Introduction
Ex situ plant populations are important sources of reintroduction material for restoration projects (
Donaldson 2009). As such, the development of improved propagation techniques is crucial for enhancing the success of reintroduced native plant material. Our proposed use of biochar represents one such improvement for
Glyceria—a native North American wetland grass. Biochar has been known to increase soil fertility and augments crop yield by (1) increasing the water-holding capacity of soil, (2) increasing soil pH (and therefore improving plant nutrient uptake), and (3) stimulating beneficial soil microbial activity in agricultural systems (
Warnock et al. 2007). More specifically, biochar’s ability to increase soil fertility is due to its effect on soil cation exchange capacity (CEC) (
Laird et al. 2010), while its capacity to increase pH (or “liming potential”) helps create a pH range more favorable for plant nutrient availability (
Warnock et al. 2007). Biochar producers suggest pre-charging biochar with nutrients; when combined with mineral fertilizer, small amounts of biochar allow plants to gain greater nutrition (
Glaser et al. 2015). Our work represents the first study of biochar’s potential benefit to ex situ wetland plant propagation.
Glyceria striata (Lam.) Hitchc. (Poaceae) is a perennial grass, growing to 100–180 cm in height (
Pan and Clay 2003;
Darris 2005), and is desirable for restoration projects as it thrives in varying wet environments (e.g., bogs) to dryer environments (e.g., marsh edges) (
Darris 2005). Although
G. striata is known to host other fungi (e.g., endophytic fungi such as
Epichloë glyceriae Schardl & Leuchtm. (
Cheplick 2004) and parasitic fungi such as
Ustilago striiformis (Westend.) Niessl or “stripe smut” (
Darris 2005)), to our knowledge mycorrhizal associations are unknown in this plant species. However, the roots of another species in the genus,
Glyceria fluitans (L.) R. Br., are known to support arbuscular mycorrhizal fungi (AMF) (
Šraj-Kržič et al. 2006) as do roots of most plants in the Poaceae (
Wang and Qiu 2006). Therefore, there is reason to suspect that a commercial AMF inoculant in combination with biochar may affect
G. striata growth ex situ. AMF colonize plant root cortical tissue and are found in all terrestrial habitats, forming root symbioses with ~80% of land plants (and facilitating plant water and mineral nutrient uptake in exchange for carbohydrates (
Bonfante and Genre 2008;
Feddermann et al. 2010)). Although this plant–fungal partnership exists in both emergent and submerged wetland plants, research suggests root colonization by AMF decreases with increasing soil moisture and subsequent lack of oxygen (
Šraj-Kržič et al. 2006). AMF hyphae can colonize smaller soil pores and access soil phosphorus, indicating that biochar porosity may modulate plant–fungal interactions and phosphorus uptake (
Hammer et al. 2014).
Domene et al. (2014) found a high biochar application rate doubled total microbial abundance in temperate soil, whereas
Conversa et al. (2015) found that high application rates decreased AMF frequency. A component of our analysis herein extends this line of inquiry by investigating the result of combining a commercial AMF inoculant with biochar in the context of
Glyceria propagation. Our primary objective was to evaluate if biochar and AMF can improve greenhouse propagation of
G. striata for future reintroduction to wetland restoration sites.
Materials and methods
Glyceria striata plants were grown in the K.C. Irving Environmental Science Centre greenhouse at Acadia University (Wolfville, Nova Scotia, Canada) from wild-collected seeds. Seeds were sterilized by plating onto agar medium (5.8 g·L−1) with 10 mL·L−1 Plant Preservative Mixture (Plant Cell Technology, Washington, D.C., USA) followed by transfer to agar only (5.8 g·L−1) after 2 d. Plants were later out-planted into small plastic cells (2 cm × 2 cm; 4.5 cm deep) containing 80:20 peat:perlite mixtures (referred to herein as “substrate”). Substrate (15 L) was fertilized with 20–8–20 (N–P–K) Plant-Prod© fertilizer at a rate of 5.4 L per 15 L (using a stock of 3 g fertilizer·L−1 reverse osmosis (RO) water) (Brampton, Ontario, Canada).
We used a total of 1.5 kg of biochar obtained from InnoTech Alberta (Vegreville, Alberta, Canada), produced from chips of spruce, pine and (or) fir trees supplied by Parkland Chip Products Limited (Acheson, Alberta, Canada). Product specifications indicated 91.92% fixed carbon content and an 8.6 pH. Biomass was slow-pyrolized in a multi-hearth carbonization reactor at 550 °C. Proximate analysis (dry basis) yielded an ash content of 1.36% and a volatile content of 6.73%. Biochar–substrate mixtures were prepared by dividing biochar equally into two containers: nutrient charged (container 1) and uncharged (container 2). To make nutrient-charged biochar, 1.44 L of the same fertilizer used above was added to container 1 and allowed to sit for 4 h. Fertilized substrate was transferred to a container with holes and drained of water overnight. The same volume of water (1.44 L) was also added to container 2 and similarly left to sit and drain. Substrate water saturation was equivalent between containers 1 and 2 at planting. A series of 100 mL cells were filled with varying proportions of biochar and substrate (10%, 50%, and 75% by volume (
v/
v)). In their meta-analysis,
Biederman and Harpole (2013) reported typical biochar application rates of 0.02% to >54.6%. Given this, we have tested biochar application rates that are in the typically tested range (i.e., 10% and 50%), and have further tested a higher rate of 75% for comparison. Three drops (0.003 g·mL
−1) of Premier Tech Myke
®Pro WP (Rivière-du-Loup, Québec, Canada) were added for each mycorrhizal treatment. This product contains 800 spores·g
−1 of the beneficial AMF fungus
Glomus intraradices N.C. Schenck & G.S. Sm.
