1. Introduction
Habitat sensitivity is a central consideration for decision-making under various environmental statutes and policies in Canada and abroad. For example, under the
Fish and Fish Habitat Protection Policy Statement (2019), which outlines Fisheries and Oceans Canada’s approach to implementing the Fish and Fish Habitat Protection provisions of the
Fisheries Act (2019), the department intends to “adopt a risk-based approach to the application of the fish and fish habitat protection provisions subject to the sensitivity of the fish and fish habitat in question” and establish ecologically significant areas to “manage fish and fish habitat that is highly sensitive, highly productive, rare, or unique” (
DFO 2019). Similarly, habitat sensitivity is a consideration for selecting sites for renewable energy projects under the
Wildlife Directive for Alberta Wind Energy Projects (
Alberta Environment and Parks 2017), and is a condition under which Indigenous governments can restrict public access to land in some modern treaties in British Columbia (
British Columbia 1999,
2013). Internationally, habitat sensitivity is considered when categorizing and protecting habitat under New Zealand’s
Exclusive Economic Zone and Continental Shelf Regulations (2013) and California’s
Coastal Act (2022), among other statutes.
Despite widespread reference to “habitat sensitivity” in policy and legislation, there is no standardized scientific method for its assessment and quantification. This absence is in part due to the fact that there is also no commonly accepted definition of habitat sensitivity, nor it is clear how habitat sensitivity relates to other concepts in ecosystem management such as impact, vulnerability, and risk. The lack of scientific consistency in the definition and quantification of habitat sensitivity could impede effective ecosystem management by complicating the development and application of new environmental policy and regulations, thwarting meaningful comparative analyses, and placing additional burden on project proponents and environmental practitioners to (re)define habitat sensitivity on a case-by-case basis (
Cullen 1990;
Stirzaker et al. 2010). It also undermines consistency in governance when key ecological states identified in policy or legislation have no established scientific definition.
As a step towards addressing this issue, we pursue two objectives. First, we briefly review the definitions of “habitat sensitivity” offered in the scientific literature and environmental policy, and identify common features of these definitions. Second, we suggest a unified definition of habitat sensitivity, and propose a framework for assessing it, including recommendations that support a consistent approach to assessing and comparing habitat sensitivity across habitats and jurisdictions. We apply this framework to a case study drawn from previously published data and include general discussion related to the challenges of assessing habitat sensitivity across diverse ecosystems and pressures.
2. Past definitions of habitat sensitivity
That natural systems vary in their response to changing environmental conditions is a fundamental concept in natural science. Based on a history of studies on ecological niches (
Strom 1946), food webs (
Huxel and McCann 1998), and comparative physiology (
Clark and Worland 2008), there are solid theoretical foundations to predict that different species and communities will show different responses to natural and anthropogenic pressures. Similarly, abiotic habitat attributes such as sediment regimes (
Abbott et al. 2018;
Rathburn et al. 2018), geomorphology (
Tooth 2018), and water temperature (
Luce et al. 2014) are dynamic and respond differently to changing environmental conditions associated with pressures (
Kail et al. 2015).
Starting in the 1990s, references to “habitat sensitivity” in the scientific literature started to bring together ideas related to how biotic and abiotic attributes of ecosystems respond to natural and anthropogenic pressures (e.g.,
Webb et al. 1994;
Michel et al. 1995;
Hiscock et al. 1999). In some jurisdictions, substantial progress has been made towards developing a systematic approach to quantifying the sensitivity of certain habitat types (
Tyler-Walters et al. 2018;
OSPAR Commission 2019), but whether these approaches are applicable to other ecosystems and jurisdictions is unclear. Establishing consistent assessments of habitat sensitivity requires agreement on the definition of habitat sensitivity. However, various authors have characterized habitat sensitivity using widely differing definitions (
Table 1).
These initial definitions can be simplified into concepts of: (1) the disturbance and recovery of habitat from a pressure; (2) the ability of that habitat to support a unique assemblage or number of species; or (3) some perceived value of the habitat related to rarity, uniqueness, or importance. To further complicate the issue, habitat sensitivity has frequently been described as a multi-component concept that included two or more of the above elements. Additionally, habitat sensitivity is sometimes differentiated from habitat vulnerability, with the latter relating to the likelihood that a pressure will occur and the former to the habitat response to the pressure (
Zacharias and Gregr 2005). Yet, other authors have included the likelihood of pressure occurrence as an integral component of sensitivity, or have considered sensitivity as a component of vulnerability (
Hiscock et al.1999;
Berry et al. 2003;
Tyler-Walters et al. 2018).
