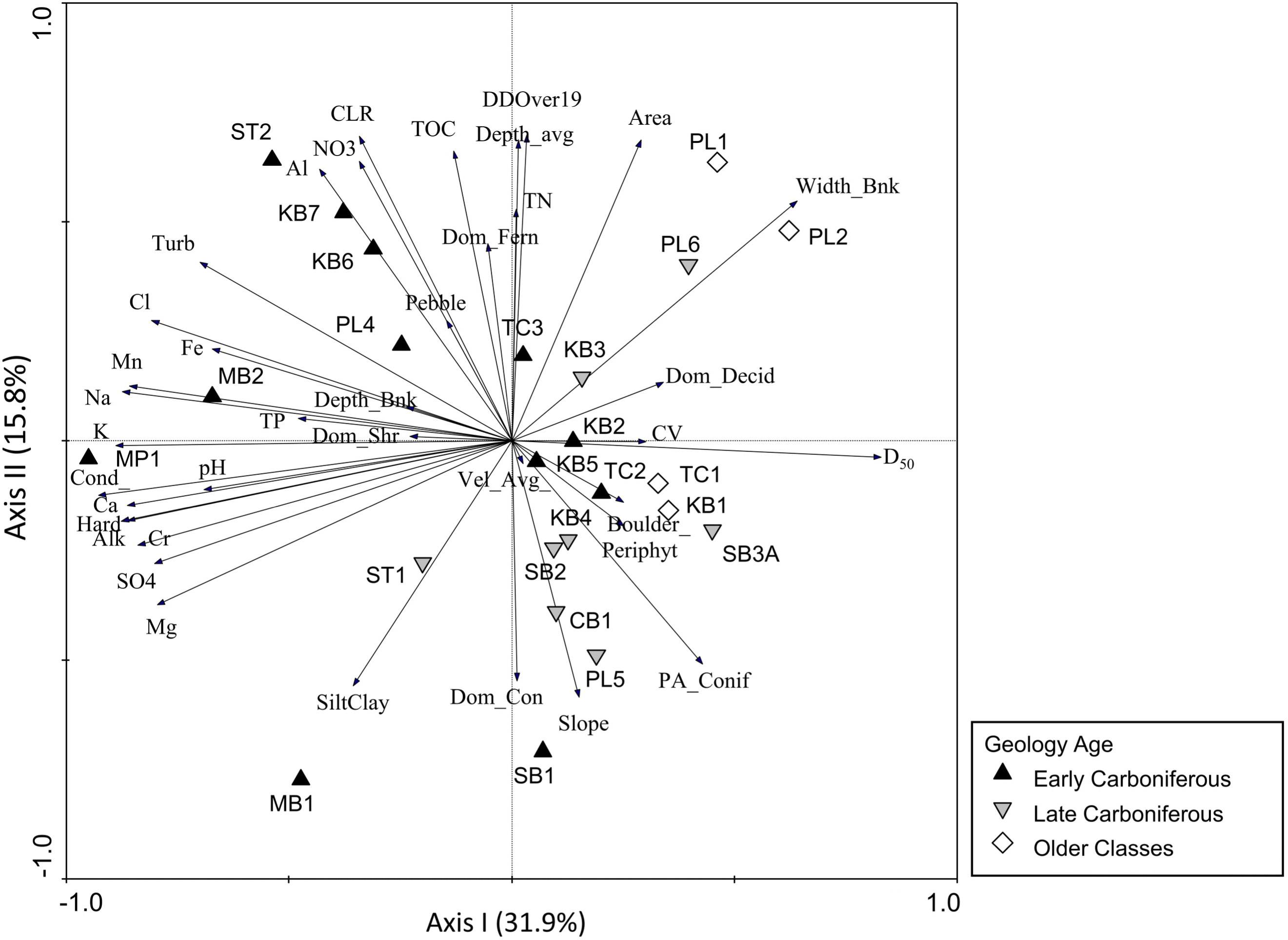
Stations underlain by different geological age classes were characterized by environmental variables that have the potential to drive gradients in biotic assemblages (e.g.,
Snelder et al. 2004). Early Carboniferous stations, which have high shale-gas potential, were strongly characterized by high levels of several water chemistry variables (e.g., ions, conductivity, turbidity, and metals), which may be driven by underlying geologic composition (
Johnson et al. 1997;
Reimann et al. 2009). In contrast, stations underlain by Late Carboniferous or older classes of bedrock were more strongly associated with a range of habitat variables that were indicative of gradients in stream width, periphyton cover, and riparian vegetation. These results supported the idea that surface and groundwaters in distinctive geological regions might display characteristic abiotic conditions with unique chemical habitats (
Stapinsky et al. 2002;
Reimann et al. 2009;
Neff and Jackson 2011,
2012). Furthermore, differences in conductivity and TP among geologic age classes in our study allowed the development of baseline criteria for monitoring based on the normal range for stations underlain by Early Carboniferous bedrock.
