Mercury exposure to red-winged blackbirds (Agelaius phoeniceus) and dragonfly (Odonata: Aeshnidae) nymphs in Prairie Pothole wetlands
Abstract
The Prairie Pothole Region (PPR) in the northern Great Plains is an area of ecological significance, serving as an important breeding site for avian wildlife. However, organisms feeding within the PPR may be at risk of mercury (Hg) exposure due to deposition of anthropogenic emissions and the high Hg methylation potential of PPR wetlands. We quantified Hg concentrations in red-winged blackbirds’ (Agelaius phoeniceus (Linnaeus, 1766); RWBLs) blood, feathers, and eggs in the spring and summer breeding season and compared our values with those from RWBLs sampled from ecoregions across North America. Hg concentrations in whole water, aeshnid dragonfly nymphs, and RWBL tissues varied by wetland and were below those considered to elicit acute effects in wildlife, and egg total Hg (THg) concentrations were significantly related to spring whole water methylmercury concentrations. Only RWBL blood THg concentrations showed a clear increase in summer compared with spring, resulting in decoupling of summer blood and feather THg concentrations. Moreover, blood THg concentrations varied by ecoregion, with those impacted by an industrial point source exhibiting high Hg levels. Our study emphasizes that tissue renewal time as well as ecological factors such as competition and diet shifts are important considerations when using RWBLs to assess biological Hg exposure.
Introduction
The Prairie Pothole Region (PPR) in the northern Great Plains is an area of ecological significance, consisting of numerous shallow, productive wetlands (Hayashi et al. 2016) that support habitat for diverse wildlife (Naugle et al. 2000; Steen et al. 2016). However, increased industrial mercury (Hg) emissions resulting from anthropogenic activities may pose a risk to organisms within these wetlands. Although far from major point sources, Hg can deposit in PPR wetlands via long-distance atmospheric transport and deposition (Selin 2009; Fleck et al. 2016; Weiss-Penzias et al. 2016), where deposited Hg(II) (Hg2+) can be methylated to the neurotoxic methylmercury (MeHg) (Paranjape and Hall 2017). MeHg is highly bioavailable and can bioaccumulate and biomagnify in organisms at higher trophic levels (Rimmer et al. 2010; Edmonds et al. 2012). Previous studies have found that although total Hg (THg) deposition is relatively low, wetlands in the PPR contain a high proportion of MeHg in sediments, suggesting that Hg exposure to wildlife may be of concern (Hoggarth et al. 2015; Fleck et al. 2016).
Wetland ponds in the PPR are generally fishless ecosystems. As such, concerns surrounding impacts of high Hg methylation rates and subsequent elevated MeHg concentrations in these systems focus on terrestrial wildlife, especially invertivorous passerines because of their potential to feed at high trophic levels and bioaccumulate MeHg concentrations that may induce adverse behavioural or health effects (Brasso and Cristol 2008; Edmonds et al. 2010). Many passerine species use wetlands in the PPR as migratory stopovers and breeding sites. As migrating birds require high protein intake to sustain the energetic costs of migration and breeding, feeding on organisms originating from the wetlands represents MeHg transfer from aquatic systems to the terrestrial food web and poses a risk to these populations. In systems too small to support permanent fish populations, dragonfly nymphs (Odonata: Anisoptera: Aeshnidae) can be responsible for a large flux of MeHg from aquatic to terrestrial food webs (Haro et al. 2013; Chumchal and Drenner 2015; Chumchal et al. 2017) and therefore may impact the health of invertivorous songbirds (Williams et al. 2017). Given that PPR wetlands and their passerine residents will likely experience further decline with persistent habitat destruction, wetland drainage, and climate change (Steen et al. 2016), it is important to assess Hg exposure to inform strategies to mitigate multiple environmental stressors on these populations.
Red-winged blackbirds (Agelaius phoeniceus (Linnaeus, 1766); RWBLs) are common throughout many North American wetlands (Bishop et al. 1995; Evers et al. 2005), and they nest in the PPR during breeding season from May to August (Robertson 1973; Yasukawa and Searcy 1995). Although typically omnivorous, stomach contents of breeding RWBLs reveal that they feed almost exclusively on invertebrates (>80%) (McNicol et al. 1982) caught within or around wetlands, including damselfly and dragonfly nymphs, spiders, and dipterans (Whittingham and Robertson 1994; Harding 2008; Gillet and Seewagen 2014). Moreover, analysis of different tissues, such as feathers, blood, and eggs yields information on past and recent exposure (Edmonds et al. 2010; Rimmer et al. 2010; Ackerman et al. 2016b). Adult RWBLs and their eggs have previously been surveyed for Hg tissue concentrations in wetlands (Tsipoura et al. 2008; Lane et al. 2012; Tyser et al. 2016). In combination with the potential for MeHg bioavailability, the tendency for RWBLs to shift to higher trophic level feeding during breeding may enhance Hg exposure. Although previous research in the PPR has assessed Hg exposure in invertebrates (Bates and Hall 2012), tadpoles (Boczulak et al. 2017), and, in larger wetlands, avian piscivores (Hall et al. 2009), exposure to invertivorous passerines remains understudied.
Here, we expand our understanding of Hg bioaccumulation in RWBL breeding in wetlands located within the PPR in Saskatchewan, Canada. Our first objective was to assess potential risk of Hg exposure to RWBLs in the PPR by quantifying Hg concentrations in RWBL tissues (i.e., blood, feathers, and eggs). We further evaluated this in context to unfiltered, whole water THg and MeHg concentrations and by comparison with aeshnid dragonfly nymphs. Second, due to potential changes in Hg availability and risk of exposure with altered diet intake, we compare concentrations between spring (prebreeding) and summer (postbreeding) and evaluate whether finer resolution proxies (e.g., blood) changed temporally. Finally, we compare RWBL breeding in the PPR with that in a previous unpublished survey from central and northeastern USA ecoregions to give broader context to RWBL Hg exposure in PPR wetlands.
Methods
Site selection and description
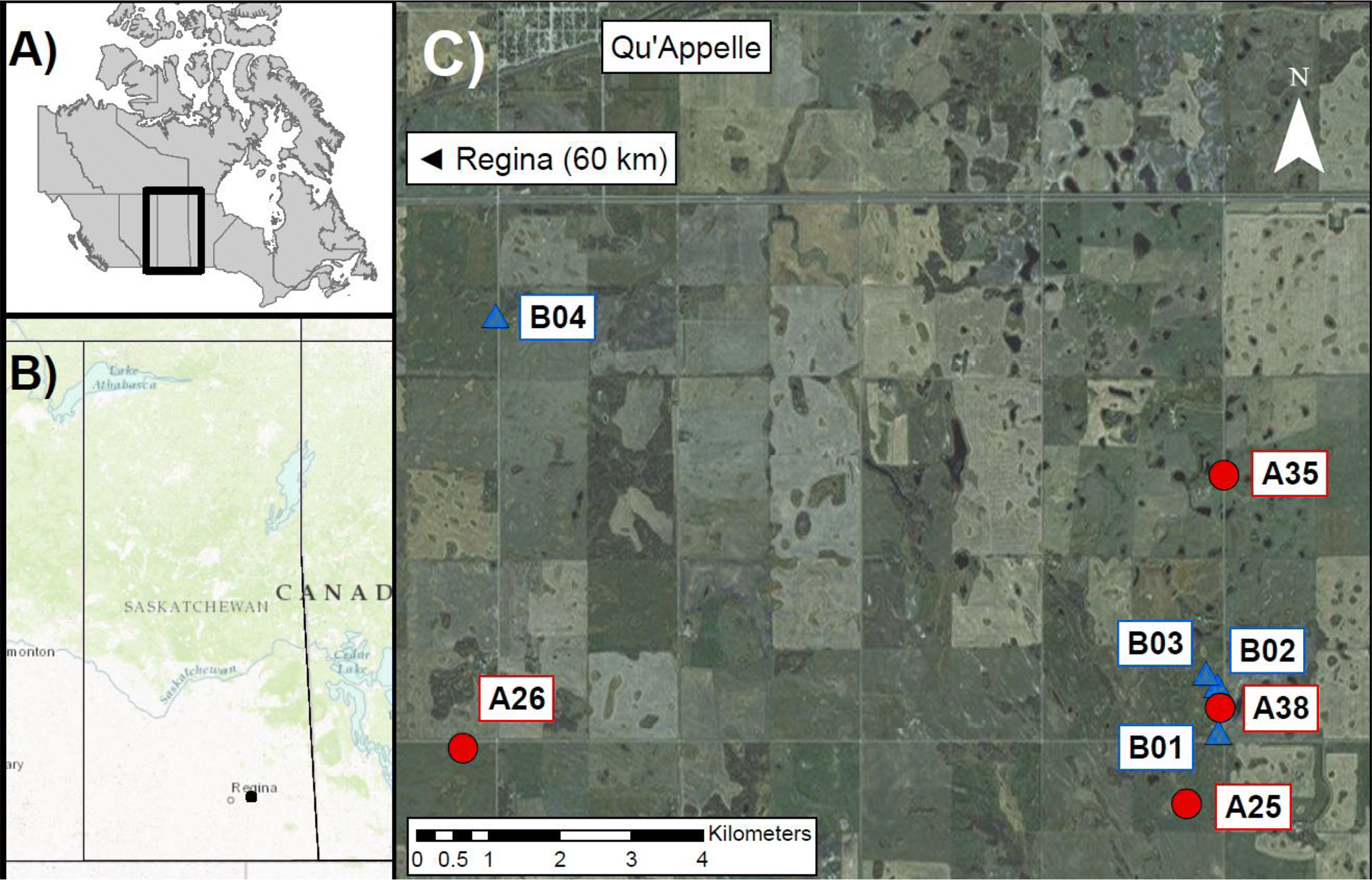
Eight wetlands were surveyed within the Saskatchewan PPR south of Qu’Appelle, Saskatchewan (Fig. 1). Sample collection occurred over 2 weeks at the end of May and in early July 2013, representing spring and summer conditions, respectively. From 1980 to 2010, the region has experienced a mean annual air temperature of 3.0 °C and mean winter and summer temperatures of −12.5 and 17.3 °C, respectively (Environment and Climate Change Canada 2016). Total annual precipitation over this period averaged ∼455 mm. Wetlands were selected based on a preliminary survey of RWBL abundance in southeastern Saskatchewan (M. Mushanski, personal communication, 2013) and further refined by the following criteria: accessibility by foot, presence of emergent vegetation, and minimal disturbance. As RWBLs are known agricultural pests and feed on grains if cropland is readily assessable (McNicol et al. 1982), birds at sites surrounded by pasture were preferentially sampled to select for birds consuming an enriched invertebrate diet. Wetlands ranged between 0.20 and 1.97 ha (one site (A25) was 8.12 ha), and they were classified as seasonal (type III) or semi-permanent (type IV) using the Stewart and Kantrud (1971) wetland classification system (Table S1).