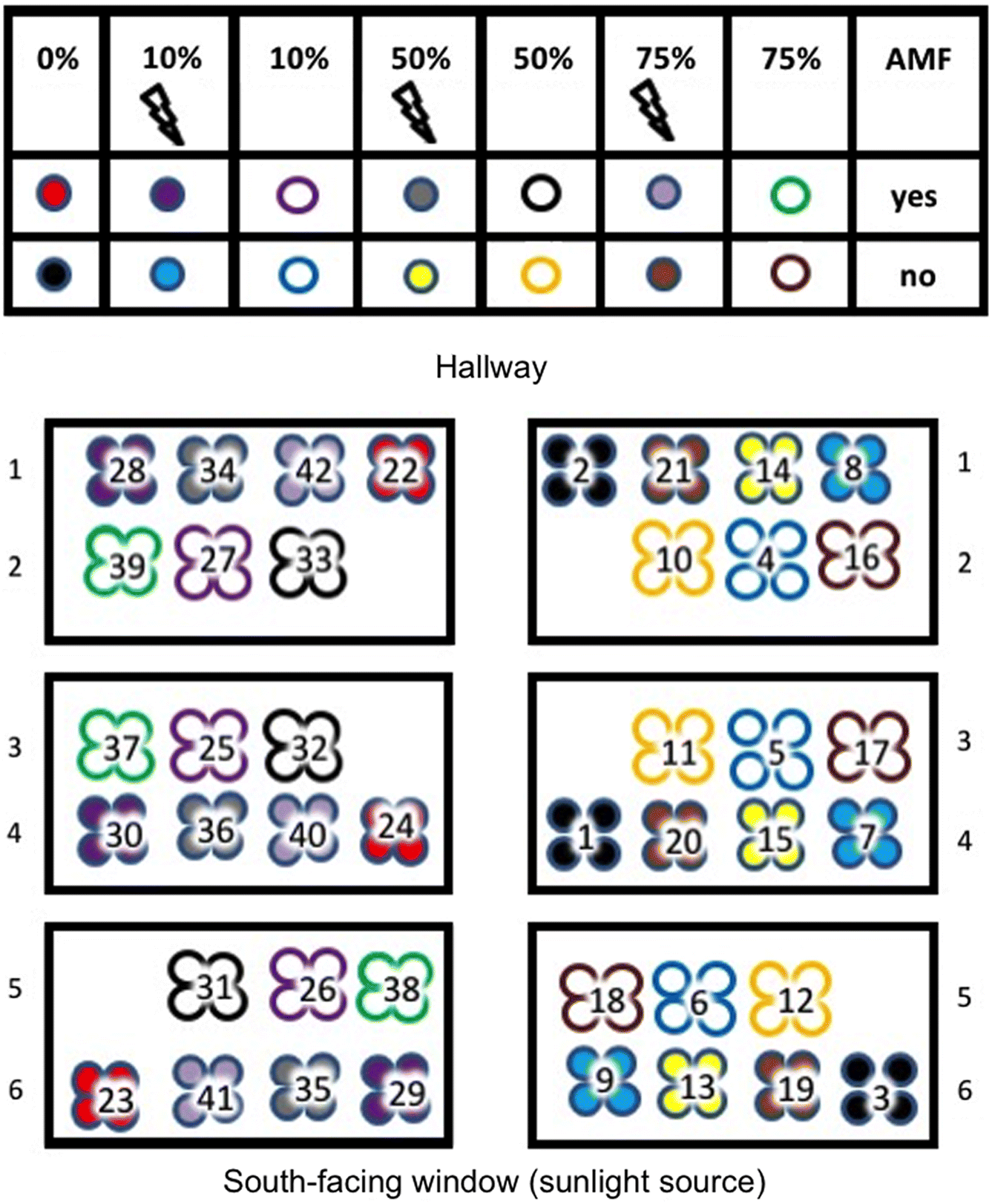
Glyceria striata plants (28 d old) were transferred from cells to individual treatments and placed in a climate-controlled greenhouse (phytotron) with sunlight as the light source (14 h of natural daylight). Fourteen treatments with twelve
G. striata specimens each (168 plants total) were used. Treatments were divided into three groups of four (
Fig. 1). For each of the 14 treatments, the three groups of four cells were distributed such that treatments were represented in each of the three vertical blocks and distributed randomly within those blocks (
Fig. 1). Plants were initially watered from above with RO water daily. Initial root length and shoot length were recorded for later comparison of these same metrics after harvest (see below). An air temperature of 21 °C was maintained throughout. Three days post-initial planting in biochar + substrate, cells were transferred onto capillary beds to ensure constant water availability. Beds consisted of Texel
® (Texel Technical Materials, Inc., Saint-Elzéar, Québec) cloth saturated in RO water (to initiate absorption) over a plastic bench sitting in a tub of RO water; the ends of the cloth hung in water. Cells were watered thoroughly from above with RO water to initiate capillary action. After 3 d, three more drops of AMF inoculant were added to the root zone of mycorrhizal treatments. After 9 d, the seal between the Texel
® cloth and the substrate was assured by pressing extra peat–perlite mixture onto the circular openings at the bottom of cells.
