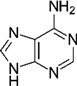
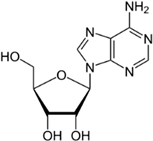
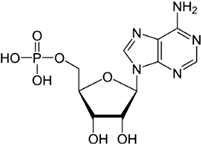
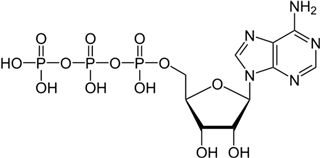
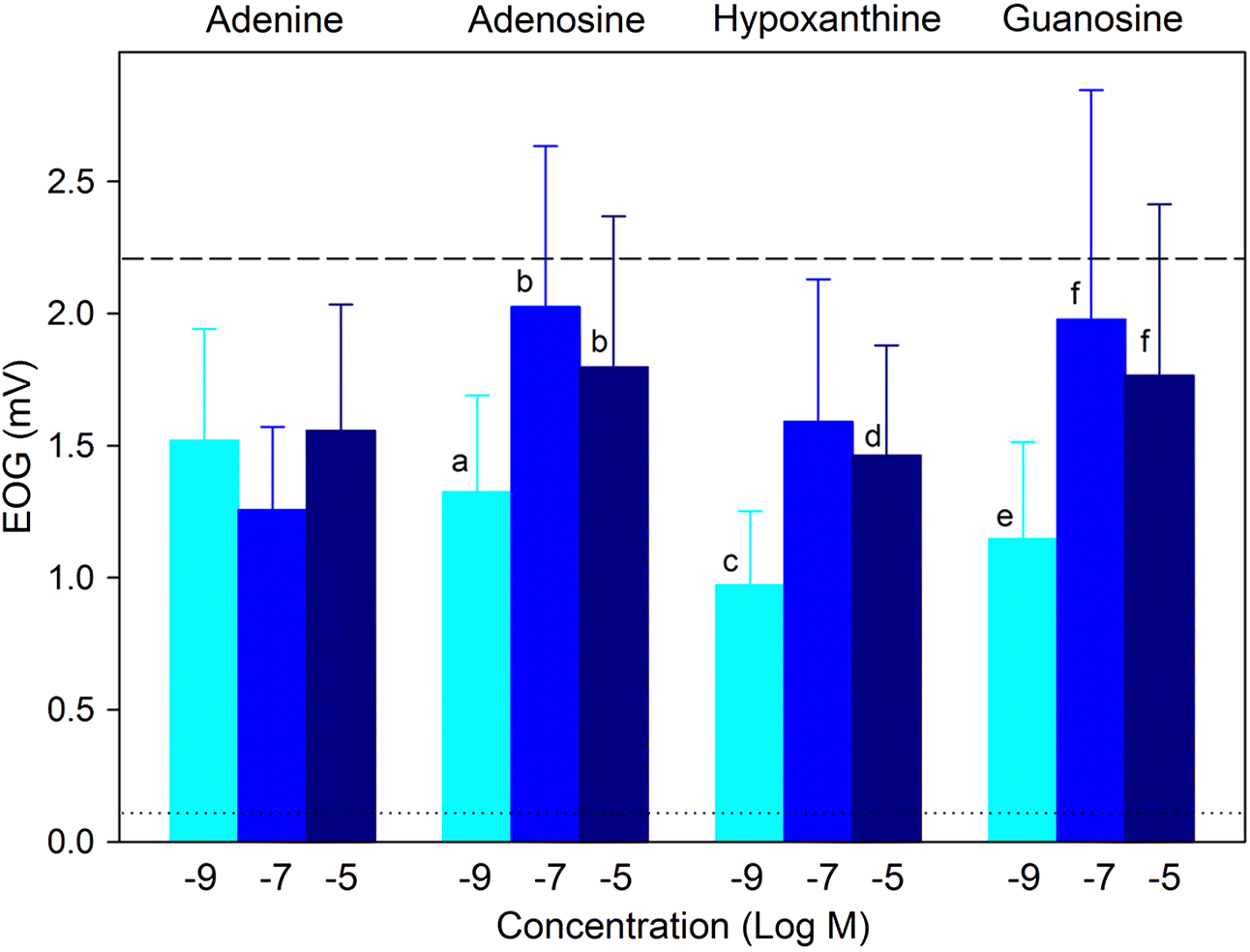
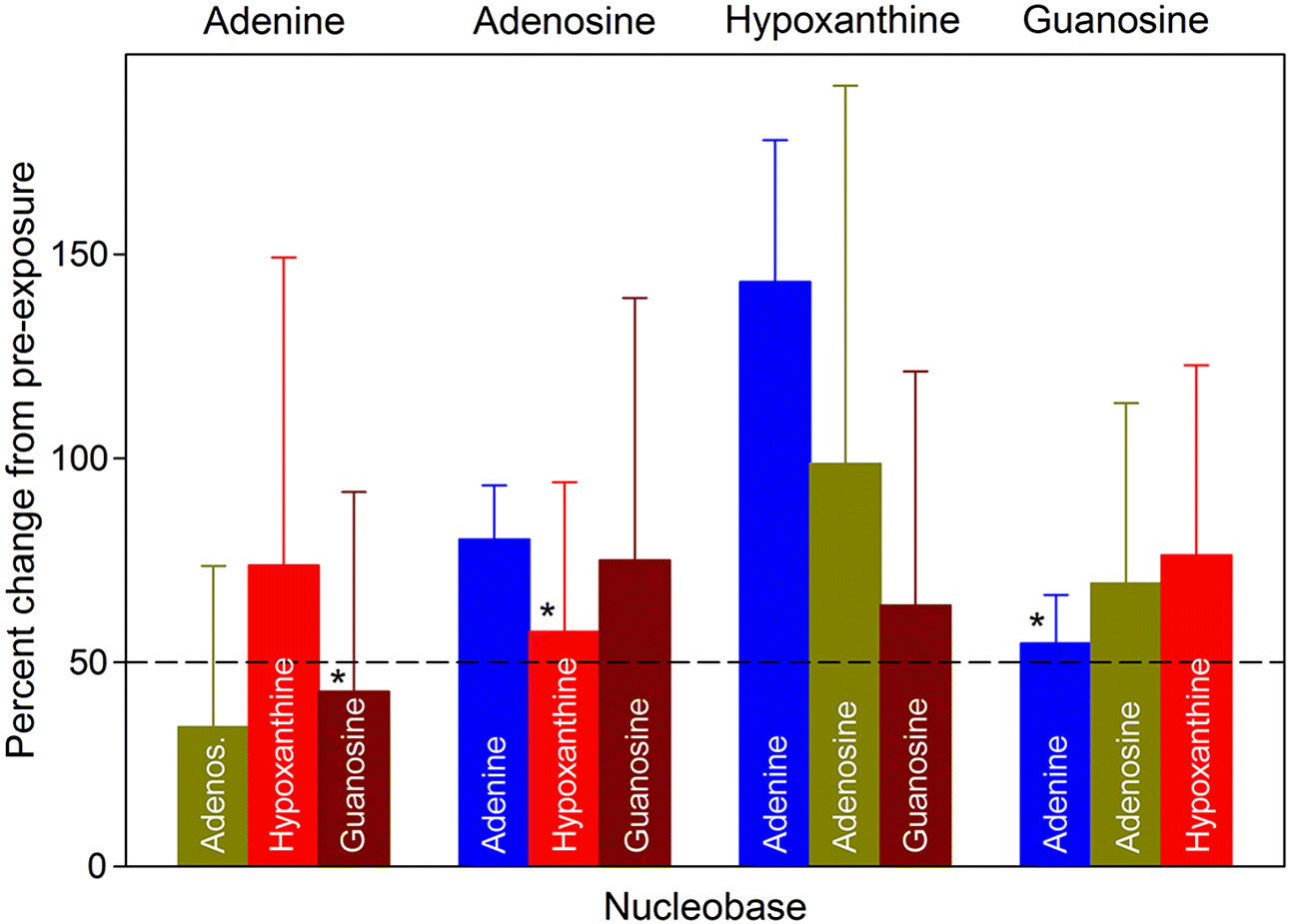
Purine nucleobases evoked behaviours, caused neuronal responses, and evoked changes in gene expression. Although some of the responses were expected, such as ATP avoidance, many were not, including neuronal responses at a very low threshold and the expression of many genes with unclear relevance to nucleobase exposure or chemosensation.
