In this study, we focused on the influence of sediment physical and geochemical factors on initial uptake of PBDEs into a variety of sediment feeders in southern British Columbia marine waters. We then examined how total and congener homologue group PBDEs changed in higher trophic levels which may be connected to benthic food sources. Understanding the initial uptake of these contaminants into the marine food chain will help to determine how they affect higher trophic levels.
Gouin and Harner (2003) modelled the environmental fate of PBDEs and suggested that they are largely partitioned to organic matter in sediments.
Li et al. (2010) found that sediment PBDE content off the coast of southeastern China was not related to the organic content of sediments, but rather to particle size, although
Zhao et al. (2010) and
Zhu et al. (2014) found that grain size and organic content were both important factors in tropical mangrove swamps. In all three aforementioned studies, sediment %fines was a stronger correlate of sediment PBDE levels than organic content. In the present study, sediment PBDEs increased with sediment organic carbon flux, content, and %fines (in decreasing order). We conclude from this that sediment geochemistry and physical structure are critical for determining PBDE storage in sediments, and fine, organic particles tend to bind these contaminants most efficiently. As a result, the coarser, less organic sediments of the Juan de Fuca Strait tend to have a lower inventory of PBDEs than the organically richer and finer sediments of the southern Strait of Georgia.
PBDEs in sediment feeders
Sediment consumers are the main entry point into the food chain of PBDEs from the marine habitat, as these contaminants are typically particle-bound. Most sediment feeders live for 1–4 years (