While some of the above definitions could allow for habitat sensitivity to be objectively quantified from empirical data, many are not sufficiently specific to promote consistent approaches across contexts. Additionally, some of the previous definitions contain subjective components, such as “habitat value” or “essential characteristics”, which cannot be objectively measured. As a result, much of the history of evaluating habitat sensitivity has been based on qualitative assessments by experts, often using “risk-matrix” like approaches to derive single estimates of sensitivity from multiple criteria (see e.g.,
La Rivière et al. 2016). However, risk matrices can have questionable validity and many limitations, including a poor ability to correctly discriminate among different quantitative values (
Cox 2008;
Duijm 2015); these issues are likely to also apply to qualitative assessments of habitat sensitivity.
Perhaps the most common definition of habitat sensitivity is that initially offered by
Laffoley et al. (2000), who stated that “A sensitive habitat or species is one that is easily affected by human activity, and is expected to only recover over a long period of time.” This definition includes two components describing (i) the resistance of habitats to change, and (ii) the resilience (i.e., recovery rate) of habitats after pressures cease. With slight modifications, this definition of habitat sensitivity has been adopted by many others (
Eno et al. 2013;
La Rivière et al. 2016;
Tyler-Walters et al. 2018;
Hodgson et al. 2022) and forms the basis for some of the more established habitat sensitivity assessment frameworks, including through the UK’s Marine Life Information Network (
Tyler-Walters et al. 2018), and OSPAR’s multilateral program to identify sensitive species and habitats in the North-East Atlantic (
OSPAR Commission 2019).
Implicit in Laffoley et al.’s definition of habitat sensitivity is the idea that there is a common understanding of the term “habitat”. However, many authors have pointed out that the definition of habitat itself is vague (
Bax and Williams 2001;
Mitchell 2005;
Kearney 2006), and some have gone so far as to suggest that our present concept of habitat is of “very limited use in basic or applied ecology” (
Mitchell 2005). When discussing habitat sensitivity, most authors adopt a broad definition of habitat inclusive of the hydrographic, physical, and chemical characteristics of an area, as well as all the species occurring in an area (
Tyler-Walters et al. 2018). In this sense, habitat sensitivity is synonymous with ecosystem sensitivity, and diverges from the narrower definition of habitat as the “arena in which chemical and biological phenomena and community dynamics act and interact” (
Minns and Wichert 2005). The challenge then is to define key attributes of habitats that are characteristic of the ecosystem and allow for quantification.
3. Towards a consistent and quantitative definition of habitat sensitivity
Building on the definitions of habitat sensitivity reviewed above, we aimed to develop a more precise definition of habitat sensitivity that would encourage a consistent approach to its quantification across ecosystems. Under our framework, habitat sensitivity is a trait of habitats (i.e., ecosystems) that modulates their response to external factors. When combined with the characteristics of a pressure (i.e., the pressure type, intensity, and duration), habitat sensitivity determines the impact to the habitat from the pressure, with higher sensitivity habitats being those that experience a greater impact from a given pressure. Importantly, this definition of habitat sensitivity is distinct from concepts of rareness/uniqueness of habitats, the value of habitats (e.g., for cultural or socioeconomic purposes), and the likelihood that a habitat is exposed to a pressure of a given type and magnitude (i.e., vulnerability). While these are also important considerations in ecosystem management, they should be considered separately from habitat sensitivity to promote clarity in terminology and methodology.