Behaviour
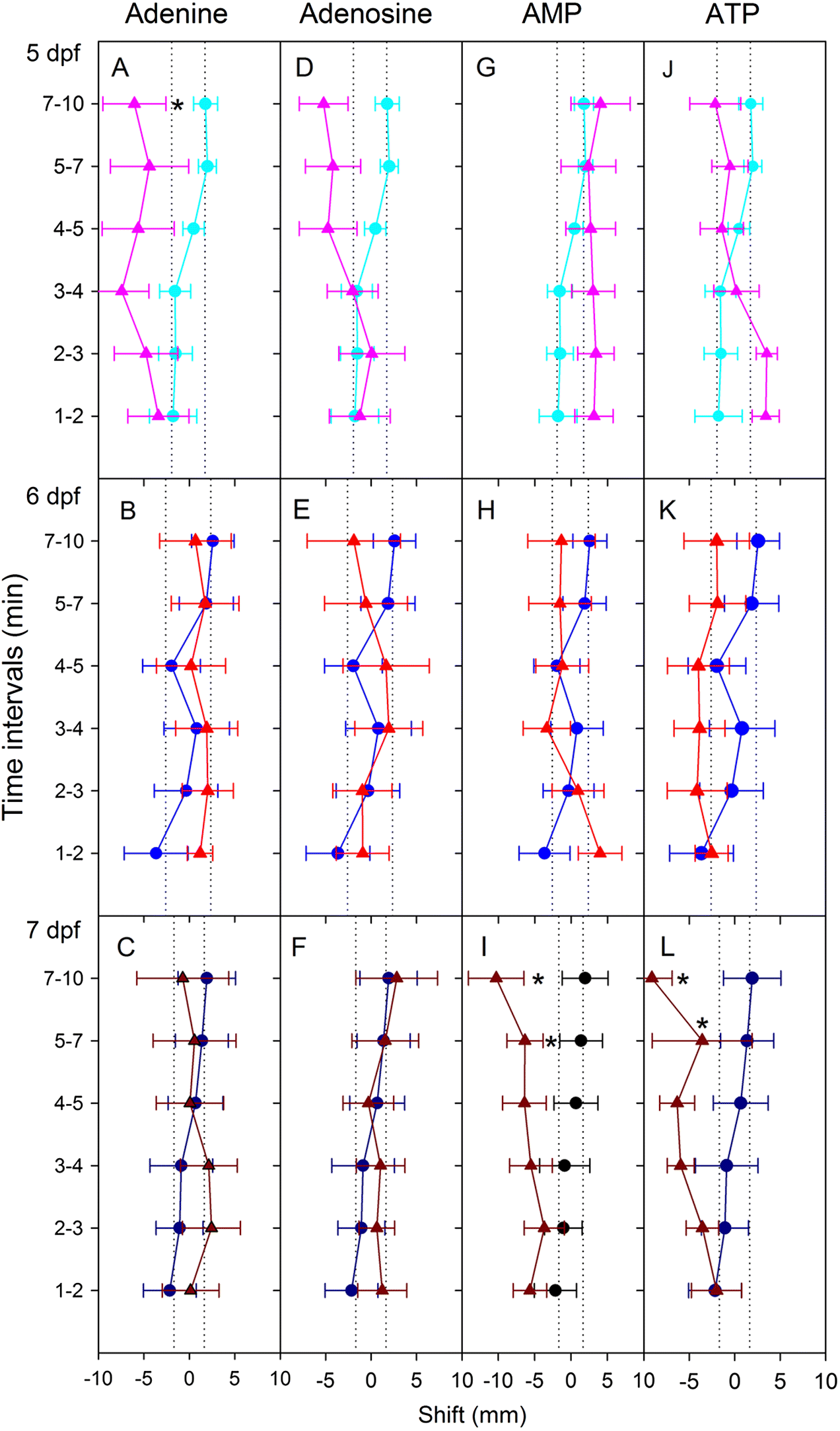
Apart from four instances among 36 different time–odourant combinations, larval zebrafish did not respond to nucleobases. When responses were observed, nucleobases evoked avoidance (with only one exception), and the responses were dependent on nucleobase structure and embryo age. The data demonstrate that adenine-containing compounds may cause behavioural change depending on dpf. Recently, it was demonstrated that ATP may be enzymatically converted to adenosine at the olfactory epithelium and that adenosine activates a novel receptor (
Wakisaka et al. 2017). Given that we noted changes in response to adenine, ATP, and AMP, it is possible that adenine is capable of stimulating the same receptor or that it has its own unique receptor. The first option may be unlikely, as
Wakisaka et al. (2017) found that adenine did not lead to activation of the same glomerulus in the olfactory bulb that adenosine did. Regardless, our findings are in agreement with previous studies that showed that adenine-containing compounds evoked the most potent responses (
Friend and Smith 1971;
Carr et al. 1986).
Attractive responses to ATP and AMP were expected owing to the ability of these compounds to evoke feeding in other species (
Friend and Smith 1971;
Smith and Griend 1976;
Mearns et al. 1987;
Miyasaki and Harada 2003;
Romero and Schal 2014). However, such responses were not observed in zebrafish larvae, likely because of fish age. A study of feeding on nucleotide-enriched agar showed that nucleotides deterred feeding in Atlantic salmon parr but stimulated feeding in adult rainbow trout (