Fig. 1.
Sample collection
Dragonfly nymphs
During the spring and summer surveys, aeshnid dragonfly nymphs were collected using dip nets from four of the eight wetlands (Table S2). Because of the relatively high unfiltered water Hg concentrations observed during spring in site B03 compared with other wetlands, dragonflies from site B03 were also collected in summer. Samples were collected near the shoreline, identified to suborder, rinsed, divided into two to three pools per site, and stored in Ziploc® bags. Nymphs were frozen at −20 °C for storage and freeze-dried prior to mechanical homogenization using an acid-washed mortar and pestle.
RWBL tissue
RWBLs were caught using mist nets between 0500 h and 0830 h at four of the eight wetlands (Table S1). Adult birds could not be caught at all wetlands and thus effort was placed on those with higher populations and easier access. Eggs were collected from additional wetlands to increase sample distribution for this proxy (see below). Birds were also surveyed at site B03 during summer, although total RWBL abundance in wetland A35 decreased in the summer due to invasion by yellow-headed blackbirds (YHBLs) (Xanthocephalus xanthocephalus (Bonaparte, 1826)). Although RWBLs establish breeding territories by late March (Orians and Willson 1964), later-arriving YHBLs are known to usurp resident RWBLs (Orians and Willson 1964; Willson 1966). To minimize detection by birds, nets were placed near vegetation and sheltered from wind. A playback of male and female RWBL mating calls was used to attract individuals to the nets.
Blood samples (∼20 μL) were collected from the cutaneous ulnar vein in Heparinized Mylar®-wrapped 75 mm Hematocrit capillary tubes, with a maximum of 3–4 tubes per bird. Vials were capped with Critocaps Disposable Micro-Hematocrit tube closures, placed in Ziploc® bags, stored on ice, and frozen at −20 °C within 24 h. Blood was dried in an oven at 60 °C prior to analysis and percent moisture calculated. Percent moisture was 77.8% ± 2.2% (mean ± SD). The right and left second secondary flight feathers were pulled from each bird and stored in Ziploc® bags until analysis. Blood THg concentrations represent MeHg exposure within the last few days or weeks (Evers et al. 2005; Edmonds et al. 2010). As MeHg is bound to keratin and sequestered in feathers during active growth, feather THg represents MeHg exposure during feather formation (Jaspers et al. 2004). RWBL molt generally occurs at the end of summer in August and thus THg concentrations indicate body burden at the end of the previous breeding season at the latest. Prior to release, each bird was marked to prevent resampling.
Egg collection occurred during mid-May, which was approximately 3–5 d postlaying. One egg per nest from each wetland was collected to a maximum of 12 eggs per wetland. Although official incubation stage data were not collected during initial surveying, using observational data we approximated the egg ages at approximately 3–5 d. Laboratory observations noted that embryo formation was not supported, which was consistent across the eggs collected. Eggs were randomly selected from each nest and placed in Ziploc® bags, and stored in a cooler for transportation. Samples were frozen at −20 °C within 24 h until analysis. Prior to analysis, eggs were freeze-dried and homogenized. Percent moisture was 83.6% ± 1.0%. Collection of animal tissues was performed using procedures approved by the Canadian Council on Animal Care under appropriate permits obtained from the University of Regina (Animal Utilization Protocol 11-04; Canadian Council on Animal Care (CCAC-CCPA) 2010).
Whole water
Water chemistry data were collected during spring and summer surveys. Parameters such as temperature (°C), conductivity (mS), pH, and dissolved oxygen (mg·L−1) were measured using an YSI 556 MPS multiprobe. We sampled surface whole water for the analysis of THg and MeHg concentrations. Unfiltered water was collected in precleaned glass bottles using clean sampling techniques (US EPA 1630, 2001; US EPA 1631, 2002). Samples were preserved by acidification (THg samples acidified to 10% HCl and MeHg to 20% HCl) and stored at 4 °C until analysis. Sulfate (SO4) concentrations were also determined in surface water using standard methods (Stainton et al. 1977).
Hg analysis
Tissue Hg
We used THg as a proxy for nymph and RWBL tissue MeHg concentrations. Previous studies on dragonfly nymph show that 75%–88% of bodily THg is MeHg (Tremblay et al. 1996; Bates and Hall 2012; Edmonds et al. 2012). Likewise, the majority of THg (>90%) in avian blood, feathers, and eggs is in the form of MeHg (Evers et al. 2005; Rimmer et al. 2005; Wada et al. 2010; Ackerman et al. 2013), so the use of THg to approximate MeHg concentrations is justified.
A Milestone Direct Mercury Analyzer (DMA)-80 equipped with a gold amalgamator quantified THg concentrations in nymph and RWBL tissues using the US EPA Method 7473 (2007) at the University of Saskatchewan. A preweighed sample amount was added to a nickel boat and combusted. The DMA-80 was calibrated with SPEX Certiprep (PLHG2-1AY) liquid Hg standard using a gradient of Hg amounts from 0.5 to 1000 ng. THg emissions were measured by absorbance at 254 nm. Certified reference materials (CRM) (DORM-4, TORT-3, and IAEA-85) for secondary check standards were included during each batch. Sample duplicates were analyzed after 10 samples to ensure replication between 10% error. Recovery of CRM was 98% ± 2% (mean ± SD) for each set analyzed, and sample duplicates were within 10% error. The lower detection limit of the DMA-80 was approximately 0.04 ng Hg. Concentrations were expressed as dry weight (dw) for nymph, fresh weight (fw) for feathers, and wet weight (ww) for blood and eggs.