Plants were harvested and dried after 13 weeks of growth in experimental substrate. One substrate sample per treatment was placed in a Ziploc
® (S.C. Johnson & Son, Inc., Brantford, Ontario) bag for pH and nutrient analyses.
Glyceria striata was stored at −20 °C until later processing. Shoot and root mass, as well as the length of the longest
G. striata shoot per plant at harvest, were recorded for all plants. Substrate samples were sent to the Nova Scotia Department of Agriculture Analytical Lab for nutrient analysis (Truro, Nova Scotia, Canada). As shown in
Fig. 1, samples 1, 7, 10, 13, 22, 31, 40, and pure biochar were analysed according to the greenhouse soil paste method (similar to that described by
Warncke (1998)) that measures electrical conductivity, pH, nitrate (N), calcium (Ca), potassium (K), magnesium (Mg), phosphorus (P), and sodium (Na).
Statistical analyses were conducted in R v3.2.3 (
R Core Team 2015). We used generalized linear models (GLMs) to model and attribute statistical significance (at
α = 0.05) to trends seen in data regarding shoot height difference (the difference between initial shoot height upon transplantation into biochar and shoot height measured at harvest) and final dry shoot mass at harvest. We used GLMs to determine the effects (and interactions) of biochar application rate, nutrient charge status, and AMF inoculation on the dependent variables of shoot height difference and of shoot mass. In creating GLMs, the two dependent variables, shoot mass and shoot height difference, were tested individually as response vectors. Shoot height difference was assumed to follow a linear relationship with shoot height over time. The correlation between shoot and root mass was tested using Spearman’s rank correlation test and found to be strong enough that dry root mass data were omitted. Gaussian distributions were assumed for shoot mass and shoot height and we log transformed the response data (i.e., log
10(shoot height difference + 1.2) and log