Mearns et al. 1987). This raises the question, is there a reason why younger fish are deterred by nucleotides? There are at least two potential explanations: (1) stimulus naivety and (2) stimulus significance. In the case of salmon parr, the fish have not yet made their oceanic migration and it is possible that the nucleotides used in the study (sourced from shrimp) were not yet familiar as food odourants. The same is possible for zebrafish larvae—they have not yet been exposed to food and therefore may not associate nucleotides with food. In support of this, adult zebrafish were attracted to adenosine-containing compounds (
Wakisaka et al. 2017). Alternatively, considering that nucleotides are found in biological tissues such as blood, perhaps salmon parr and zebrafish larvae are prey (
Mearns et al. 1987;
Carr et al. 1996;
Engeszer et al. 2007;
Romero and Schal 2014), and so ATP indicates an alarm cue (e.g., recent release from a nearby sibling).
A relationship between the number of phosphates and the strength of the behavioural response was not observed. In studies of insects, decreased phosphorylation was associated with decreased stimulatory efficacy (
Friend and Smith 1971;
Smith and Griend 1976;
Romero and Schal 2014). In the present study, there was no observed relationship between the number of phosphates and the capacity to evoke behaviours. However, we did not directly compare responses to AMP and ATP and so cannot draw definitive conclusions. To confirm our finding, future studies should include dual odourant trials (i.e., comparison of two odourants, one on each side of the attraction–avoidance apparatus).