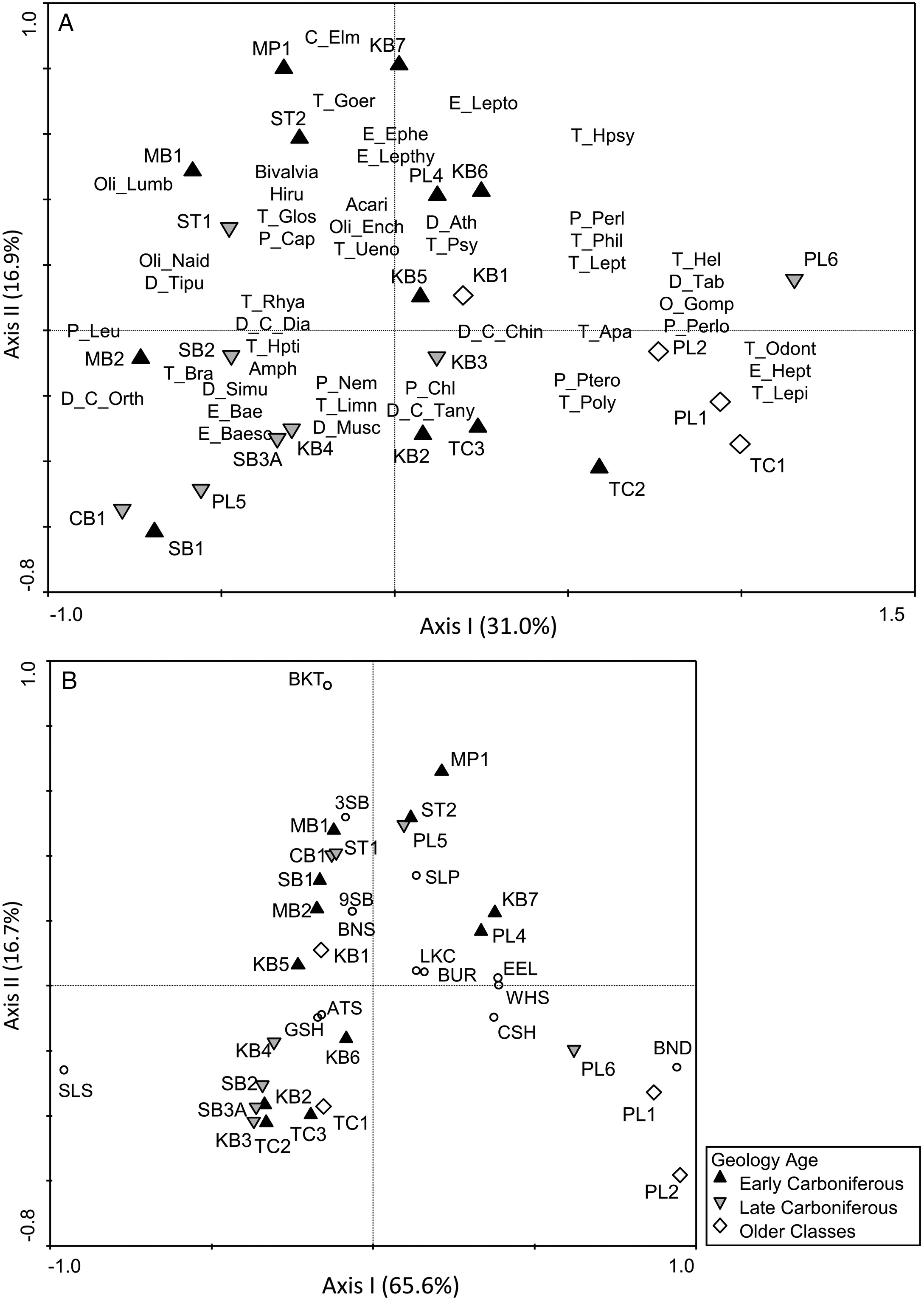
There was clearly a stronger association of BMI with conductivity, which was a defining feature of stations underlain by Early Carboniferous bedrock geology, whereas fish were more strongly associated with system size (catchment area and depth) and a gradient in nutrients that was not specific to a particular geologic age class.