10(shoot mass)) to produce a normal distribution of the residuals. We also used the GLMs’ associated ANOVA statistics to help interpret the degree of significance for the effects of biochar application, nutrient charging, and AMF inoculation on the dependent variables. The
ggplot2 package in R was used to create boxplots of the true shoot height and shoot mass means with predicted shoot height and shoot mass means generated by GLMs overlaid after back-transforming them. Major data sets are provided in
Supplementary Material 1. Additional data are available upon request from the corresponding author.
Results
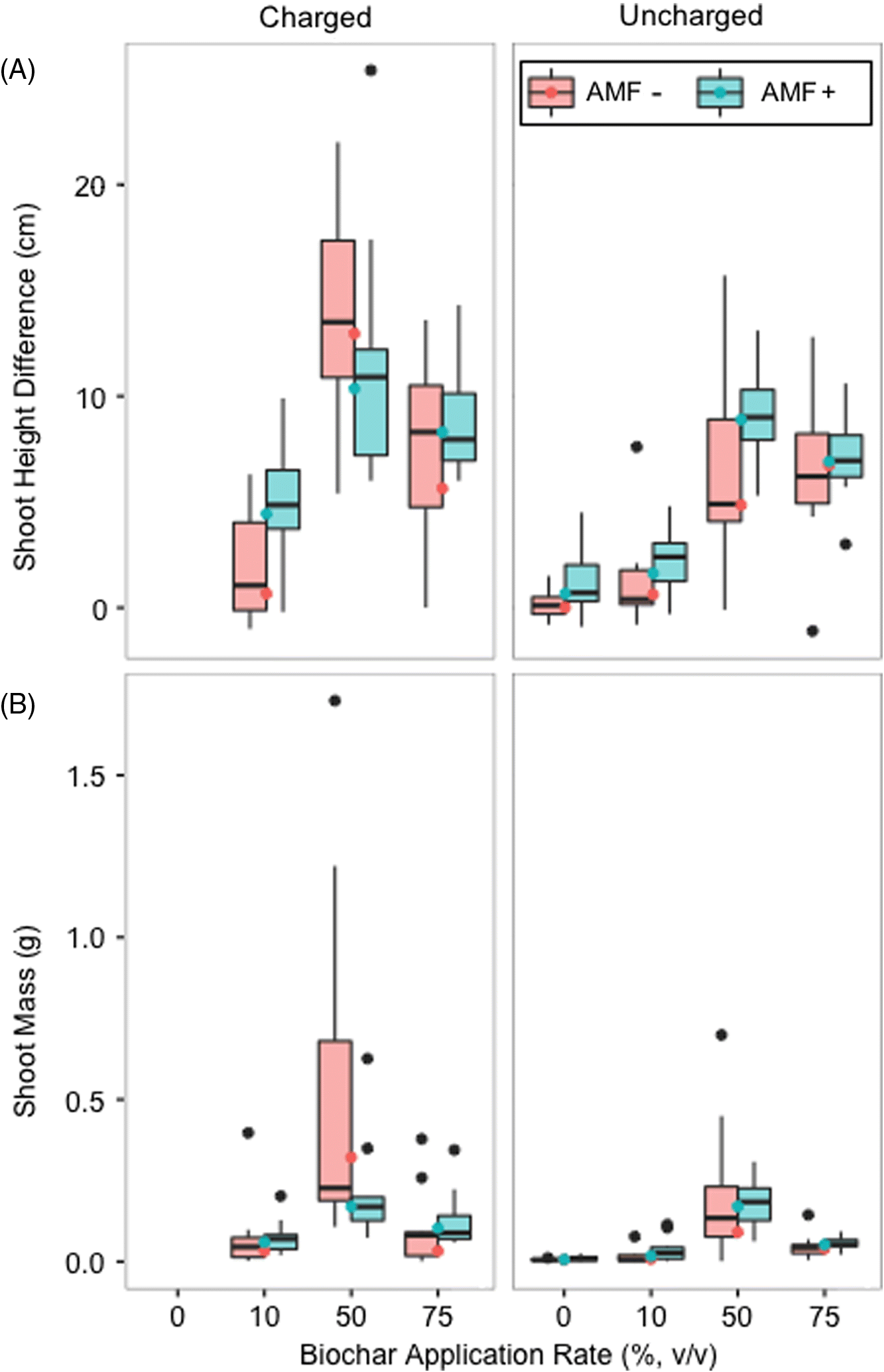
Throughout the experiment
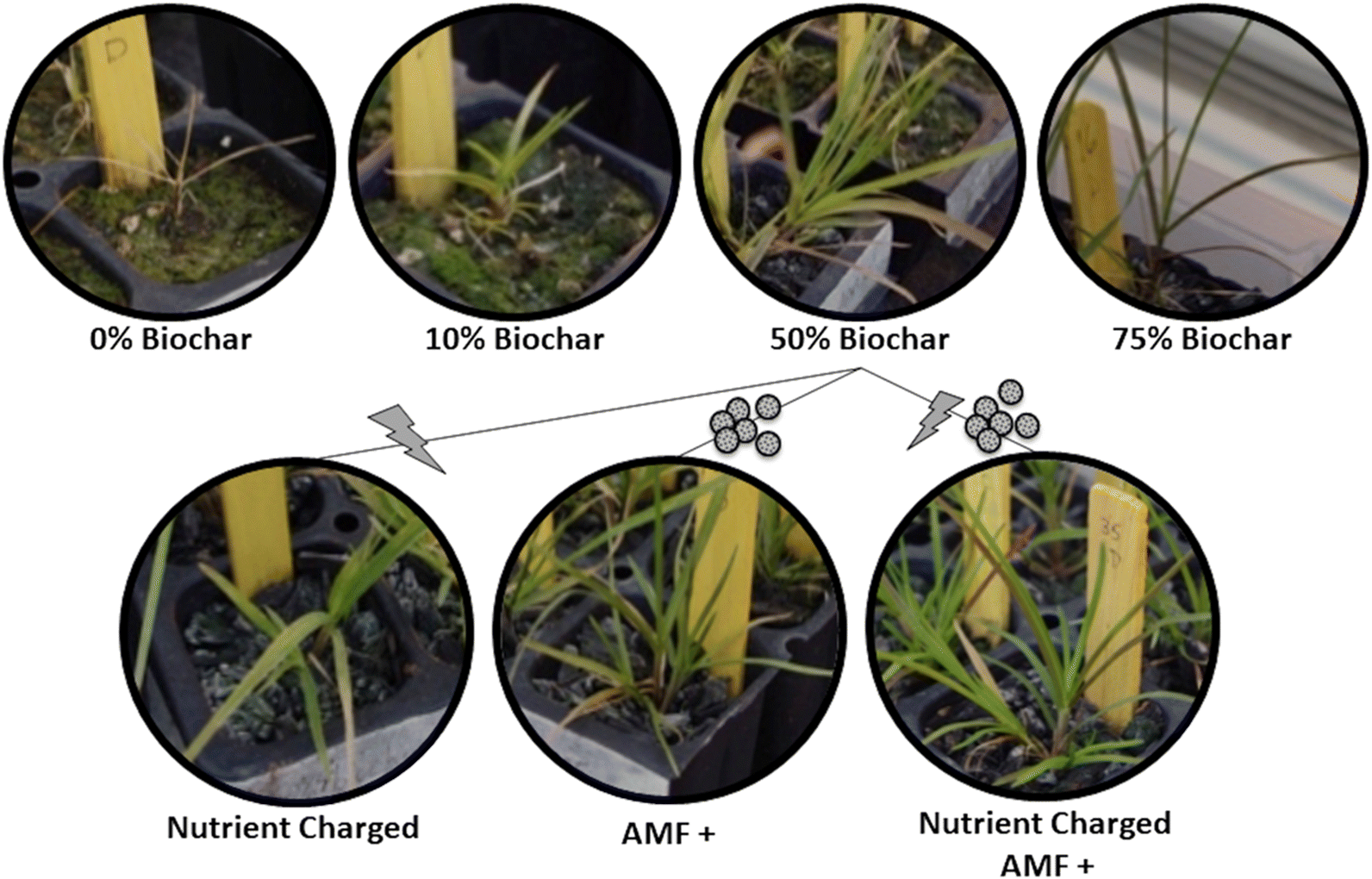
G. striata grown in biochar without added nutrients or AMF displayed healthier characteristics than plants grown without biochar (i.e., greener leaves, early emergence of multiple shoots, and greater height;
Figs. 2 and
S1). The 50% (
v/
v) biochar application rate yielded the greatest qualitative leaf/shoot health and aboveground biomass (
Fig. 2). Quantitatively, the 50% (
v/
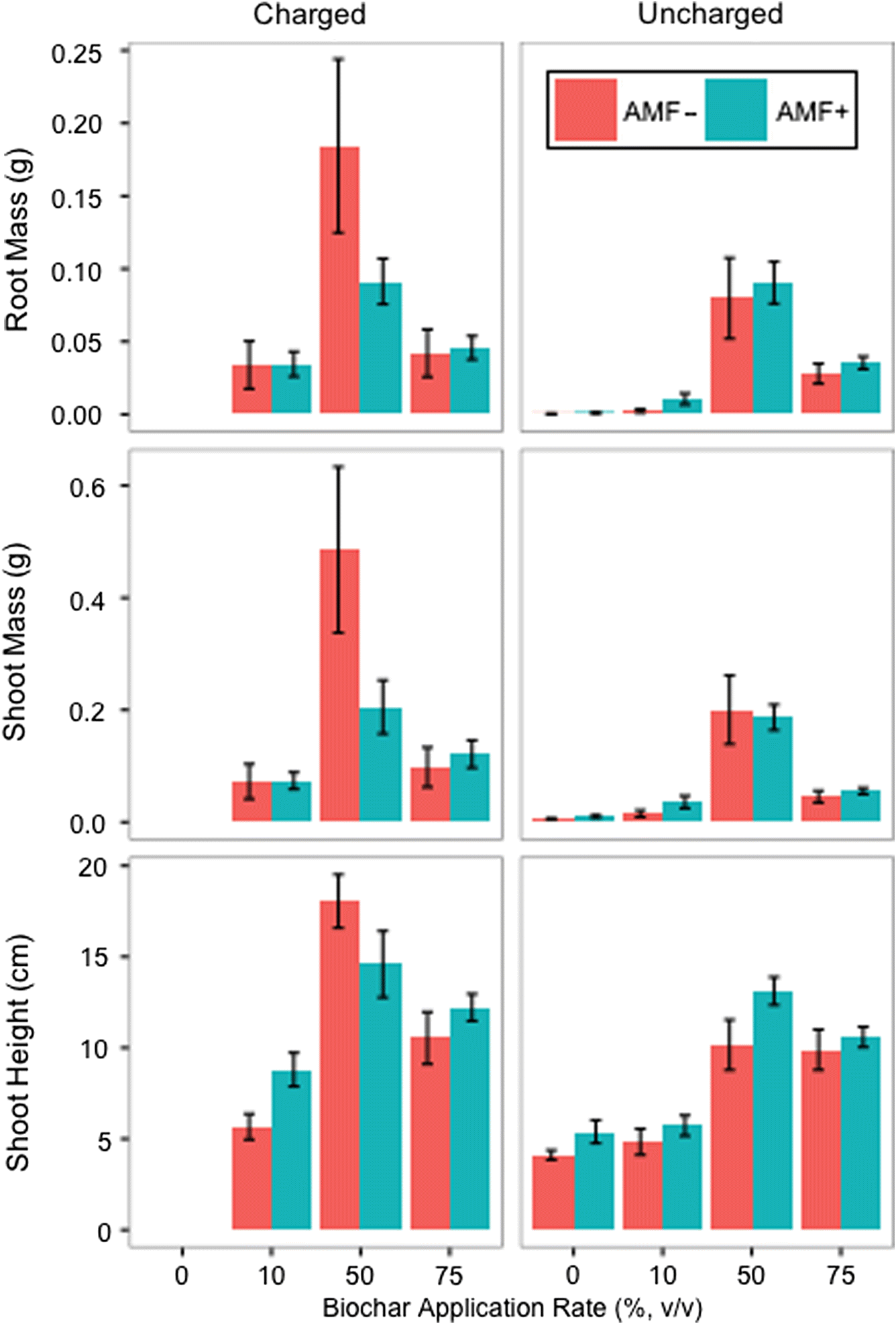
v) biochar application rate yielded the greatest mean increases in dry root mass, dry shoot mass, and shoot height, with the greatest values occurring for “nutrient charged and non-AMF-inoculated” treatments (
Fig. 3). Root mass was omitted as it had a strong positive correlation with shoot mass (
R = 0.96) (see
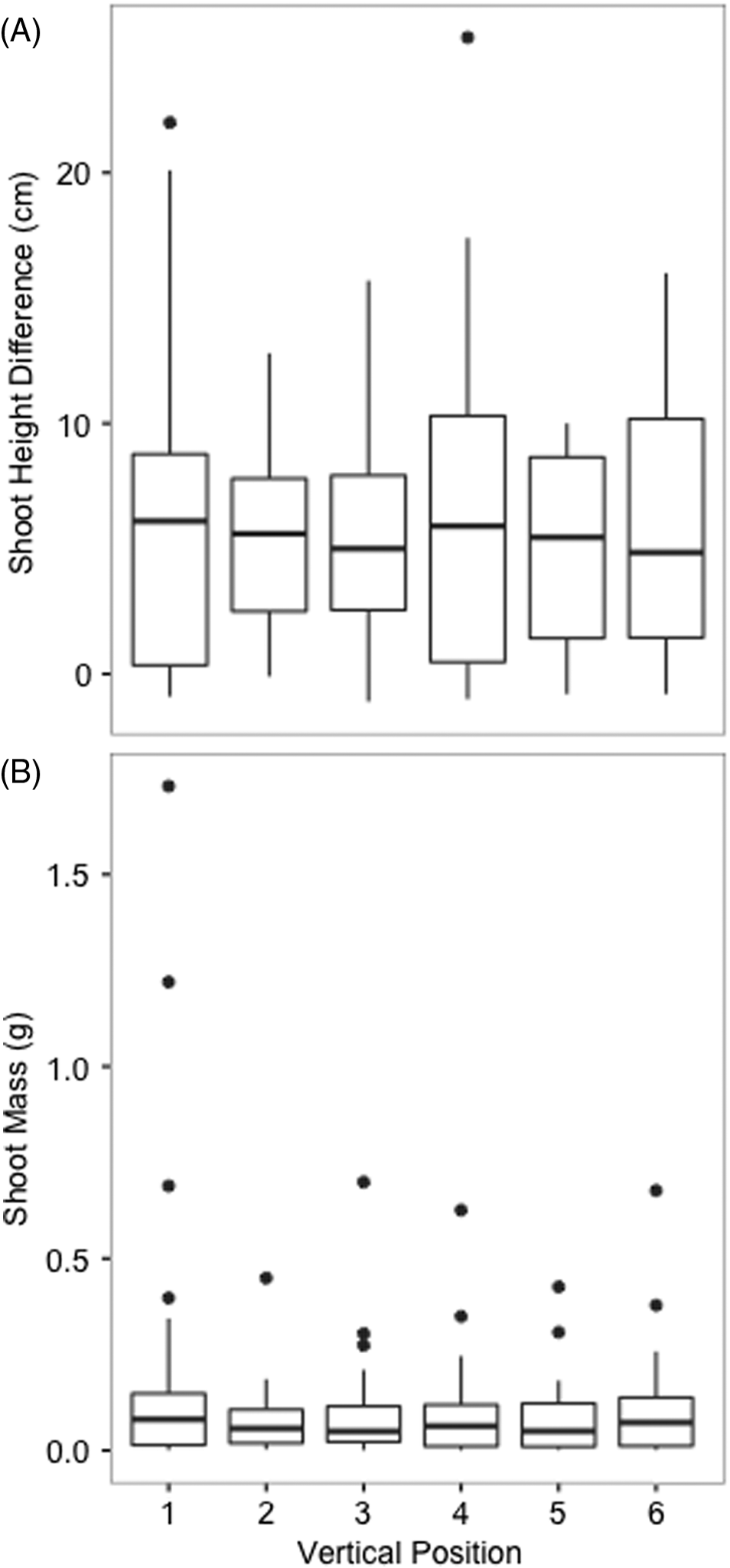
Table 1 for root mass summary ANOVA statistics). Position in the greenhouse was also omitted as distributions for shoot height difference data and shoot mass data for different greenhouse positions were similar (
Figs. 4A and
4B, respectively). The significance of shoot height difference and shoot mass results were therefore analyzed further (see
Fig. 5 for boxplots of true sampling data with predicted means calculated by the GLM).
The GLM for shoot height difference and shoot mass of