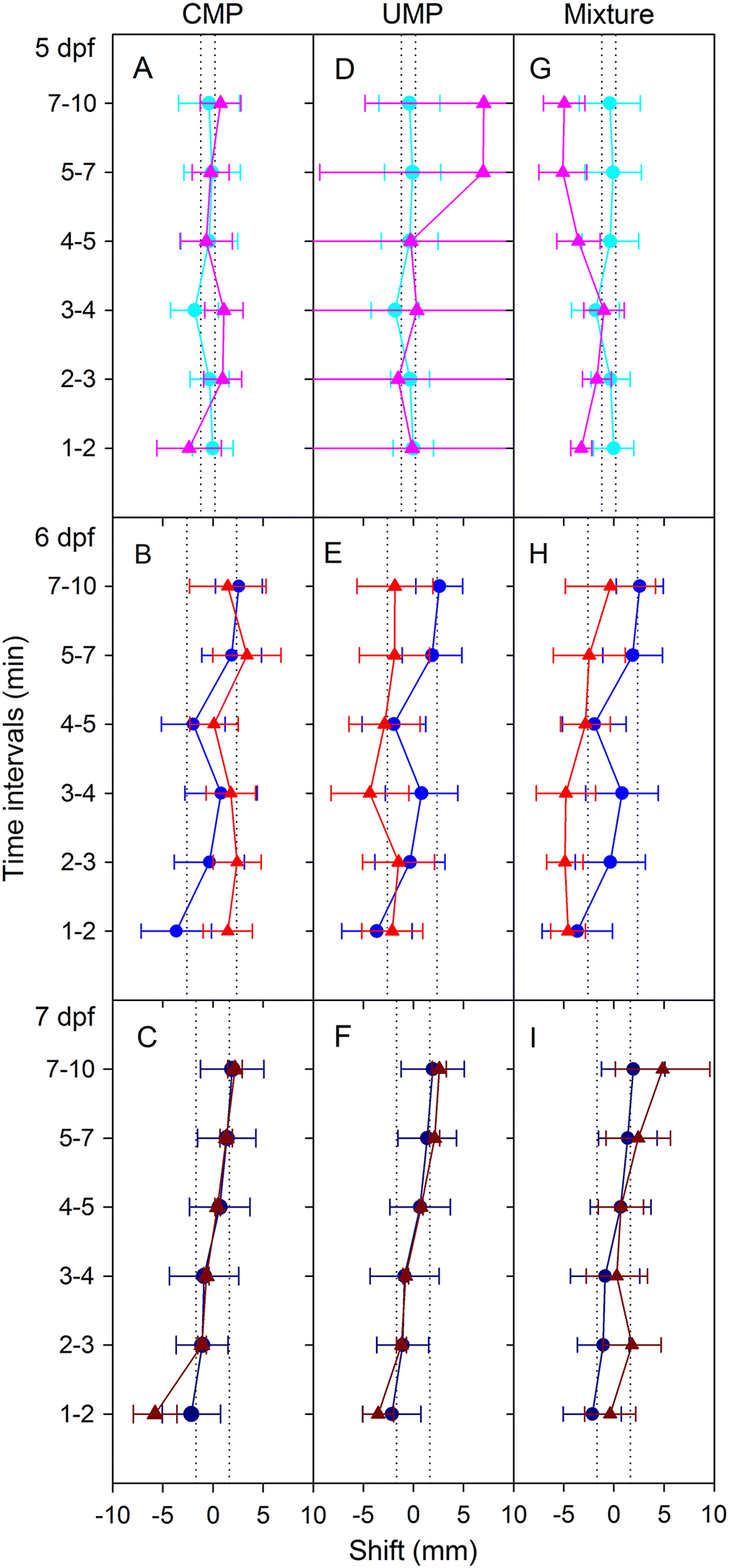
We were surprised that no pyrimidines evoked responses, as cytidine- and uridine-containing nucleotides initiated gorging behaviour in the kissing bug, and CTP stimulated spiny lobster chemosensory cells (
Friend and Smith 1971;
Smith and Griend 1976;
Carr et al. 1996). Based on these studies, we expected that the phosphate groups in nucleotides would drive the behavioural responses. We found the opposite, as it appears that for embryonic zebrafish, nucleobase structure is the determining factor. Interestingly, in abalone and oriental weatherfish (
Misgurnus anguillicaudatus (Cantor, 1842)), neither CMP nor UMP increased feeding responses; however, the pyrimidine thymidine was effective (
Brown et al. 2001). We suggest that pyrimidine responses be tested in adult zebrafish.