Water Hg
Water THg concentrations were analyzed using a Tekran® Series 2600 Cold Vapour Atomic Fluorescence Spectrophotometer (CVAFS) at the University of Regina Mercury Lab, using protocols outlined by the US EPA Method 1631 (2002). Quality assurance and quality control (QA/QC) procedures were performed regularly, including Hg standards, duplicates, and spike recoveries. Duplicates were within 10% of each other, and the recovery range for spiked samples was between 88% and 115%. For MeHg determination, water was distilled using Tekran® Series 2750 Mercury Distillation System at the University of Regina Mercury Lab using protocols in the US EPA Method 1630 (2001). Ammonium 1-pyrrolidine carbodithionate was added to each vial to dissociate Hg bound to organic matter and then distilled over 2 h. Samples were shipped overnight for analysis at the University of Western in Ontario by CVAFS. Machine stability measures were similar for MeHg CVAFS as those described for THg determination, with spike recoveries within 94%–101%.
Statistical analysis
Seasonal differences in THg concentrations were determined using one-tailed Student’s t test (α = 0.05). Data were log-transformed prior to analysis, and Shapiro–Wilk’s test was used to assess normality of residuals. Generalized linear models (GLM) were used to assess the relationship of Hg proxies, and the probability distribution of the data was assessed prior to analysis. As such, a GLM fitted using a gamma distribution with an inverse link-function assessed the relationship between surface water MeHg concentrations and the arithmetic mean of dragonfly nymph and RWBL blood, feather, and egg THg concentrations (package glm2 in R Studio version 3.3.3; Marschner 2011). A GLM (gamma distribution; inverse link) was used to evaluate seasonality in the relationship between blood and feather THg concentrations. Model fit was assessed using pseudo-R2 values, Akaike information criterion, and residual normality with comparison with null and reduced models.
Results
Tissue Hg concentrations
Dragonfly nymphs
Dragonfly THg concentrations ranged between 6.06 and 445.12 ng·g−1, with lowest and highest levels in nymphs collected from sites A25 and A26, respectively. Mean nymph THg concentrations in spring and summer were 123.36 ± 117.26 and 136.24 ± 132.87 ng·g−1, respectively. These concentrations were not significantly different between seasons (t = 0.22, df = 20.89, and p = 0.83) (Fig. 2A).
Fig. 2.

Blood
THg concentrations in RWBL blood ranged between 17.06 and 247.05 ng·g−1 THg (Fig. 2B) and were significantly lower in spring (70.70 ± 57.11 ng·g−1) than in summer (173.54 ± 44.18 ng·g−1) (t = −5.18, df = 16.03, and p < 0.05). There was no significant difference between sexes in blood THg concentrations (Fig. S1). A few individuals from wetland A35 exhibited higher concentrations (>0.17 ng·g−1) compared with other sites, which contributed to greater variation in spring THg concentrations; however, no birds were caught at this site during summer. Only one bird was caught at B03 in summer, and it had similar THg concentration as birds from A25 and A26.
Feathers
Feather THg concentrations ranged from 99.26 to 1432.60 ng·g−1 (Fig. 2C). Mean feather THg concentrations were 755.89 ± 402.07 ng·g−1 in spring and 611.15 ± 467.37 ng·g−1 in summer. There were no significant differences in feather THg concentrations between seasons (t = 1.06, df = 13.20, and p = 0.31).
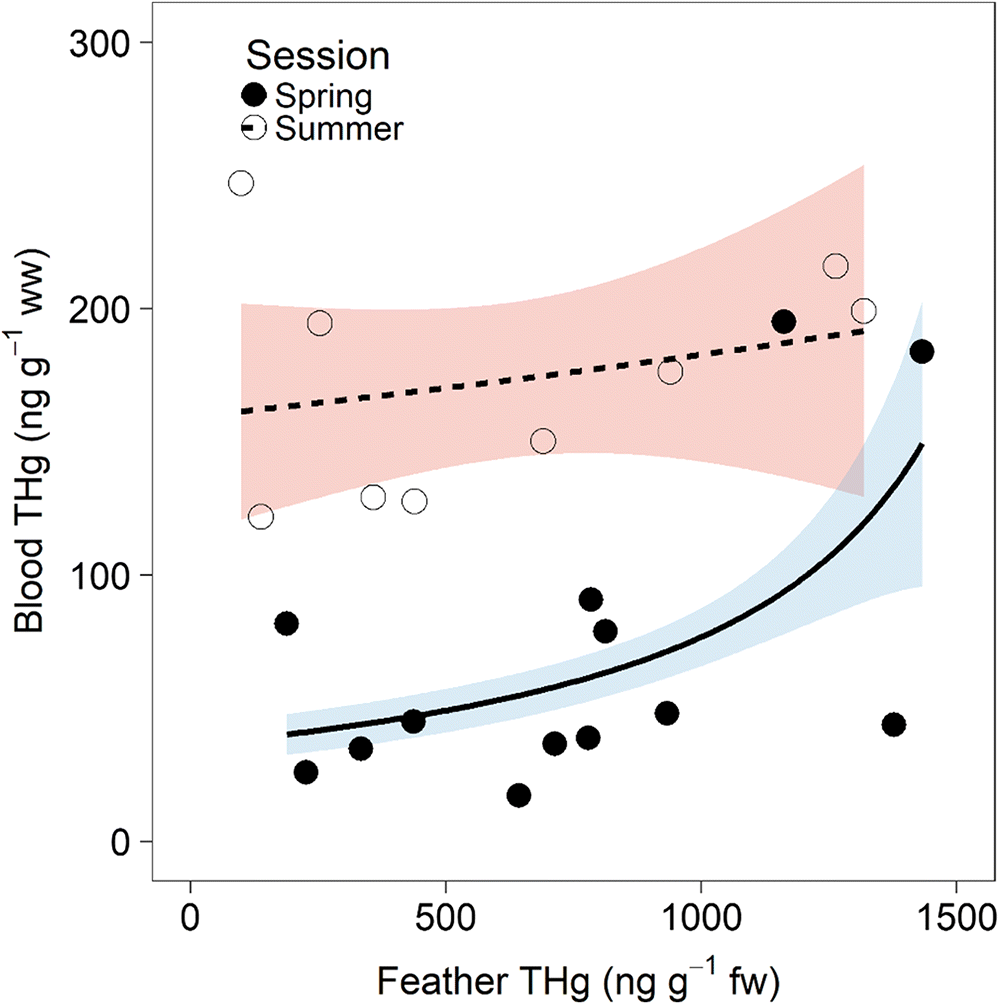
The relationship between RWBL blood and feather THg concentrations was related to season. GLM analysis indicated that season, feather THg concentration, and the interaction between these variables were predictive of blood THg concentration (pseudo-R2 = 0.59) (Table 1). Specifically, feather THg concentrations were positively related to blood THg concentration in spring (Fig. 3); however, this relationship decoupled later in summer when no relationship between tissues was observed.
Table 1.
| Term | Estimate | SE | t-value | p-value |
|---|---|---|---|---|
| (Intercept) | 2.77 × 102 | 5.60 × 10−3 | 4.95 | <0.01 |
| Season | −2.14 × 102 | 5.86 × 10−3 | −3.66 | <0.01 |
| Feather | −1.47 × 105 | 4.82 × 10−6 | −3.04 | <0.01 |
| Season × feather | 1.39 × 105 | 5.28 × 10−6 | 2.63 | 0.02 |
Fig. 3.