Neff and Jackson (2013) noted similar patterns when comparing stream systems on the Precambrian Shield and off-Shield, with BMI responding more strongly to water chemistry differences across geology types and fish predominantly responding to physical habitat variables. Conductivity and alkalinity played a dominant role in distinguishing between Shield and off-Shield stations in their study, and
Neff and Jackson (2011) found an association of several noninsect groups (e.g., Gastropoda, Amphipoda, mites, Hirudinea, and Isopoda) with the higher ion levels in off-Shield stations, similar to our results. Furthermore,
Johnson et al. (2015) sampled streams in active shale-gas development areas in the southern United States and found their BMI assemblages to be dominated by Oligochaeta, Chironomidae, and tolerant genera of Ephemeroptera, which corresponds to the BMI patterns seen in our study for Early Carboniferous stations. The predominance of noninsect groups in high conductivity waters underlain by Early Carboniferous bedrock may relate to the dependence of taxa such as gastropods and bivalves on uptake of calcium from the water to maintain shell growth (
Havas and Rosseland 1995;
Neff and Jackson 2013). Though conductivity appeared to be a less dominant driver for fish, it was positively correlated with both Brook Trout and Threespine Stickleback. Conductivity has been found to correlate with abundance (
Scarnecchia and Bergersen 1987) and size (as outlined in
Copp 2003) of some fish species including trout, with high conductivity stations equated to more productive systems. The positive association of Brook Trout relative abundance with both conductivity and periphyton in these streams appears to support this idea.
Physical habitat differences among stations were reflected by taxonomic associations of both BMI and fish with gradients in temperature and system size. For example, stations that were cooler were associated with Plecoptera taxa such as Leuctridae, Nemouridae, and Chloroperlidae, which prefer small cool-water streams (
McCafferty 1998). Brook Trout were similarly associated with small cool-water streams, consistent with their preferred temperature of 16 °C (
Coker et al. 2001). The strongest gradient in the fish PCA was a negative relationship along the first axis between Slimy Sculpin, which have a cool-water preference (11–16 °C;
Lyons 1990), and stations PL1, PL2, and PL6, which were large, warm systems, with higher maximum summer temperatures than other systems (ranging from 26 to 27.5 °C). These stations had more degree days above 19 °C, and they were positively correlated with Pteronarcyidae, Perlidae, and Perlodidae, which inhabit larger systems and are more tolerant of warm temperatures (
McCafferty 1998). Blacknose Dace and American Eel were the fish species that were most strongly associated with these warm-water stations, and these species have preferred temperatures of 24.6 °C and 19 °C, respectively (
Coker et al. 2001). Temperature in our study was not specifically related to particular geological age classes, though
O’Sullivan et al. (2019) found that surficial geology, slope, and areas of geologic contact could be used to predict stream temperatures. The Pollett River stations, which had the highest degree days above 19 °C and the most variable temperatures in our study, were large stations that also had low percent cover of riparian vegetation and low percent forest in the upstream catchment.