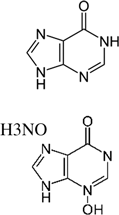
It was also surprising that H3NO, a potent alarm-inducing compound, did not evoke avoidance. However, when zebrafish larvae were placed in a flow-through chamber and forced to experience H3NO, they responded rapidly (≤1 min) with increased swimming activity and speed. Typically in adult fishes, alarm response is apparent in dashing, which may be followed by immobility or “freezing”. However, in adult zebrafish, H3NO has only been observed to evoke increases in activity (
Speedie and Gerlai 2008;
Parra et al. 2009;
Mathuru et al. 2012;
Kalueff et al. 2013;
Gallus et al. 2016). Our study agrees with the adult data and demonstrates that alarm-like behaviour can be evoked by H3NO just days into zebrafish development.
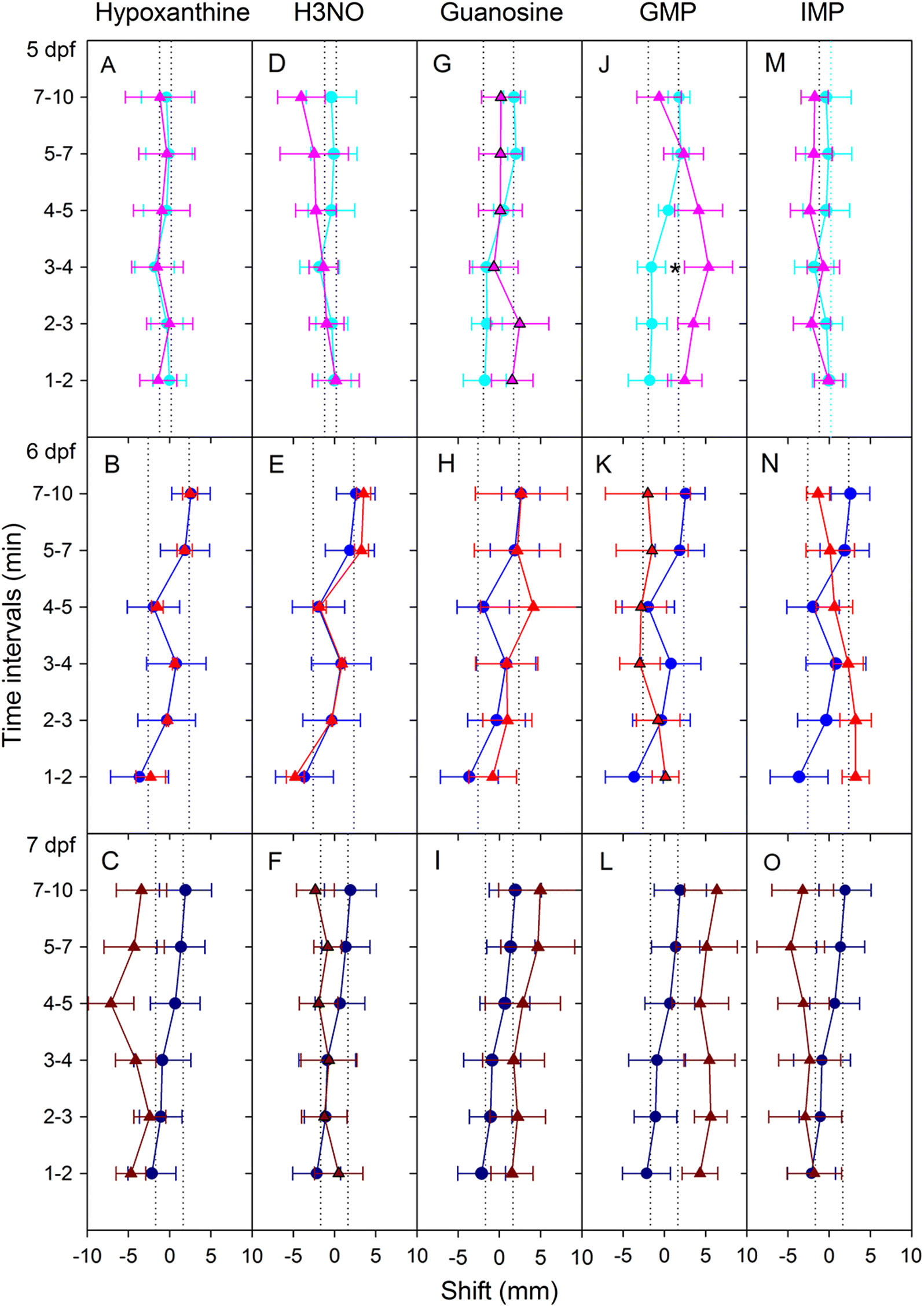
Hypoxanthine did not evoke avoidance, which confirms earlier work in fathead minnows (
Pimephales promelas Rafinesque, 1820) and finescale dace (
Chrosomus neogaeus (Cope, 1867)), in which compounds that lacked a nitrogen-oxide functional group, including hypoxanthine, failed to evoke alarm responses (
Brown et al. 2000). The results of the present study suggest that the nitrogen-oxide group is likely related to an alarm response and the basic purine ring structure is related to an avoidance response.
The nucleotide mixture did not evoke any avoidance or attraction response despite containing behaviourally active AMP and GMP. This may be a result of the ratio and combination of compounds within the mixture, or it may be because the active stimuli were lost in the noise of the inactive. Because nucleotides are most likely to appear in nature as mixtures, additional combinations should be tested that better represent the nucleotide composition of food and conspecific tissue extracts.
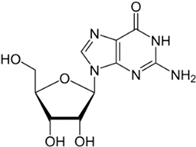
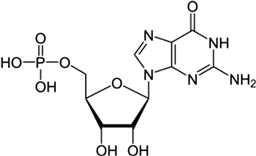