Eggs
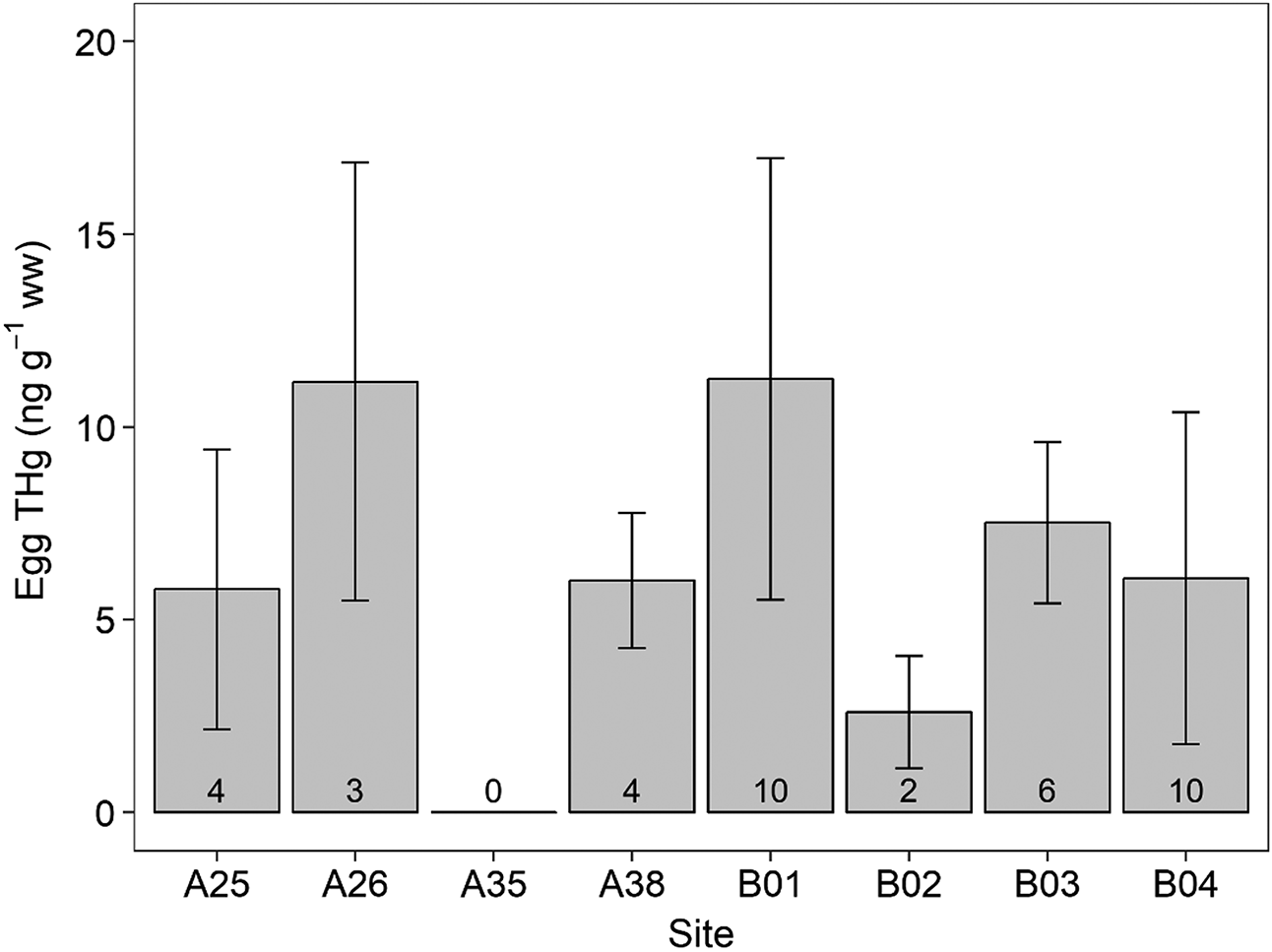
Mean egg concentration was 7.60 ± 4.87 ng·g−1 and ranged from 0.78 to 24.65 ng·g−1. Eggs from sites A26 and B01 had the highest mean concentrations at 11.17 and 11.24 ng·g−1, respectively, whereas those from B02 had the lowest at 2.60 ng·g−1 (Fig. 4). Only one egg was collected from A35, which had a THg concentration below detection limit. There was a significant difference in egg THg concentrations among sites (F(6,32) = 2.49; p = 0.05).
Fig. 4.
THg concentrations were highest in feathers followed by blood and then eggs. The ratio of THg concentrations in feather:blood:egg was 7:1:0.1.
Wetland water THg and MeHg concentrations
Apart from one wetland (B03), both THg and MeHg concentrations in unfiltered water did not demonstrate a consistent, directional change with season (THg: t = 0.39, df = 13.93, p = 0.70; MeHg concentrations: t = 0.38, df = 11.25, p = 0.71; Table 2). Overall, unfiltered water THg concentration (excluding B03) ranged from 2.2 to 6.7 ng·L−1 with means of 5.81 ± 4.32 ng·L−1 in spring and 5.00 ± 4.03 ng·L−1 in summer, whereas MeHg concentrations ranged between 0.3 and 1.9 ng·L−1 with means of 2.22 ± 2.90 ng·L−1 and 1.77 ± 1.69 ng·L−1 in spring and summer, respectively. On average, %MeHg was one-third of THg in both spring and summer (32.0% ± 16.9% and 32.5% ± 8.6%, respectively), with no consistent change between seasons. Wetland B03 had substantially higher concentrations in spring (15.9 ng·L−1 THg and 9.3 ng·L−1 MeHg) and summer (14.7 ng·L−1 THg and 5.7 ng·L−1 MeHg), but %MeHg was comparable with other sites (58.2% in spring and 39.1% in summer).
Table 2.
| Site | Season | Temperature (°C) | pH | Conductivity (μS·cm−1) | DO (mg·L−1) | SO42− (mg·L−1) | THg (ng·L−1) | MeHg (ng·L−1) | MeHg% |
|---|---|---|---|---|---|---|---|---|---|
| A25 | Spring | 17.9 | 7.05 | 0.43 | 4.8 | 75.2 | 3.26 | 0.85 | 26.05 |
| Summer | 18.2 | 6.30 | 0.55 | 7.8 | 29.4 | 3.96 | 1.48 | 37.33 | |
| A26 | Spring | 15.9 | 7.18 | 0.42 | 5.3 | 40.0 | 6.67 | 1.83 | 27.46 |
| Summer | 19.8 | 6.86 | 0.42 | 4.7 | 18.5 | 3.04 | 1.08 | 35.59 | |
| A35 | Spring | 13.4 | 7.24 | 1.04 | 4.8 | 223.8 | 2.35 | 0.30 | 12.75 |
| Summer | 20.7 | 7.16 | 1.17 | 3.6 | 255.5 | 4.30 | 1.45 | 33.72 | |
| A38 | Spring | 13.0 | 7.18 | 0.44 | 5.9 | 15.2 | 5.00 | 1.47 | 29.44 |
| Summer | 16.9 | 6.43 | 0.64 | 7.8 | 5.4 | 2.21 | 0.39 | 17.59 | |
| B01 | Spring | 14.3 | 7.87 | 0.73 | 5.9 | 130.5 | 3.18 | 1.84 | 57.82 |
| Summer | 17.9 | 6.54 | 1.09 | 6.8 | 68.6 | 5.22 | 1.87 | 35.79 | |
| B02 | Spring | 14.4 | 7.14 | 0.61 | 5.2 | 142.8 | 4.86 | 1.07 | 21.90 |
| Summer | 20.3 | 6.81 | 0.68 | 4.0 | 73.4 | 4.22 | 1.71 | 40.60 | |
| B03 | Spring | 10.8 | 6.79 | 0.82 | 5.8 | 190 | 15.94 | 9.29 | 58.25 |
| Summer | 17.6 | 6.76 | 1.03 | 3.6 | 96.1 | 14.65 | 5.73 | 39.12 | |
| B04 | Spring | 16.3 | 7.08 | 0.36 | 6.7 | 27.8 | 5.25 | 1.16 | 22.03 |
| Summer | 19.6 | 6.72 | 0.38 | 5.4 | 10.6 | 2.37 | 0.49 | 20.48 |
Water and tissue Hg concentrations
When tissues were compared with wetland water MeHg concentration, only egg THg concentrations were significantly related. When data for B03 were removed, our model indicated a significant, positive relationship with spring water MeHg and RWBL egg THg concentrations (pseudo-R2 = 0.68) (Table 3). However, the overarching model was not significant (pseudo-R2 = 0.04) as B03 contained high (>4 times) MeHg water concentrations. In contrast, egg THg concentration was not related to summer water MeHg concentration or %MeHg. Feather, blood, and nymph THg concentrations were not related to either Hg metric (Table 3).
Table 3.
| Term | Estimate | SE | t-value | Pseudo-R2 | p-value |
|---|---|---|---|---|---|
| Nymph | −7.93 × 10−4 | 1.27 × 10−4 | −0.63 | 0.04 | 0.55 |
| Blood | 2.29 × 10−3 | 3.05 × 10−3 | 0.75 | 0.09 | 0.50 |
| Feather | 1.37 × 10−3 | 1.28 × 10−3 | 1.07 | 0.24 | 0.35 |
| Egg | −2.28 × 10−2 | 6.87 × 10−2 | −0.33 | 0.04 | 0.75 |
| Egg (no B03) | −5.40 × 10−2 | 1.78 × 10−2 | −3.08 | 0.68 | 0.04 |
Discussion
Objective 1: To assess the potential risk of Hg exposure to RWBLs and aeshnid dragonflies
Overall, surveyed RWBLs and dragonfly nymphs in the PPR of North America were indicative of background to low-risk Hg exposure. All metrics (whole water, nymph, and RWBL tissues) were below those considered to elicit acute effects in wildlife (Ackerman et al. 2016b), although absolute Hg concentrations did vary by wetland.