Johnson (2004) found evidence that shading affected maximum temperatures in small stream systems, and the lack of riparian vegetation at the Pollett River stations may have contributed to increased temperatures, driving differences in BMI and fish assemblages.
Community concordance in relation to environmental drivers
The detection of significant community concordance among BMI and fish has two potential implications: (
i) that the current range of environmental drivers in the study systems acts on BMI and fish in a similar manner, resulting in similar groupings of stations regardless of the organism examined and (
ii) that there is redundancy in the evaluation of both BMI and fish assemblage structures within the same system, as they both lead to the same conclusion (
Jackson and Harvey 1993). In the case of the Kennebecasis and Pollett River watersheds, the evaluation of both BMI and fish assemblages cannot be considered fully redundant as these groups of organisms appeared to respond most strongly to different environmental drivers. Other studies have noted significant concordance among organism groups despite evidence of differing responses to localized drivers (e.g.,
Jackson and Harvey 1993;
Dolph et al. 2011;
Bae et al. 2014). In particular,
Neff and Jackson (2013) found significant community concordance between fish and BMI assemblages in Shield and off-Shield stream systems despite a stronger association of BMI with water chemistry variables and fish with physical habitat variables. Similar to our study,
Neff and Jackson (2013) assessed streams within a localized region where there were distinct geological differences that had a significant effect on the physical and chemical habitat of the stream systems. Where the importance of drivers differs among groups that are concordant (e.g., fish and BMI), each assemblage could be expected to respond differently to future perturbation, which indicates that they provide complementary information about ecosystem condition.
Previous studies that have noted weaker concordance at smaller spatial scales (e.g., within drainage basins or within ecoregions) have attributed the difference in response of organism groups to strong localized differences in small-scale environmental drivers such as water chemistry and physical habitat (
Paavola et al. 2003,
2006;
Larsen et al. 2012). However,
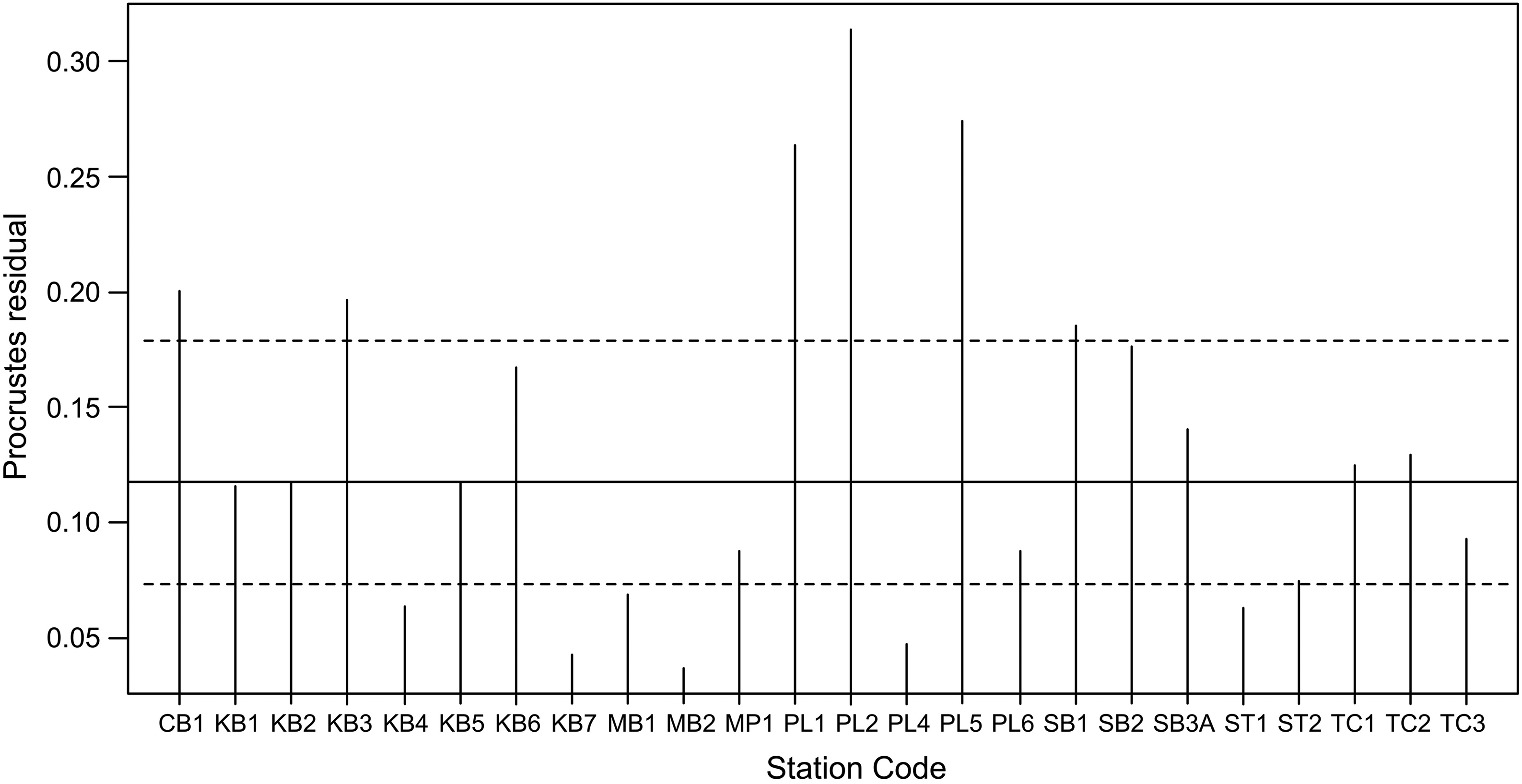
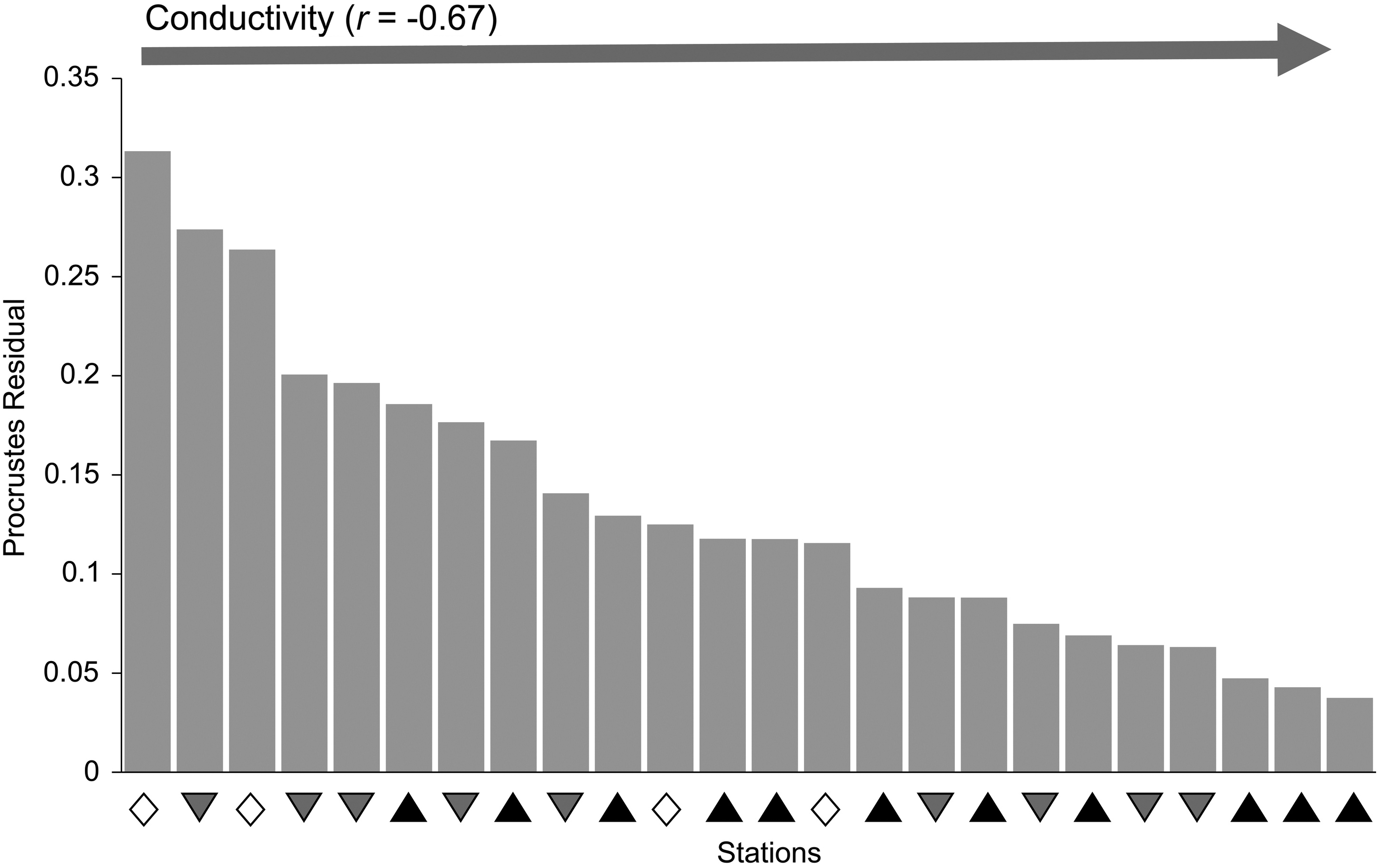
Paavola et al. (2006) suggested that concordance might be evident in small-scale assessments if there is a single, strong environmental gradient driving assemblages in a similar way. In our study, the geological topologies characterized by Early Carboniferous, Late Carboniferous, and older classes of bedrock may have acted in such a manner. The concordance gradient that we identified across stations indicated that the strongest concordance or consistency among fish and BMI was evident in small systems with small substrate size and high conductivity. This association with system size is contrary to the findings of
Paavola et al. (2006), who found increased concordance in larger systems in their broadscale regional assessment of streams. However, this may speak to the strength of the geological gradient in the current study and the important distinction of Early Carboniferous (shale-gas producing) areas from those underlain by other geological formations, providing further evidence of the dominant role geology can play in characterizing freshwater habitats (e.g.,