There was just one example of zebrafish attraction response—to GMP—and it was a true oddity because it occurred 3–4 min into the trial (no other responses were seen at this time). In contrast to GMP, guanosine evoked no response, suggesting that the presence of phosphate has the potential to alter odourant efficacy. However, because not all of the tested nucleotides evoked a response (UMP and CMP did not), the presence of phosphate alone does not appear sufficient to evoke a behavioural response. This leaves three possibilities: (1) odourant–receptor interaction is based on three-dimensional structure and is therefore influenced by the complete form of the nucleotide, (2) GMP circumvents the odourant–receptor interaction, or (3) this finding was a statistical aberration.
Olfactory-evoked behaviours vary with age
Odourant responses were observed almost exclusively at 5 and 7 dpf, not 6 dpf. This is interesting because previous studies have shown that zebrafish develop kin odour recognition at 6 dpf (
Gerlach et al. 2008;
Hinz et al. 2013). At 6 dpf, zebrafish larvae exhibit an increase in thyroid hormone receptor β mRNA and whole-body thyroxine (
Liu and Chan 2002;
Chang et al. 2012). In a salmonid species (
Oncorhynchus kisutch Walbaum, 1792) known to imprint to the odours of the natal stream, elevated plasma thyroxine was associated with olfactory epithelium proliferation (
Lema and Nevitt 2004). This behavioural and hormonal evidence has led to the theory that olfactory imprinting occurs at 6 dpf. If imprinting occurs at 6 dpf, it is possible that the dynamic status of the olfactory system at this time affects odourant interpretation. An alternative explanation for temporal changes in responses may be asynchronous expression of olfactory receptors, as studies have shown that the onset of expression of odourant receptors varies (
Barth et al. 1996;
Argo et al. 2003).