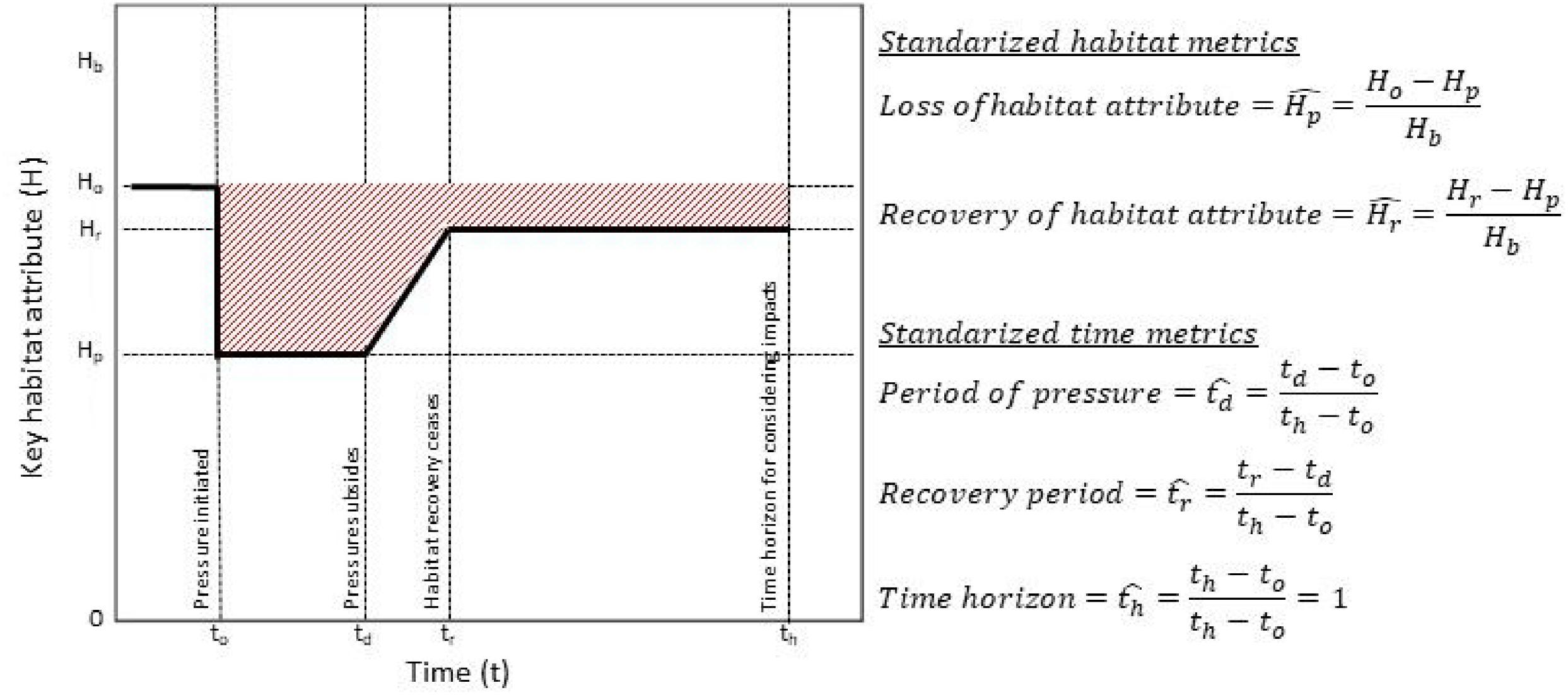
Quantification of habitat sensitivity requires consideration of the way in which pressures cause impacts to habitats. In a general sense, the impact of a pressure on a habitat will be a function of the magnitude and persistence of change in habitat state. The change in habitat state can occur (i) when the pressure is acting on the habitat, (ii) during the period after the pressure subsides and when habitat recovery is occurring, and (iii) continuing through a post-recovery period if the habitat attribute does not achieve full recovery to the pre-pressure state (i.e., if the habitat demonstrates hysteresis;
Litzow and Hunsicker 2016). Based on this framework, we define habitat sensitivity as being composed of three components, (i) habitat resistance, which describes the magnitude of change in habitat state to a given pressure, (ii) habitat resilience, which describes the rate at which habitat state recovers from change, and (iii) habitat recoverability, which describes the degree to which habitat state recovers from change.
Quantification of habitat sensitivity then requires a means to measure resistance, resilience, and recoverability in real-world ecosystems, which are composed of a variety of species (each with a number of different life stages), abiotic traits, and a number of environmental and ecological processes acting at different timelines. Under our suggested framework, habitat sensitivity can be quantified by evaluating the resistance, resilience, and recoverability of a set of key habitat attributes (
Fig. 1), which are metrics that characterize the state of important structural, functional, or characteristic species (
Tyler-Walters et al. 2018), geological, chemical, or physical features, or measures of biological function (e.g., productivity).