Robertson 1979). PBDE uptake from sediments and subsequent incorporation into the food chain is limited by sediment feeder lifespan and habitat conditions, but has been shown to be higher in situ than in short-term uptake experiments (
Klosterhaus et al. 2011). This finding suggests that tissue uptake may be cumulative over the life span of the animals.
Johannessen et al. (2008) noted that regardless of the actual rate of input to the sediments, benthic organisms are exposed to contaminants in proportion to sediment concentrations (e.g.,
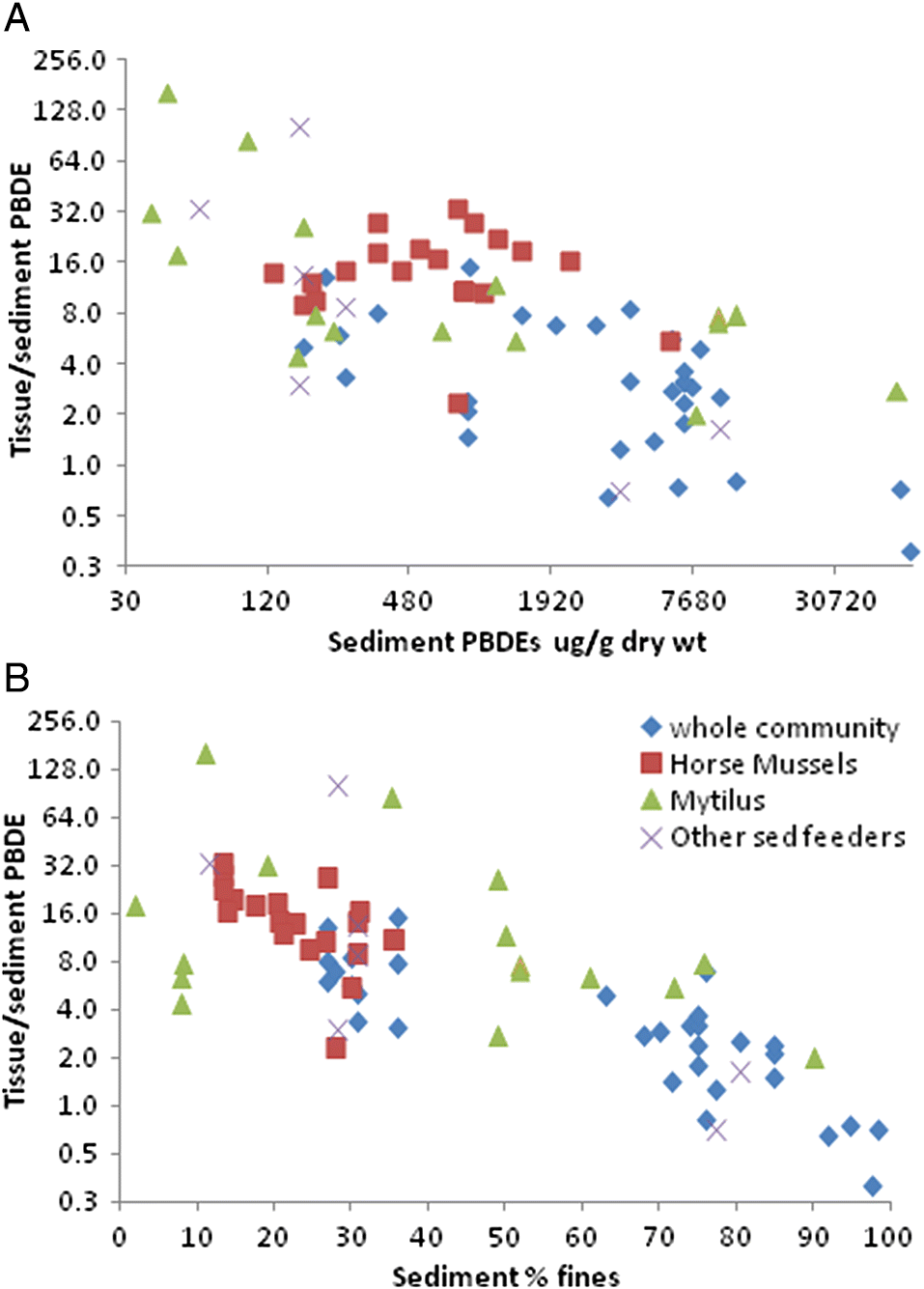
Reynoldson 1987). In the present study, sediment feeders clearly took up contaminants in proportion to sediment concentrations, but this relationship is not simple or linear, and other factors modify uptake.
Zhao et al. (2010) also noted that although marine sediment PBDE concentration are often correlated with both %TOC and %fines, benthos tissue concentrations are not. In the present study, tissue concentrations were correlated with sediment AVS, %TOC, δ
15N, and organic carbon flux and quality. Unlike sediments, tissue PBDE concentrations in sediment feeders had a negligible correlation with %fines.
Although sediment PBDEs are often TOC-normalized for interpretation of biotic uptake (
Dinn et al. 2012a), organic input and burn-down cannot always be interpreted from sediment %TOC. Low %TOC can occur where considerable, labile organic material is being input if the relative inorganic flux is high (
Burd et al. 2012a,
2012b). In the present study, increasing organic content and input (%TOC, organic carbon flux), organic lability (δ
15N) and bacterial metabolism (inferred from AVS
3) enhanced tissue PBDE uptake. This pattern is illustrated by the considerably higher tissue PBDEs relative to sediment PBDEs near municipal wastewater outfalls (see also