G. striata were used to calculate predicted means. With a slightly under-dispersed dispersion value (0.38) for shoot height difference and an over-dispersed dispersion value (1.25) for shoot mass, this fit the trend of observed means well (
Figs. 5A and
5B, respectively).
Table 1 shows an ANOVA table summarizing the
F values and probabilities for the overall effects of biochar application, nutrient charging, and AMF inoculation on
Glyceria plant shoot height difference and shoot mass. Based on the values in
Table 1, it is clear that each single treatment (biochar, nutrient charging, or AMF inoculation) had a significant effect on both shoot height difference and shoot mass (
p < 0.05 for all three treatments individually). However, combining treatments did not affect the two growth measurements (
p > 0.05;
Table 1). This outcome may be the result of varying biochar concentrations having differing effects in the presence of other treatments (i.e., when biochar is pre-charged with nutrients or combined with an AMF inoculant). For example,
Fig. 3 shows there is clearly a discrepancy between the mixed effects at the biochar application rates of 10% and 50%. As such, the subsequent statistical analyses are based on observed growth changes with respect to the different application rates of biochar.
The following data interpretations are based on a significance threshold of
α = 0.05. Furthermore, the
p-values reported are in reference to the probabilities for each variable returned using the GLMs (i.e., the variables of 0%, 10%, 50%, and 75% biochar, as well as nutrient charging and (or) inclusion of the AMF inoculant). The greatest mean difference in shoot height and the greatest shoot mass were recorded for the 50% (
v/
v) application rate of non-AMF inoculated, nutrient-charged biochar (
Figs. 3 and
5). Final shoot height was on average 4.4× greater and shoot mass on average was 85× greater for this treatment compared with negative controls (0% biochar (