We propose defining habitat resistance as the ability of a habitat to maintain its state in the face of a pressure. It is measured as the ratio of the intensity of the pressure to the magnitude of change in a key habitat attribute:
where
R is the habitat resistance,
is the standardized intensity of the pressure (see
Box 1), and
is the change in a key habitat attribute as a result of the pressure, standardized to the baseline (reference) value for the attribute. Habitat resistance therefore represents the intensity of pressure required to produce a given level of change in a habitat attribute, and is high when habitats are able to maintain key attributes in the face of pressures. Furthermore, this definition of habitat resistance is the inverse of the slope of pressure–response functions, a common paradigm for describing the effect of stressors on ecological variables (
Rosenfeld et al. 2022).
Habitat resilience is the rate at which a habitat recovers following the cessation of a pressure:
where
L is the resilience of the habitat attribute,
is the standardized magnitude of recovery in a key habitat attribute after the pressure has subsided, and
is the time period over which recovery occurs, standardized relative to the time horizon (
th) over which impacts are considered. Note that
th is not a function of habitats or pressures, but a practical or policy consideration required for quantification.
Habitat recoverability is the amount of habitat recovery following a pressure relative to the amount of change as a result of the pressure:
where
C is the recoverability of a key habitat attribute, and
and
are as defined above.
Using these definitions and the framework outlined in
Fig. 1, we propose that the impact of a given pressure on a key habitat attribute be defined as
where
is the duration of the pressure, standardized to the time horizon. Because both the time variables and habitat variables are standardized, impact ranges between 0 and 1, and reflects the proportion of the maximum possible impact for a given habitat attribute and time horizon.
Substituting in the relationships defined by
eqs. 1–
3, the impact to a habitat attribute can also be expressed in terms of the pressure intensity, pressure duration, and the habitat sensitivity (i.e., resistance, resilience, and recoverability):
For further information on the derivation of
eqs. 4 and
5, see the Electronic Supplementary Material.
Equation 5 relates the impact of pressures directly to our three components of habitat sensitivity (namely, habitat resistance, habitat resilience, and habitat recoverability), and demonstrates that for a given level of pressure intensity and pressure duration, impact is higher when habitat resistance and habitat resilience are low. Similarly, impact is high when habitat recoverability is low, because
increases faster than
decreases, with increasing
C (since
C is bound between 0 and 1). Because we define habitat sensitivity as a trait that determines the level of impact for a given pressure, habitat sensitivity can therefore be said to be high when habitat resistance, habitat resilience, and habitat recoverability are low, which is consistent with a common use of the terms.
The simple geometric framework for estimating the impact of a pressure can be extended to consider impacts that do not occur instantaneously or impacts with delayed effects that continue to increase beyond the release of the pressure (see the supplementary material). While adding these complexities can be valid extensions of this framework, they do not change the main conclusions drawn from the simplified version presented.
4. Practical considerations for assessing habitat sensitivity
Ecosystems are complex, containing many component species, physical and chemical properties, and biological processes. The framework outlined above describes a conceptual basis for understanding how changes in the state of each key habitat attribute can be described in terms of resistance, resilience, and recoverability. However it is infeasible to consider the sensitivity of every attribute in a focal habitat, which is why we recommend that assessments of habitat sensitivity focus on key attributes that characterize important features of the habitat in question (e.g., keystone or regionally important species, variables of management focus). Selection of habitat attributes for assessment should ideally be driven by the purpose of the habitat assessment and be consistent among habitats to support comparisons. For example, under Canada’s Fisheries Act (2019) and the Fish and Fish Habitat Protection Program Policy Statement (2019), impacts on fish habitat are considered with respect to the habitat’s capacity to “support the life processes of fish”. Key habitat attributes appropriate for characterizing sensitivity under this Act and Policy Statement could therefore focus on the compositional and functional attributes of habitat that have a strong conceptual link to fish vital rates and life history, especially those linking to the vital rates and life history of species of regional importance.