Dragonflies
The majority of dragonfly nymphs in this survey had THg concentrations below 150 ng·g−1. These concentrations are lower than aeshnid nymphs collected farther north in the PPR (∼200 ng·g−1 dw; Bates and Hall 2012) and in boreal Canada (∼190 ng·g−1 dw; Hall et al. 1998). Values are also lower than those from odonate nymphs in the northeastern Great Lakes (230–1200 ng·g−1 dw; Sinclair et al. 2012; Haro et al. 2013), but comparable with nymphs collected from ephemeral and semi-permanent ponds in Texas (120 ng·g−1 dw MeHg; Chumchal et al. 2017). Moreover, mean THg concentrations in nymphs from our sites were <500 ng·g−1 dw, suggesting that organisms feeding on dragonfly nymphs are not at great risk of accumulating high Hg concentrations. However, studies show that odonates contribute a large proportion of MeHg flux from ponds in the Great Plains (Chumchal and Drenner 2015). This flux can be greater in fishless ponds, such as in our study (compared with fish consuming invertebrates; Chumchal et al. 2017), which may result in some risk to more vulnerable organisms such as passerine nestlings (Williams et al. 2017).
RWBLs
Surveyed RWBLs from the PPR were indicative of background to low-risk Hg exposure. The maximum blood concentration observed was 247 ng·g−1, substantially below the benchmark value of high-risk exposure for passerines (>3000 ng·g−1; Ackerman et al. 2016b). Likewise, these concentrations were generally below values known to cause adverse ecological effects such as a 10% reduction in nesting success with blood concentrations >∼700 ng·g−1 (Jackson et al. 2011; Lane et al. 2012). Egg THg concentrations were also substantially below the lethal concentration for passerines (tree swallows (Tachycineta bicolor (Vieillot, 1808)): LC50 = 250 ng·g−1 ww; Heinz et al. 2009) as well as levels associated with a 10% reduction in nest success (110 ng·g−1; Jackson et al. 2011). However, two birds had blood concentrations above 200 ng·g−1, which may indicate moderate physiological and behavioural alterations such as oxidative stress (Custer et al. 2000) and impaired reproductive success (Ackerman et al. 2016b). There are few studies examining feather, blood, and egg THg concentrations in RWBLs that we found to occur as a ratio of 7:1:0.1. Similar THg concentrations of feather:blood:egg ratios have been reported for the common loon (Gavia immer (Brunnich, 1764)) (6:1:0.3; Evers et al. 2005) and tree swallow (9:1:0.4; Brasso and Cristol 2008). Mean blood (113 ng·g−1) and feather (697 ng·g−1) THg concentrations were within the range found in marshes of northeastern USA (blood: 23–279 ng·g−1; feather: 93–826 ng·g−1; Tsipoura et al. 2008; Lane et al. 2012). Likewise, Bicknell’s thrush (Catharus bicknelli (Ridgway, 1882)) within boreal forests has similar blood (90 ng·g−1 ww) and feather (700 ng·g−1 fw) concentrations (Rimmer et al. 2005). Assuming 80% moisture, egg concentrations in the PPR were similar to mean concentrations observed in RWBL eggs from Voyageurs National Park, Minnesota, USA (∼13.2 ng·g−1 ww; Tyser et al. 2016), but they were much lower than those from urban wetlands in New Jersey, USA (48 ng·g−1 ww; Tsipoura et al. 2011).
Trophic level position affects biomagnification of Hg in passerines. Hg concentrations in RWBLs were much lower than those for rusty blackbirds (Euphagus carolinus (Statius Muller, 1776)) in the Acadian Forest (blood: 940 ng·g−1, feather: 8260 ng·g−1), which are almost exclusively invertivorous (Edmonds et al. 2010). Despite feeding mainly on insects during the breeding season (McNicol et al. 1982), omnivory by RWBLs may reduce their overall biomagnification potential. Indeed, in a synthesis of passerine Hg concentrations across North America, RWBL blood concentrations fall within similar values as those of other omnivores (Jackson et al. 2015). Overall, based on three tissues, ecological impediments to the surveyed RWBLs within the PPR as a result of Hg exposure were likely minimal.
Whole water MeHg concentrations and relationships to concentrations in bird tissues
Unfiltered water MeHg concentrations within the wetlands were similar to those found in other prairie systems, which have been found to be elevated compared with other systems (0.02 to over 9 ng·L−1; Sando et al. 2007; Hall et al. 2009; Bates and Hall 2012; Hoggarth et al. 2015). Of particular concern, wetland B03 had significantly higher spring THg (15.9 ng·L−1) and MeHg (9.3 ng·L−1) concentrations compared with other wetlands (∼4.2 and 1.3 ng·L−1, respectively). We did not find a relationship between summer unfiltered water MeHg and blood concentrations, even in B03 where high whole water MeHg concentrations were not reflected by similarly elevated concentrations in nymph or RWBL tissue compared with other wetlands (Table S2). The lack of relationship could be due to spatial and temporal variation in factors such as temperature, sediment redox potential, organic content, and sulfate concentration, which influence Hg methylation and hence accessibility to biota in shallow, PPR wetlands (Lavoie et al. 2013; Hoggarth et al. 2015; Paranjape and Hall 2017). The lack of relationships could also be because we examined relationships with MeHg concentrations in whole water and not those in the dissolved phase. Likely the lack of relationship of unfiltered water concentrations to other tissues reflects complexity in Hg biogeochemistry, spatiotemporality, and ecology (e.g., diet shifts and competition) that influences tissue THg concentrations (Edmonds et al. 2012; Paranjape and Hall 2017). As such, further research is needed to determine how these conditions affect the extent of Hg bioaccumulation through food webs.
Our results show that egg THg concentration was significantly related to spring whole water MeHg concentrations, whereas RWBL blood and feathers were not (Table 2). These results support previous indications that data from eggs may be useful indices for Hg risk assessment (Tsipoura et al. 2008; Peck et al. 2016; Tyser et al. 2016). In their review, Ackerman et al. (2016b) placed priority on using blood and egg THg concentrations as proxies for recent Hg exposure. This is partially because collecting eggs is relatively easy and because maternal THg blood concentrations are correlated with those in eggs (Ackerman et al. 2016a). As a result, eggs provide insight into Hg risk of passerines during the breeding season, a sensitive life history event. Clutch initiation date influences egg THg concentrations due to current Hg body burden and diet as well as subsequent depuration into eggs (Tyser et al. 2016). As spring whole water MeHg was correlated with egg THg and overall concentrations were relatively low, we argue that early clutches are not at risk of elevated Hg exposure resulting from greater biomagnification potential in breeding RWBLs. However, further research is needed to investigate the impacts of prolonged exposure to Hg-contaminated food sources of RWBLs on later clutches in the PPR, which may increase with clutch initiation date (e.g., Tyser et al. 2016).
Objective 2: A comparison of THg concentrations between spring and summer
Only RWBL blood THg concentrations showed an increase in summer compared with spring, whereas nymph and feather concentrations did not change with season (Fig. 2). Because blood generally reflects exposure over the previous 1–3 weeks and feather THg concentrations are indicative of past exposure specifically during molting (which for RWBLs is generally during August after breeding) (Evers et al. 2005), there was decoupling of blood and feather THg concentrations in summer (Fig. 3), emphasizing the importance of tissue renewal in assessing Hg exposure.
Seasonal differences in blood THg likely represented a trophic shift with breeding, as there is a seasonal increase to higher biomagnification potential resulting from shifting to higher trophic level feeding as birds feed on a greater proportion of arthropods to cope with higher energy demands during breeding (McNicol et al. 1982; Lane et al. 2012). This shift to greater proportional intake of insects during breeding resulting in higher blood THg concentrations and reduced association between blood and feather concentrations has been observed elsewhere in other songbirds (e.g., the rusty blackbird), a relative to RWBL (Edmonds et al. 2010), and the Bicknell’s thrush (Rimmer et al. 2005) whose diet varies temporally.
Personal observation indicated that RWBLs from all sites were noticeably less active in the summer compared with spring, presumably because intraspecific competition for breeding territory diminished. As most nestlings hatched before the summer survey, female flight activity may also have decreased. These observations highlight the importance of ecological interactions, such as interspecific and intraspecific competition, that can complicate the application of potential sentinel species. Our data underline that understanding sentinel ecology is imperative to accurately assess Hg exposure, and priority should be placed on tissues with fast turnover (blood) or more timely occurrence (eggs) when assessing Hg risk (Ackerman et al. 2016b; Jackson et al. 2016).