v/
v) + non-AMF inoculated) (
Fig. 3;
Table S1). Charging biochar with nutrients significantly increased shoot height difference and shoot mass for the 50% (
v/
v) biochar application rate (shoot height difference
p = 0.007; shoot mass
p = 0.028). Charging biochar with nutrients also significantly increased shoot mass at the 10% (
v/
v) biochar application rate (
p = 0.012). The interaction of all treatment conditions (nutrient charged, AMF inoculated, and the 50% (
v/
v) application rate) was significant for shoot height difference (
p = 0.049) and for shoot mass (
p = 0.022). Mean shoot height difference and mean shoot mass also increased with increasing biochar application rate until the 50% (
v/
v) application rate and then decreased between the 50% (
v/
v) and 75% (
v/
v) biochar treatments (
Fig. 5). The 50% (
v/
v) and 75% (
v/
v) treatments were significant: shoot height difference at 50% (
v/
v)
p = 2.26e–09 and at 75% (
v/
v)
p = 1.83e–11; shoot mass at 50% (
v/
v)
p = 4.60e–09 and at 75% (
v/
v)
p = 2.65e–05. This indicates that the change in shoot height difference from the 0% biochar treatment to the 50% biochar treatment was significant and the change in shoot height difference from the 0% biochar treatment to the 75% biochar treatment was significant. The shoot height difference between 0% and 10% biochar treatments was not significant (
p > 0.05). Other than with the 50% biochar treatment using charged biochar (recall shoot height difference
p = 0.049; shoot mass
p = 0.022), the commercial AMF treatment did not significantly influence shoot height response nor shoot mass in
G. striata in our study. It should be noted that although we could not confirm AMF presence in our experimental plant roots using root staining, nested polymerase chain reaction (PCR) with AMF-specific primers following the method of
Krüger et al. (2009) indicated the presence of AMF DNA in some of our greenhouse propagated
G. striata plant root samples (
Fig. S1).