Burd et al. 2014). Elevated sediment AVS is an indicator of enhanced burn-down of this organic material, primarily by bacteria. The organic material from wastewater outfalls is fresh and labile, and it is expected that benthos would selectively feed on this material (
Burd et al. 2014). Biomass turnover of benthos is, therefore, increased near municipal wastewater outfalls relative to background because of high bacterial metabolism, as well as dominance by smaller, rapid-turnover invertebrates (
Burd et al. 2013), so that contaminant uptake rates are also expected to be higher than in background areas (
Burd et al. 2014).
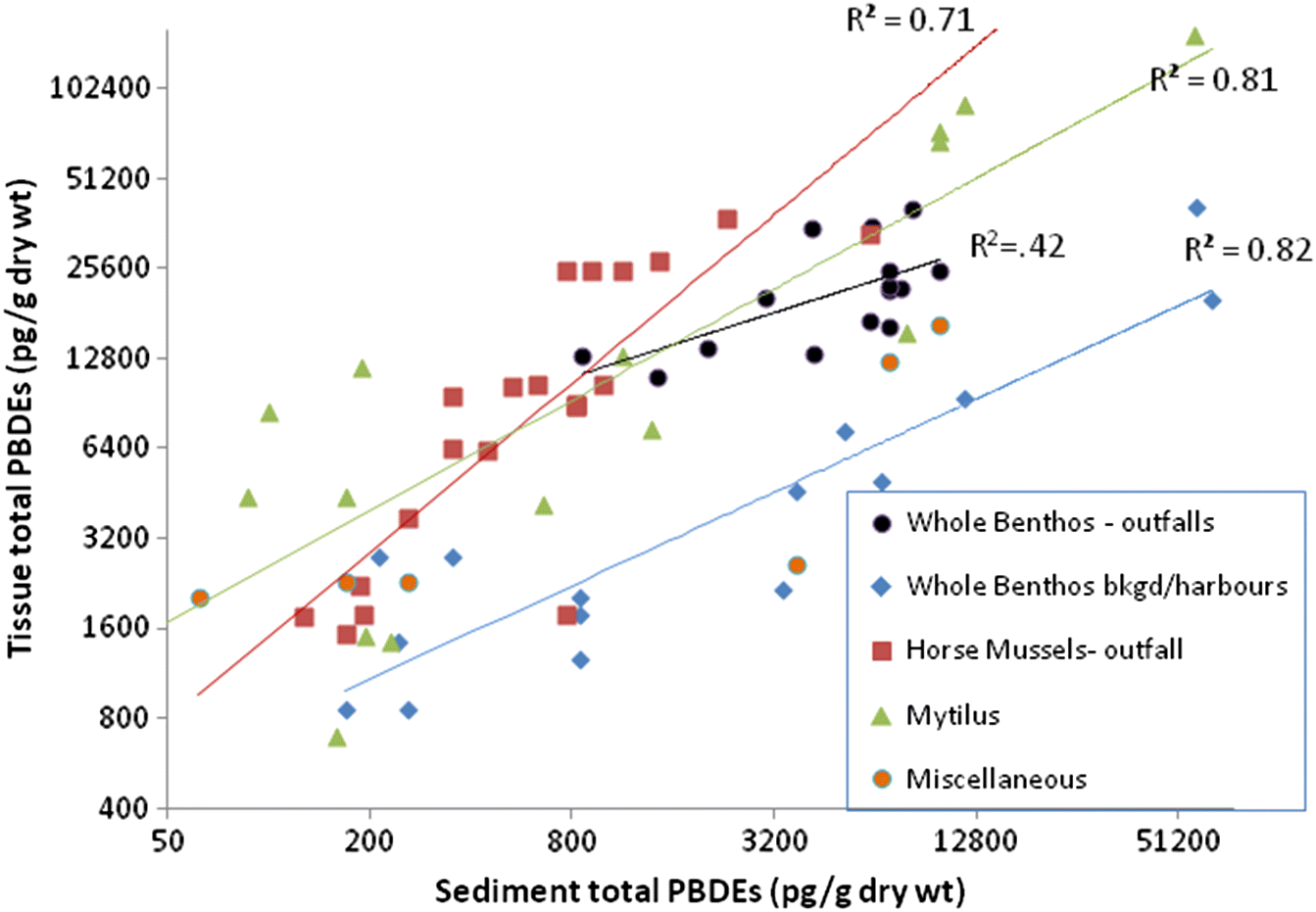
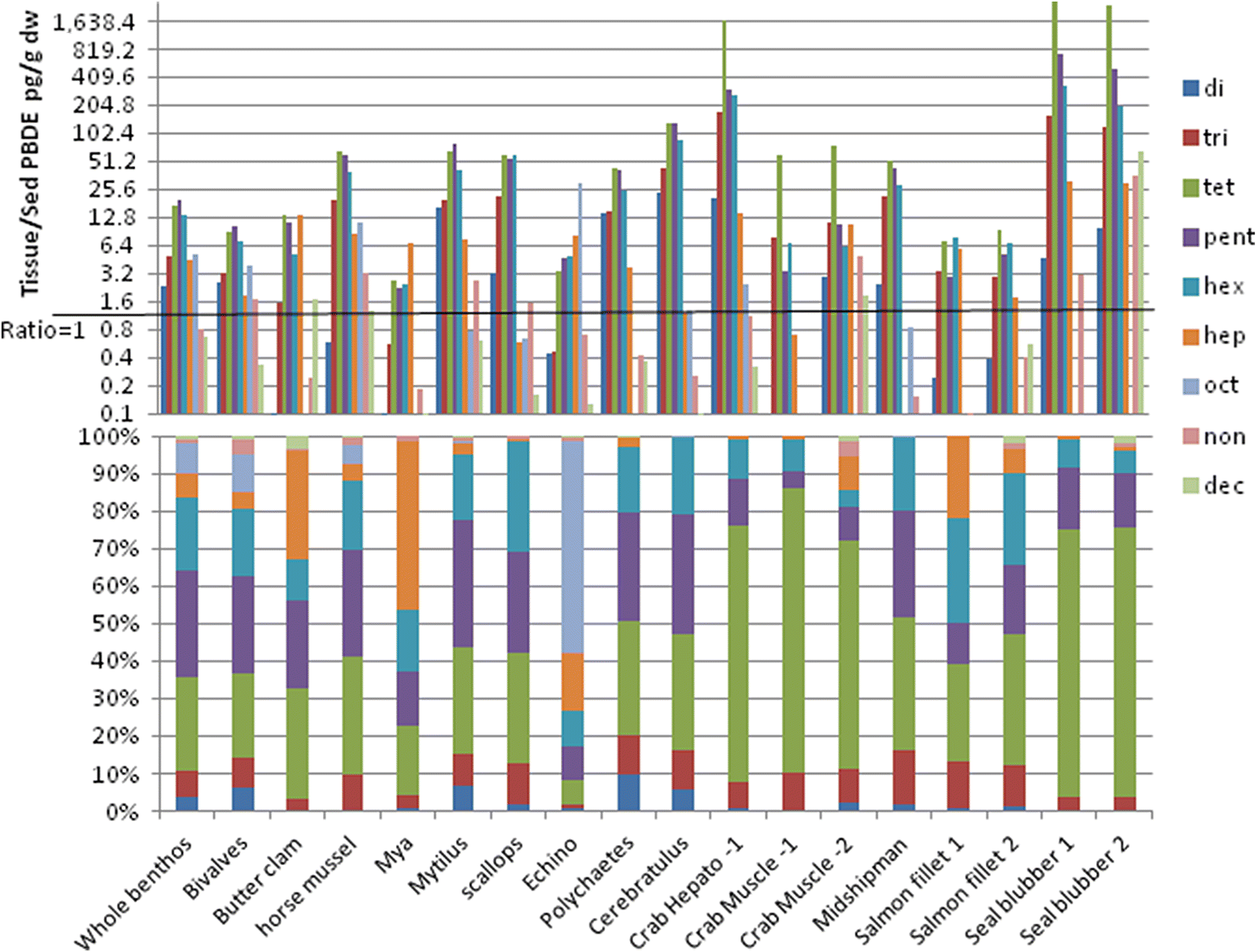
The accumulation or uptake ratio of PBDEs in sediment feeders is illustrated herein as the dry-weight ratio of tissue/sediment PBDEs. Tissue accumulation showed a general negative trend as sediment PBDEs increased, so that above sediment total PBDE concentrations of about 10 000 pg/g dry wt, a dilution effect (ratio <1) was evident. This dilution was most evident in the highly PBDE-contaminated urban harbour samples. Similarly, tissue PBDE accumulation decreased progressively with increasing %fines, resulting in a regional dichotomy in PBDE tissue accumulation rates between the Juan de Fuca Strait (typically sandy, low depositional rate, and low organic carbon flux) and the Strait of Georgia (more silty and a high depositional rate with higher organic carbon flux;
Burd et al. 2012a). Therefore, despite the lower sediment inventory of PBDEs in the Juan de Fuca Strait, the rate of uptake into sediment feeders is higher than in the Strait of Georgia.