Objective 3: How do THg concentrations in RWBLs in the PPR compare with those from central and northeastern USA ecoregions and give broader context to RWBL Hg exposure in PPR wetlands?
Staff at the Biodiversity Research Institute, Portland, Maine, USA collected and analyzed blood samples for THg from over 500 RWBLs using similar methods as in the present study. Birds were sampled in the central and eastern United States, and locations were grouped according to US Environmental Protection Agency (EPA) Level II classification of ecoregions (Commission for Environmental Cooperation 1997). This resulted in categorizing locations into one of seven ecoregions: Southeast US Plains, Ozark, Ouachita–Appalachian Forest, Mixed Wood Plains, Mississippi Alluvial and Southeast USA Coastal Plains (Level I: Eastern Temperate Forest), Atlantic Highlands (Northern Forests), Western Cordillera (Northeastern Forested Mountains), and Cold Desert (North American Desert). THg was measured using EPA-certified methods with appropriate QA/QC procedures (a list of laboratories where samples were analyzed is found in Table S3). Data were further divided into sites with and without known industrial point sources. The majority of the sites were sampled during the summer breeding season, with the exception of the southeast plains, which were also sampled during winter. Differences in log-transformed blood THg concentrations by ecoregion and impact status were assessed using a mixed-effects GLM, with site and year included as random effects.
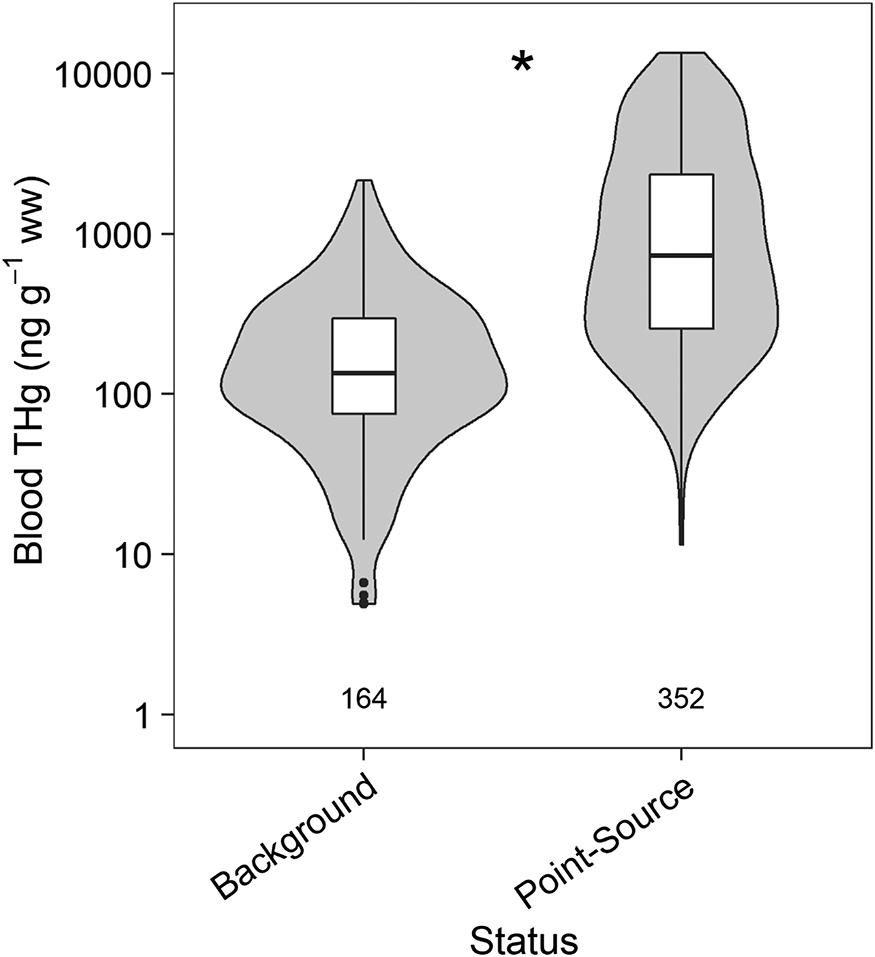
Comparison of THg concentrations in birds sampled from the PPR suggests that Hg exposure differs by ecoregion (F8,30 = 3.19 and p < 0.01) (Fig. 5; Table S4) and that birds from areas impacted by point sources exhibit greater Hg bioaccumulation than those in areas receiving background exposure (F1,461 = 37.19, p < 0.01) (Fig. 6; Table S5). Surprisingly, RWBLs captured in forested ecoregions (e.g., Atlantic Highlands and Appalachian Forest) had similar blood THg concentrations to PPR birds. However, PPR birds exhibited comparable concentrations to birds captured during breeding in the southeastern Plains. The fact that RWBLs captured from wintering sites in the Southeast Plains exhibited the lowest blood THg concentrations supports the influence of diet on bioaccumulation, with spring PPR concentrations falling intermediate of respective winter and summer surveys (Fig. 5). Thus, exposure at the breeding site is the combination of MeHg availability as well as the re-accumulation of tissue Hg concentration resulting from consumption of relatively more contaminated food sources.
Fig. 5.
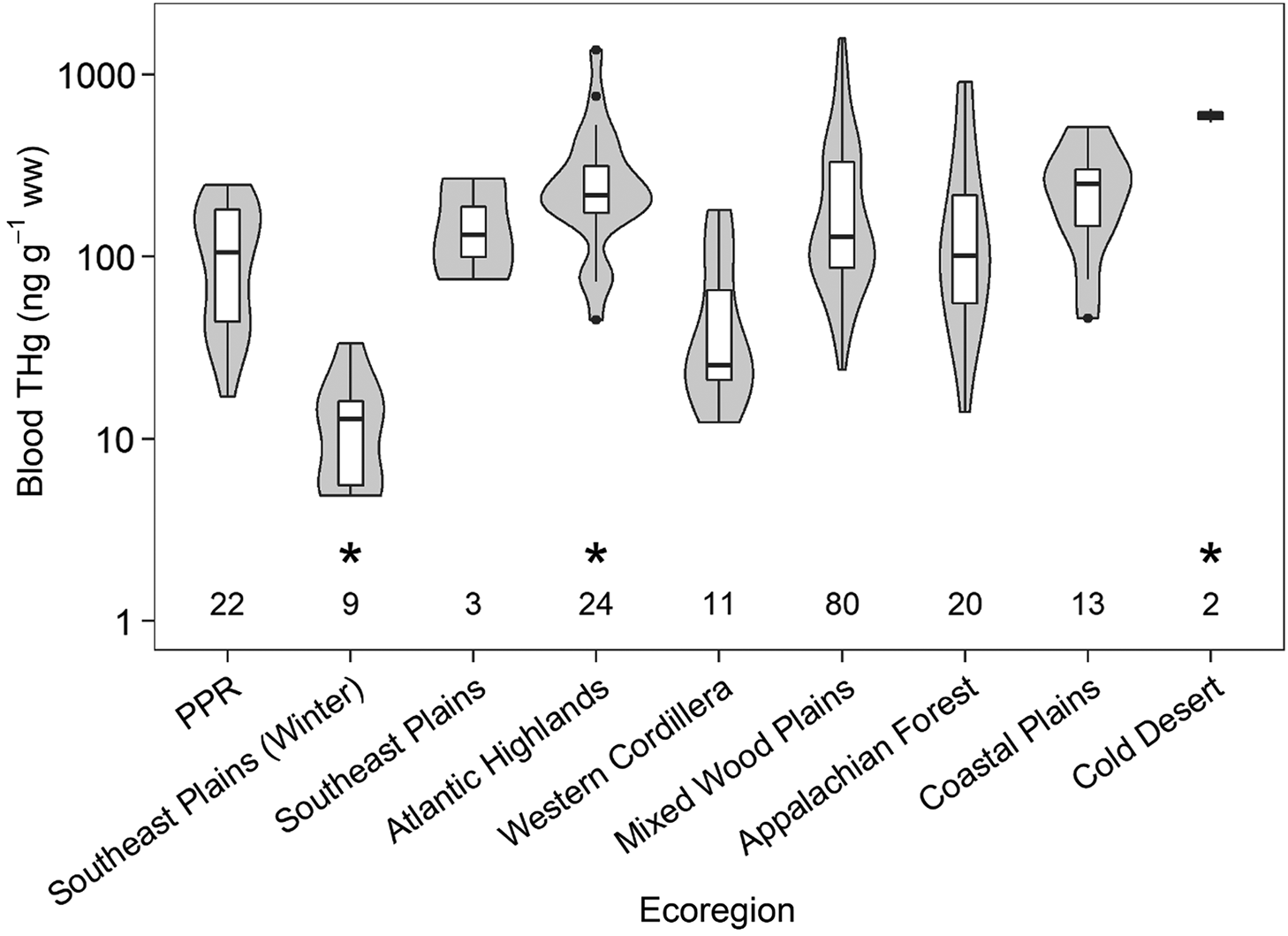
Fig. 6.