Substrate analysis
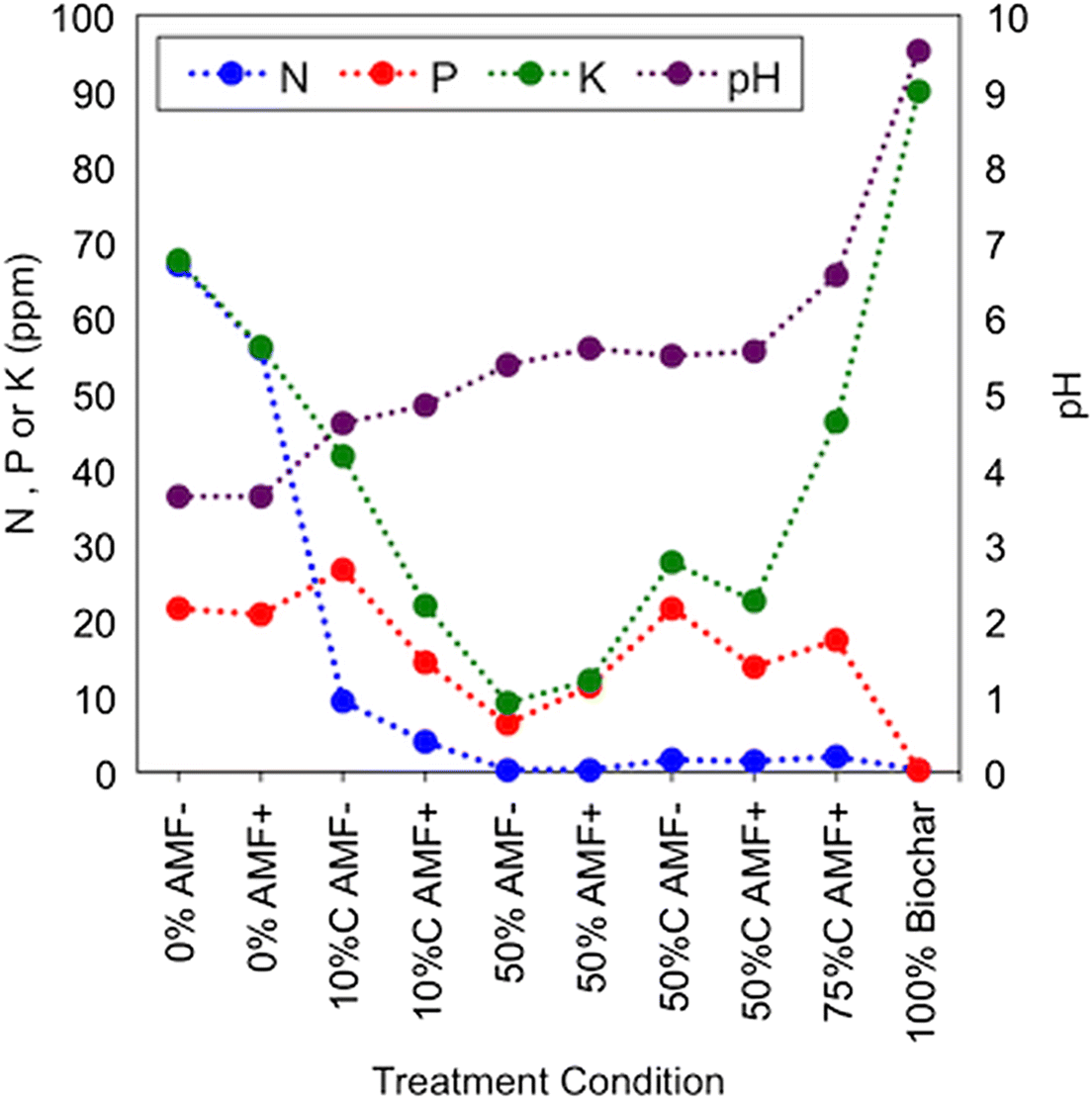
Substrate pH increased as the application rate of biochar increased, with a corresponding decrease in nitrate-N (
Table 2;
Fig. 6). Pure biochar also showed a relatively high level of potassium but displayed trace amounts of nitrate-N, phosphorus, and sodium (
Table 2;
Fig. 6). Electrical conductivity, a measure of the salinity of the soil that can serve as a proxy for soil fertility—i.e., it positively correlates to soil fertility indicators, such as CEC (
USDA and NRCS n.d.)—was highest in the 0% biochar treatments but variable for the other samples tested (
Table 2). Note that single measurements were taken for each treatment.