Neff and Jackson 2011,
2012,
2013).
Monitoring surface waters in shale-gas formations
Though differences in system size, substrate size, and temperature were evident within and among geology age classes, water chemistry was the most important set of variables for characterizing Early Carboniferous stations. In a PCA including only water chemistry data (excluding physical habitat data), the first two axes explained 71.8% of the variance among sites (results not shown) further indicating the importance of water chemistry to distinguish among geology classes. Water chemistry also represents one of the characteristics of surface waters that is most likely to be impacted by potential spills, contamination by untreated flowback fluids (hydraulic fracturing fluids that return to the surface), or the release of treated effluent from shale -gas production (
Entrekin et al. 2011;
Vengosh et al. 2014). In their review of the potential impacts of hydraulic fracturing activities,
Vengosh et al. (2014) noted that surface spills in the United States have caused high levels of organics, metalloids (Se, As), radionuclides (Ra), methane, pH, salts (total dissolved salts, and Cl and Br in particular), and specific conductivity.
Wilson et al. (2014) found Cl levels in produced water from shale gas activities to be on average 133 733 mg/L, several orders of magnitude higher than the average Cl levels in the Early Carboniferous stations in the current study (8.38 mg/L), which were elevated compared to Cl levels in Late Carboniferous and Older Class stations (2.34 and 1.63 mg/L, respectively).