Conclusions
Despite being a potential source of bioavailable MeHg (Hoggarth et al. 2015; Fleck et al. 2016), little research within the PPR has focused the movement of MeHg from aquatic to terrestrial systems and the resulting exposure to insectivorous passerines. Our study expands the understanding of Hg exposure to wildlife in the PPR by comparing seasonal THg concentrations in dragonfly nymphs to those in the RWBL. Moreover, when combined with a broader spatial survey, RWBL Hg exposure varies by ecoregion and is higher in sites impacted by disturbance. These results suggest that blood THg concentrations from breeding RWBLs and their eggs may be useful invertivorous biosentinels for assessing Hg contamination; however, to ensure accuracy, assessment must be sensitive to not only environmental concentrations but also to ecological aspects that influence Hg exposure and capture success (e.g., diet changes and competition). As such, RWBL eggs may be a preferred option for evaluating PPR passerine Hg exposure (Ackerman et al. 2013). Although despite increased biomagnification potential of RWBLs during the breeding season, both dragonfly nymph and RWBL tissues indicated low exposure to Hg in the PPR, with minimal risk of these organisms to adverse impacts of Hg.
Acknowledgements
This work was supported by a National Science and Engineering Research Council Undergraduate Student Research Award to JDW. We thank M. Mushanski for providing information on study sites and A. Wolfe, E. Helmond, and C. Hoggarth for assisting in field and laboratory work. Thank you to Dr. T. Jardine’s lab at the University of Saskatchewan for DMA-80 analysis. Finally, we thank the landowners who provided access to their land.
References
Ackerman JT, Herzog MP, and Schwarzbach SE. 2013. Methylmercury is the predominant form of mercury in bird eggs: a synthesis. Environmental Science & Technology, 47: 2052–2060.
Ackerman JT, Eagles-Smith CA, Herzog MP, and Hartman CA. 2016a. Maternal transfer of contaminants in birds: mercury and selenium concentrations in parents and their eggs. Environmental Pollution, 210: 145–154.
Ackerman JT, Eagles-Smith CA, Herzog MP, Hartman CA, Peterson SH, Evers DC, et al. 2016b. Avian mercury exposure and toxicological risk across western North America: a synthesis. Science of the Total Environment, 568: 749–769.
Bates LM, and Hall BD. 2012. Concentrations of methylmercury in invertebrates from wetlands of the Prairie Pothole Region of North America. Environmental Pollution, 160: 153–160.
Bishop CA, Koster MD, Chek AA, Hussell DJT, and Jock K. 1995. Chlorinated hydrocarbons and mercury in sediments, red-winged blackbirds (Agelaius phoeniceus) and tree swallows (Tachycineta bicolor) from wetlands in the Great Lakes–St. Lawrence River Basin. Environmental Toxicology and Chemistry, 14: 491–501.
Boczulak SA, Vanderwel MC, and Hall BD. 2017. Survey of mercury in boreal chorus frog (Pseudacris maculata) and wood frog (Rana sylvatica) tadpoles from wetland ponds in the Prairie Pothole Region of Canada. FACETS, 2: 315–329.
Brasso RL, and Cristol DA. 2008. Effects of mercury exposure on the reproductive success of tree swallows (Tachycineta bicolor). Ecotoxicology, 17: 133–141.
Canadian Council on Animal Care (CCAC-CCPA). 2010. Guide to the care and use of experimental animals [online]: Available from ccac.ca/en_/standards/guidelines.
Chumchal MM, and Drenner RW. 2015. An environmental problem hidden in plain sight? Small human-made ponds, emergent insects, and mercury contamination of biota in the Great Plains. Environmental Toxicology and Chemistry, 34: 1197–1205.
Chumchal MM, Drenner RW, Greenhill FM, Kennedy JH, Courville AE, Gober CAA, et al. 2017. Recovery of aquatic insect-mediated methylmercury flux from ponds following drying disturbance. Environmental Toxicology and Chemistry, 36: 1986–1990.
Commission for Environmental Cooperation. 1997. Ecological regions of North America: toward a common perspective (scale 1:12,500,000). Commission for Environmental Cooperation, Montreal, Quebec.
Custer TW, Custer CM, Hines RK, Sparks DW, Melancon MJ, Hoffman DJ, et al. 2000. Mixed-function oxygenases, oxidative stress, and chromosomal damage measured in lesser scaup wintering on the Indiana Harbor Canal. Archives of Environmental Contamination and Toxicology, 38: 522–529.
Edmonds ST, Evers DC, Cristol DA, Mettke-Hofmann C, Powell LL, McGann AJ, et al. 2010. Geographic and seasonal variation in mercury exposure of the declining rusty blackbird. The Condor, 112: 789–799.
Edmonds ST, O’Driscoll NJ, Hillier NK, Atwood JL, and Evers DC. 2012. Factors regulating the bioavailability of methylmercury to breeding rusty blackbirds in northeastern wetlands. Environmental Pollution, 171: 148–154.
Environment and Climate Change Canada. 2016. Canadian Climate Normals 1981–2010 [online]: Available from climate.weather.gc.ca/climate_normals/index_e.html.
Evers DC, Burgess NM, Champoux L, Hoskins B, Major A, Goodale WM, et al. 2005. Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicology, 14: 193–221.
Fleck JA, Marvin-Dipasquale M, Eagles-Smith CA, Ackerman JT, Lutz MA, Tate M, et al. 2016. Mercury and methylmercury in aquatic sediment across western North America. Science of the Total Environment, 568: 727–738.
Gillet A-MTY, and Seewagen CL. 2014. Mercury exposure of a wetland songbird, Agelaius phoeniceus, in the New York metropolitan area and its effect on nestling growth rate. Environmental Monitoring and Assessment, 186: 4029–4036.
Hall BD, Rosenberg DM, and Wiens AP. 1998. Methyl mercury in aquatic insects from an experimental reservoir. Canadian Journal of Fisheries and Aquatic Sciences, 55: 2036–2047.
Hall BD, Baron LA, and Somers CM. 2009. Mercury concentrations in surface water and harvested waterfowl from the prairie pothole region of Saskatchewan. Environmental Science & Technology, 43: 8759–8766.
Harding LE. 2008. Non-linear uptake and hormesis effects of selenium in red-winged blackbirds (Agelaius phoeniceus). Science of the Total Environment, 389: 350–366.
Haro RJ, Bailey SW, Northwick RM, Rolfhus KR, Sandheinrich MB, and Wiener JG. 2013. Burrowing dragonfly larvae as biosentinels of methylmercury in freshwater food webs. Environmental Science & Technology, 47: 8148–8156.
Hayashi M, van der Kamp G, and Rosenberry DO. 2016. Hydrology of prairie wetlands: understanding the integrated surface-water and groundwater processes. Wetlands, 36(Suppl. 2): 237–254.
Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR, Kondrad SL, and Erwin CA. 2009. Species differences in the sensitivity of avian embryos to methylmercury. Archives of Environmental Contamination and Toxicology, 56: 129–138.
Hoggarth CGJ, Hall BD, and Mitchell CPJ. 2015. Mercury methylation in high and low-sulphate impacted wetland ponds within the prairie pothole region of North America. Environmental Pollution, 205: 269–277.
Jackson AK, Evers DC, Folsom SB, Condon AM, Diener J, Goodrick LF, et al. 2011. Mercury exposure in terrestrial birds far downstream of an historical point source. Environmental Pollution, 159: 3302–3308.
Jackson AK, Evers DC, Adams EM, Cristol DA, Eagles-Smith C, Edmonds ST, et al. 2015. Songbirds as sentinels of mercury in terrestrial habitats of eastern North America. Ecotoxicology, 24: 453–467.
Jackson AK, Evers DC, Eagles-Smith CA, Ackerman JT, Willacker JJ, Elliott JE, et al. 2016. Mercury risk to avian piscivores across western United States and Canada. Science of the Total Environment, 568: 685–696.