Discussion
For both shoot height and shoot mass, GLMs predicted a significant increase in growth that was positively correlated with increasing application rate until 75% biochar, at which point mean shoot height and shoot mass decreased relative to the 50% application rate. There are several reasons why biochar may have influenced both root mass and shoot mass/height, and why particular trends regarding AMF inoculant usage were seen, including (1) the increased liming effect of biochar, (2) the general nutrient availability as well as plant growth inhibition at higher biochar concentrations, and (3) AMF symbiosis in relation to nutrient availability. The observed increase in growth response for
G. striata may be due to the liming effect of biochar. Our substrate was acidic following all biochar applications, with pH values favoring maximum nutrient availability (i.e., pH = 5.5–7.0) (
Warnock et al. 2007) at 50% and 75% biochar application rates (
Table 2;
Fig. 6). For
G. striata, a 50% application rate may be favorable over higher and lower application rates, but this does not suggest that trials with other plants would show the same preference. As restored wetlands may have higher pH values (e.g., pH 5.76,
Kluber et al. 2014) similar to the values we recorded for substrate with biochar, there is the potential for our propagation methodology to help condition
G. striata for re-introduction into restored wetland sites. However, this would require further greenhouse and field testing using field soils.
Our general linear models supported a hypothesis whereby nutrient availability is a significant factor in improving growth, as we demonstrated that pre-charging biochar with nutrients provided a statistically significant increase in plant growth. We fertilized substrate without biochar with the same proportions of nutrients as substrate with biochar. Our results suggest charged biochar improved the availability, rather than the quantity, of nutrients. This may be due to biochar’s documented ability to absorb nutrients (
Mukherjee and Zimmerman 2013). In a practical sense (i.e., for wetland restoration), such absorptive property may impede nutrient leaching by other means (e.g., irrigation). In addition, we also observed that an increase in nutrient availability was not reflected in the change in conductance value (
Table 2). Conductance correlates positively with CEC, which is an indicator of soil fertility; charged biochar should have resulted in an increased CEC (
Glaser et al. 2015). However, this was not reflected in the conductance values of charged biochar calculated during our substrate analysis (
Table 2). Possibly, the biochar required a longer period of aging in nutrients to elevate the CEC (
Lehmann et al. 2011;
LeCroy et al. 2013), thus introducing another avenue for future research. It is important to note that water holding capacity is one of the proposed significant benefits of biochar (
Jeffery et al. 2011). By using capillary beds our plants had a consistent water supply, thus, the water holding feature of biochar was controlled among treatments and unlikely to influence our results. Furthermore, we showed a decline in plant growth response for both charged and uncharged biochar at the 75% application rate, indicating that plant growth inhibition might occur when biochar application rates exceed 50%. At high application rates, biochar can increase pH to unfavorable levels for plant growth (
de la Rosa et al. 2014). However, our substrate chemistry analysis indicated the 75% biochar application rate still yielded an ideal pH for plant nutrient uptake. Therefore, an unknown mechanism inhibited plant growth at the highest biochar concentration tested.
The statistical significance of the interaction between nutrient-charged and AMF-inoculated biochar at a 50% application rate suggests a relationship between fungal symbiosis and nutrient availability. However, it did not appear that the mycorrhizal inoculant benefitted plant growth. The 50% application rate of nutrient-charged and AMF-negative biochar yielded a significant increase in mean shoot mass and mean shoot height compared with the same treatment of biochar with AMF (
Figs. 3 and
5). The increased nutrient availability from charged biochar might have resulted in an increase of phosphorus availability (expected with elevated pH), thereby reducing the need for AMF symbiosis (
Treseder 2004;
Warnock et al. 2007;
Hammer et al. 2014).
LeCroy et al. (2013) noted that when necessary soil nutrients are available, AMF species may not benefit the plant host. In such cases a parasitic relationship, rather than a symbiotic one, between the AMF and its host plant may be induced or competition for resources may result. However, results of our substrate analysis suggest that nitrogen was relatively low in samples of 50% biochar (
Table 2;
Fig. 6), indicating that nutrients were not present in excess. In general, no significant beneficial effect of the commercial AMF inoculant on plant growth was noted; but rather, a significant decrease in growth response for charged + AMF-inoculated treatments was observed. It is also important to point out (as suggested in the Introduction), that no previous AMF relationship has been described for
G. striata specifically, but such a relationship is known for another member of the genus,
G. fluitans (
Šraj-Kržič et al. 2006). Our study of the AMF relationship in
G. striata is ongoing with preliminary microscopic work showing mycorrhizae in wild-collected
G. striata roots from Nova Scotia (e.g., using ink vinegar staining techniques of
Vierheilig et al. 1998). Although we could not confirm AMF presence in our experimental plant roots using root staining, nested PCR with AMF-specific primers did indicate the presence of AMF DNA in some of our greenhouse propagated
G. striata plant root samples. Nonetheless, the potential effect of biochar on mycorrhizal symbiosis with
Glyceria is likely complex, with biochar pore size, source material, and pyrolysis temperature contributing to the interaction (
Hammer et al. 2014).
Acknowledgements
The assistance of T. d’Entremont, D. Stewart, J.C. Lopez, T. Avery, D. Dunnington, D. Quinn, J. Walker, D. Kristie, P. Romkey, and A. Greene is acknowledged. L.O’Halloran, K. Heo, and B. Holmes are also gratefully thanked. An NSERC USRA and an Arthur Irving Academy Scholarship in Environmental Science to SM, as well as an Arthur Irving Academy of Environmental Science Research Grant to R. Evans, RN, and RB supported this work.