In addition, assessing multiple habitat attributes would provide a broader understanding of the overall response of habitats, and would better align with an ecosystem approach to management, relative to assessing single habitat attributes. Depending on the purpose of the habitat assessment, the sensitivity of multiple habitat attributes may need to be combined into a single value to facilitate comparisons among habitats (e.g., during the consideration of candidate areas for protection). In such a case, a possible approach is to define the overall habitat sensitivity as equivalent to the sensitivity of the most sensitive key habitat attribute, with the argument that harm to a single key habitat attribute threatens ecological integrity. Alternatively, overall habitat sensitivity could be defined as the (geometric or arithmetic) mean value of the key habitat attributes under consideration. Additional methods of combining the sensitivity of multiple key habitat attributes into a single synthetic measure could pull from theory on multiple stressors (e.g.,
Liess et al. 2016;
Schäfer and Piggott 2018) and ecological interactions, with the basis that impacts on multiple nodes within a network can cause emergent effects that are not easily predicted (
Terborgh et al. 2001). However, the development of such approaches is beyond the scope of this paper.
We recognize that the framework outlined above does not address all practical and theoretical challenges associated with the assessment of habitat sensitivity. For example, when habitats respond nonlinearly to pressures (i.e., pressure–response curves that are nonlinear), the impact of a pressure of a given intensity will depend on the initial state of the habitat (
Hunsicker et al. 2016), which further complicates comparisons among and within habitats. Similarly, exposure to other co- or previously occurring stressors can impact the magnitude of response to a focal pressure (
Piggott et al. 2015;
Jackson et al. 2016;
Dey and Koops 2021), and it is not clear how these (potentially non additive) pressure interactions should be accounted for in habitat sensitivity assessment. Future research focused on how resistance, resilience, and recoverability can be quantified for systems experiencing multiple, interacting pressures would be valuable.
Additionally, our framework assumes (i) that the response of habitats to pressures is immediate, (ii) that recovery of habitat attributes occurs linearly, and (iii) that habitat attributes demonstrate some response to pressures (i.e., that the responses are nonzero). While these assumptions are not likely to hold across all systems, we include them in our framework to simplify the definition (and quantification) of impact, habitat resistance, resilience, and recoverability. Extending this framework to accommodate alternate shapes in the response and recovery of habitat attributes to pressures is theoretically possible, but is unlikely to change the general conclusions or applicability of the framework. As an example of such an approach, we demonstrate how relaxing our assumption of an immediate response in habitat attributes to pressures fails to produce meaningful differences in the quantification of impact in the Electronic Supplementary Materials.
5. Case study of the quantification of habitat sensitivity
To demonstrate the application of our framework, we review two case studies focusing on the impact of (i) a flood (
Milner et al. 2018) and (ii) a pesticide spill (
Reiber et al. 2021) on aquatic habitat in Wolf Point Creek, Alaska and the Holtemme River, Germany, respectively. The first step to assessing habitat sensitivity based on our framework is to define key habitat attributes that characterize the ecosystem. Based on their important role in aquatic food webs, and on data availability for these examples, the abundance and richness of macroinvertebrates will serve as key habitat attributes. In addition, we include the density of juvenile coho salmon as a key habitat attribute for the Alaskan system, as salmon represent a keystone aquatic species in many Alaskan rivers.
Next, we define benchmark pressure intensities for the pressures acting in each case study. In a mature habitat assessment program, such benchmark values would be set a priori (i.e., outside of any particular habitat sensitivity assessment) based on a solid evidentiary basis, and related to the probability distribution of the intensities of the focal pressure (i.e., considering their central tendencies). That is, a benchmark value should indicate the likely intensity of a pressure given that it occurs, such that a particular pressure event can be said to be of high or low intensity (e.g., above or below the average intensity value). Our goal here is not to define scientifically robust benchmark values, but instead to assign example benchmarks for illustrative purposes. In
Milner et al. (2018), the flood under consideration is considered a “>1 in 100 year flood,” but no additional information on flood level or change in flow is provided. Assuming annual maximum flow levels follow a Weibull distribution (with shape and scale parameters of 1), then such a flood is at least 6.79 times larger than a flood with a 50% chance of annual occurrence (i.e., a 2 year flood). Therefore, we consider the standardized pressure intensity to be 6.79 benchmarks (i.e.,
= 6.79) for our reanalysis of data from
Milner et al. (2018). In
Reiber et al. (2021), the concentration of the focal pesticide cypermethrin increased from 0.2 to 30 ng/g in sediment downstream of the spill site. While we could not find data related to environmental concentrations of cypermethrin from other spills, the 10-day lethal concentration
50 for
Hyalella azteca exposed to cypermethrin in sediment was 3.6 ng/g (
Maund et al. 2002). For illustrative purposes, we use this value (3.6 ng/g) as the pressure benchmark, which means that the focal spill of cypermethrin represents a standardized pressure intensity (
) of 8.28 benchmarks (i.e., 29.8 ng/g increase ÷ 3.6 ng/g benchmark = 8.28 benchmarks).