Lento et al. (2019) found a number of ions had elevated levels in Early Carboniferous stations, consistent with the strong positive association of these stations with conductivity and ions in the present study. Given the already elevated levels of Cl and conductivity in the Early Carboniferous (high shale-gas potential) stations relative to other geological formations in the current study, this suggests the potential for even stronger geological distinction of stations if shale-gas production were to cause impacts to surface waters through wastewater spills. To support monitoring and detection of such impacts, baseline criteria can be developed by identifying the range of values outside which there may be evidence of impairment (normal range;
Munkittrick et al. 2009). In our study, confidence intervals for conductivity provided evidence of the distinction among geologic age classes with respect to this chemical parameter, but also indicated the normal range for Early Carboniferous stations within which 95% of the conductivity values fell. With additional monitoring of streams in areas of potential shale gas development, these baseline criteria could be further refined to allow greater accuracy in the detection of impacts.
The stronger association of BMI with conductivity has important implications for biomonitoring in areas with high shale-gas potential, as it suggests that any changes to ion levels that might result from shale-gas production through spills or overland runoff may be most evident as impacts on BMI assemblages. Noninsect taxa and some families of Ephemeroptera and Trichoptera were highly associated with ion levels and could act as indicators of shifts in conductivity, whereas many Plecoptera in particular were more related to temperature gradients. BMI abundance, taxonomic richness, and the percent composition of Chironomidae, EPT, and Oligochaeta were all significantly different in stations underlain by Early Carboniferous bedrock relative to older classes of bedrock geology, similar to the patterns noted by
Lento et al. (2019), and the baseline criteria developed for these metrics can be used to monitor how test stations compare with the expected normal range in the absence of impacts from shale-gas development. Fish assemblages provided complementary information by strongly reflecting other physical and chemical habitat characteristics that might be altered with shale-gas development (e.g., increased turbidity from development activities;
Entrekin et al. 2011) and natural gradients (e.g., nutrients) that did not relate as strongly to geologic age. Shifts in fish assemblages may be expected to reflect impacts to the system that are related to more general land use and development changes, such as changes to nutrient and sediment regimes or habitat fragmentation caused by clearing the land for construction of wells and development of supporting infrastructure (
Entrekin et al. 2011;
Brittingham et al. 2014). Thus, monitoring of both BMI and fish may provide a measure of the relative strength of different anthropogenic impacts, including multiple aspects of shale-gas development, as taxonomic preferences and tolerance levels inform assessment of community shifts.
This study characterized the abiotic habitat and biotic communities of streams in geologic areas with high shale-gas potential, contrasting with streams on neighbouring geologic formations. There was clear evidence of differences in the abiotic habitat across geological age classes, and these differences were associated with variability in BMI and fish assemblage structure, with BMI more strongly reflecting temperature and conductivity and fish relating more to system size and gradients in nutrients and turbidity. Despite the differences in dominant drivers across organism groups, there was evidence of significant community concordance among BMI and fish assemblages, and this concordance increased with increasing conductivity and decreasing system size and substrate size. Geological age, differentiating Early Carboniferous, Late Carboniferous, and older classes of bedrock, appeared to act as a large-scale driver in these systems, contributing to concordance within the biotic community despite differences in localized environmental drivers (i.e., physical and chemical habitat variables), and the results of this study suggest that BMI and fish provide complementary information about ecosystem condition across the range of geology types examined. Effective environmental monitoring in areas of shale-gas development should therefore include assessment of both BMI and fish assemblages to allow for the detection of impacts related to shale-gas extraction as well as general perturbation related to development, with deviations from concordance used to detect assemblage-specific driver–response relationships. Importantly, baseline criteria developed in this paper provide estimates of the normal range for chemical and biotic metrics that might be affected by shale-gas development, thereby supporting the development of diagnostic tools for monitoring. This study highlights the importance of examining biotic response to environmental drivers in multiple assemblages to understand and interpret baseline conditions and ultimately measure meaningful change to adequately, and appropriately, inform management and biomonitoring of freshwater systems.