Jaspers V, Dauwe T, Pinxten R, Bervoets L, Blust R, and Eens M. 2004. The importance of exogenous contamination on heavy metal levels in bird feathers. A field experiment with free-living great tits, Parus major. Journal of Environmental Monitoring, 6: 356–360.
Lane OP, Edmonds S, Atwood JL, Regan K, Buck D, and Evers DC. 2012. Assessment of mercury in birds at Onondaga Lake: 2008–2009 breeding season final report. Biodiversity Research Institute, Gorham, Maine. 64 p.
Lavoie RA, Jardine TD, Chumchal MM, Kidd KA, and Campbell LM. 2013. Biomagnification of mercury in aquatic food webs: a worldwide meta-analysis. Environmental Science & Technology, 47: 13385–13394.
Marschner IC. 2011. glm2: fitting generalized linear models with convergence problems. The R Journal, 3: 12–15.
McNicol DK, Robertson RJ, and Weatherhead PJ. 1982. Seasonal, habitat, and sex-specific food habits of red-winged blackbirds: implications for agriculture. Canadian Journal of Zoology, 60: 3282–3289.
Naugle DE, Johnson RR, Estey ME, and Higgins KF. 2000. A landscape approach to conserving wetland bird habitat in the Prairie Pothole Region of eastern South Dakota. Wetlands, 20: 588–604.
Orians GH, and Willson MF. 1964. Interspecific territories of birds. Ecology, 45: 736–745.
Paranjape AR, and Hall BD. 2017. Recent advances in the study of mercury methylation in aquatic systems. FACETS, 2: 85–119.
Peck LE, Gilchrist HG, Mallory CD, Braune BM, and Mallory ML. 2016. Persistent organic pollutant and mercury concentrations in eggs of ground-nesting marine birds in the Canadian high Arctic. Science of the Total Environment, 556: 80–88.
Rimmer CC, McFarland KP, Evers DC, Miller EK, Aubry Y, Busby D, et al. 2005. Mercury concentrations in Bicknell’s thrush and other insectivorous passerines in montane forests of northeastern North America. Ecotoxicology, 14: 223–240.
Rimmer CC, Miller EK, McFarland KP, Taylor RJ, and Faccio SD. 2010. Mercury bioaccumulation and trophic transfer in the terrestrial food web of a montane forest. Ecotoxicology, 19: 697–709.
Robertson RJ. 1973. Optimal niche space of the redwinged blackbird: spatial and temporal patterns of nesting activity and success. Ecology, 54: 1085–1093.
Sando SK, Krabbenhoft DP, Johnson KM, Lundgrean RF, and Emerson DG. 2007. Mercury and methylmercury in water and bottom sediments of wetlands at Lostwood National Wildlife Refuge, North Dakota, 2003–2004. USGS Scientific Investigations Report 2007-5219. US Geological Survey, Reston, Virginia. 66 p.
Selin NE. 2009. Global biogeochemical cycling of mercury: a review. Annual Review of Environment and Resources, 34: 43–63.
Sinclair KA, Xie Q, and Mitchell CPJ. 2012. Methylmercury in water, sediment, and invertebrates in created wetlands of Rouge Park, Toronto, Canada. Environmental Pollution, 171: 207–215.
Stainton MP, Capel MJ, and Armstrong FAJ. 1977. The chemical analysis of fresh water. Fisheries and Marine Services Miscellaneous Special Publications No. 25. 2nd edition. Freshwater Institute, Winnipeg, Manitoba. 166 p.
Steen VA, Skagen SK, and Melcher CP. 2016. Implications of climate change for wetland-dependent birds in the Prairie Pothole Region. Wetlands, 36: 445–459.
Stewart RE, and Kantrud HA. 1971. Classification of natural ponds and lakes in the glaciated prairie region. Resource Publication 92. Bureau of Sport Fisheries and Wildlife, US Fish and Wildlife Service, Washington, D.C. 57 p.
Tremblay A, Lucotte M, and Rheault I. 1996. Methylmercury in a benthic food web of two hydroelectric reservoirs and a natural lake of Northern Québec (Canada). Water, Air, and Soil Pollution, 91: 255–269.
Tsipoura N, Burger J, Feltes R, Yacabucci J, Mizrahi D, Jeitner C, et al. 2008. Metal concentrations in three species of passerine birds breeding in the Hackensack Meadowlands of New Jersey. Environmental Research, 107: 218–228.
Tsipoura N, Burger J, Newhouse M, Jeitner C, Gochfeld M, and Mizrahi D. 2011. Lead, mercury, cadmium, chromium, and arsenic levels in eggs, feathers, and tissues of Canada geese of the New Jersey Meadowlands. Environmental Research, 111: 775–784.
Tyser RW, Rolfhus KR, Wiener JG, Windels SK, Custer TW, and Dummer PM. 2016. Mercury concentrations in eggs of red-winged blackbirds and tree swallows breeding in Voyageurs National Park, Minnesota. Archives of Environmental Contamination and Toxicology, 71: 16–25.
United States Environmental Protection Agency (US EPA). 2001. Method 1630: methyl mercury in water by distillation, aqueous ethylation, purge and trap, and cold vapor atomic fluorescence spectrometry. US EPA, Washington, D.C.
United States Environmental Protection Agency (US EPA). 2002. Method 1631: mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry. Revision E. EPA 821-R-95-027. US EPA, Washington, D.C.
United States Environmental Protection Agency (US EPA). 2007. Method 7473 (SW-846): mercury in solids and solution by thermal decomposition, amalgamation, and atomic absorption spectrometry. US EPA, Washington, D.C.
Wada H, Yates DE, Evers DC, Taylor RJ, and Hopkins WA. 2010. Tissue mercury concentrations and adrenocortical responses of female big brown bats (Eptesicus fuscus) near a contaminated river. Ecotoxicology, 19: 1277–1284.
Weiss-Penzias PS, Gay DA, Brigham ME, Parsons MT, Gustin MS, and ter Schure A. 2016. Trends in mercury wet deposition and mercury air concentrations across the U.S. and Canada. Science of the Total Environment, 568: 546–556.
Whittingham LA, and Robertson RJ. 1994. Food availability, parental care and male mating success in red-winged blackbirds (Agelaius phoeniceus). Journal of Animal Ecology, 63: 139–150.
Williams EB, Chumchal MM, Drenner RW, and Kennedy JH. 2017. Seasonality of odonate-mediated methylmercury flux from permanent and semipermanent ponds and potential risk to red-winged blackbirds (Agelaius phoeniceus). Environmental Toxicology and Chemistry, 36: 2833–2837.
Willson MF. 1966. Breeding ecology of the yellow-headed blackbird. Ecological Monographs, 36: 51–77.
Yasukawa K, and Searcy WA. 1995. Red-winged blackbird (Agelaius phoeniceus), version 2.0. In The birds of North America. Edited by PG Rodewald. Cornell Lab of Ornithology, Ithaca, New York.
Supplementary material
Supplementary Material 1 (DOCX / 208 KB)
- Download
- 207.71 KB
Information & Authors
Information
Published In

FACETS
Volume 3 • Number 1 • October 2018
Pages: 174 - 191
Editor: Nelson O’Driscoll
History
Received: 28 June 2017
Accepted: 6 November 2017
Version of record online: 22 February 2018
Copyright
© 2018 Wolfe et al. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Data Availability Statement
All relevant data are within the paper and in the Supplementary Material.
Key Words
Sections
Subjects
Authors
Author Contributions
JDW, RMB, and BDH conceived and designed the study.
JDW and OPL performed the experiments/collected the data.
JDW and BDH analyzed and interpreted the data.
All contributed resources.
All drafted or revised the manuscript.
Competing Interests
The authors have declared that no competing interests exist.
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Jared D. Wolfe, Oksana P. Lane, R. Mark Brigham, and Britt D. Hall. 2018. Mercury exposure to red-winged blackbirds (Agelaius phoeniceus) and dragonfly (Odonata: Aeshnidae) nymphs in Prairie Pothole wetlands. FACETS.
3(1): 174-191. https://doi.org/10.1139/facets-2017-0086
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
Cited by
1. Do songbirds in wetlands show higher mercury bioaccumulation relative to conspecifics in non-wetland habitats?
2. Long-term monitoring of mercury in adult saltmarsh sparrows breeding in Maine, Massachusetts and New York, USA 2000–2017
3. Mercury exposure to swallows breeding in Canada inferred from feathers grown on breeding and non-breeding grounds