We consider the standardized change in each habitat attribute, using the pre-pressure value (
Ho) as equivalent to the baseline value (
Hb), and a time horizon (
th) of 20 years after the pressure was initiated. The response of the habitat attributes varied among and within habitats (
Fig. 2), with macroinvertebrate richness decreasing from 17 to 16 taxa in response to the flood in Wolf Point Creek (
= 0.06 units) and from 36 to 5 taxa in response to the pesticide spill in the Holtemme River (
= 0.86 units). Other key habitat attributes also showed relatively large responses (
Fig. 2), with calculated resistance values for macroinvertebrate abundance being slightly higher in response to the pesticide spill in the Holtemme River compared to the flood in Wolf Point Creek, due to the higher pressure intensity at that site (
Table 2).
Habitat resilience, estimated as a linear rate of recovery between the time that the pressure ceases (here, the same as the date the pressure initiated, because
= 0) and the time that recovery ceased, was relatively high in response to the pesticide spill compared to the flood. Additionally, both the recoverability and the resilience of macroinvertebrate abundance in response to flooding at Wolf Point Creek was low, with abundance still 67.5% below the pre-pressure state nearly 8 years after the pressure occurred (
Fig. 2). Other habitat attributes showed recoverability values of 1, with recovery achieved between 69 and 1005 days post-pressure, depending on the habitat attribute (
Fig. 2).
6. Conclusion
The sensitivity of habitats to anthropogenic and natural pressures is a primary consideration for many ecosystem management decisions. We suggest that a universal definition of habitat sensitivity, as well as a standardized approach to its quantification, could improve the implementation of environmental policy and legislation, as well as improve the body of evidence concerning how ecosystems respond to pressures. The definition and framework presented herein precisely define the components of habitat sensitivity in a manner that allows for quantitative assessment, while also directly integrating the concept of habitat sensitivity with that of (environmental) impact. Such a link is helpful because impact (also called harm;
DFO 2019) is an important consideration for ecosystem managers when considering whether to authorize development projects, as well as when considering whether habitat offsetting or banking activities provide sufficient compensation for anticipated alteration, destruction, or disruption of habitats (
Clarke and Bradford 2014;
Doka et al. 2022). Additionally, our framework for habitat sensitivity could be integrated with concepts of vulnerability and risk by applying probability distributions to define the likelihood of occurrence of pressures of different intensities and durations, and variance in the estimate values of resistance, resilience, and recoverability, and avoids a variety of issues associated with risk-matrix-like approaches that have poor discriminatory ability and high subjectivity.
A drawback in our framework is that the relationship between the components of habitat sensitivity and impact is complex as a result of (nonlinear) interactions between variables (
Levin 1998). Therefore, comparison of sensitivity among different habitats is not straightforward unless there are systematic differences across all components of habitat sensitivity. That is, if one habitat has higher resistance, resilience, and recoverability values than another, it can be said to have lower sensitivity. However, the relative importance of resistance, resilience, and recoverability in determining impact depends on the dynamics of the pressure (i.e., its intensity and duration), as well as on the time horizon. As a result, it is not currently possible to define a single value for habitat sensitivity and comparison of sensitivity among different habitats will require engaging with its multiple components (i.e., resistance, resilience, and recoverability). Nonetheless, we believe this framework represents a significant step forward for generating repeatable and comparable estimates of habitat sensitivity, and that such an approach could provide great value in informed ecosystem management decisions.