The above patterns imply that at very high sediment PBDE levels, only likely to be found in samples with high %fines, most of the PBDEs are not bioavailable, possibly because they are bound to inorganic particles. PBDEs bound to inorganic fine particles are unlikely to be incorporated into tissues due to selective feeding of benthos on organic particles.
It is also expected that there would be a difference in contaminant loads between suspended particles and deposited (sediment) particles, although this was not measured in the current study. In suspended particles, there is less dilution from coarse, inorganic sands (which sink faster), and suspension feeders may, therefore, be exposed to a more concentrated source of organically bound contaminants than deposit feeders. This is supported by the finding that total PBDEs in tissues (relative to sediment) of the suspension feeders Mytilus and Modiolus were similar to whole benthos near municipal wastewater outfalls, all of which were higher than whole benthos from background areas. Although some of the Modiolus were sampled near a municipal wastewater outfall, the Mytilus samples were not.
To summarize, selective feeding on fresh municipal wastewater outfall deposits versus older organic particulates and on organic versus inorganic particulates likely accounts for much of the variability in the relationship between total tissue PBDEs and sediment PBDEs and explains why accumulation of PBDEs from sediments is not linear or predictable from sediment concentrations alone.
Trophic transfer
There has been considerable literature focusing on trophic transfer and bio-magnification of hydrophobic organic contaminants such as PBDEs in marine food chains (
Natale 2007;
Ross et al. 2009;
Cullon et al. 2012). There are a number of factors that contribute to trophic transfer in secondary consumers. For example, the sex and age of animals clearly contribute significantly to tissue content in marine mammals (
Wolkers et al. 2006;
Mongillo et al. 2012;
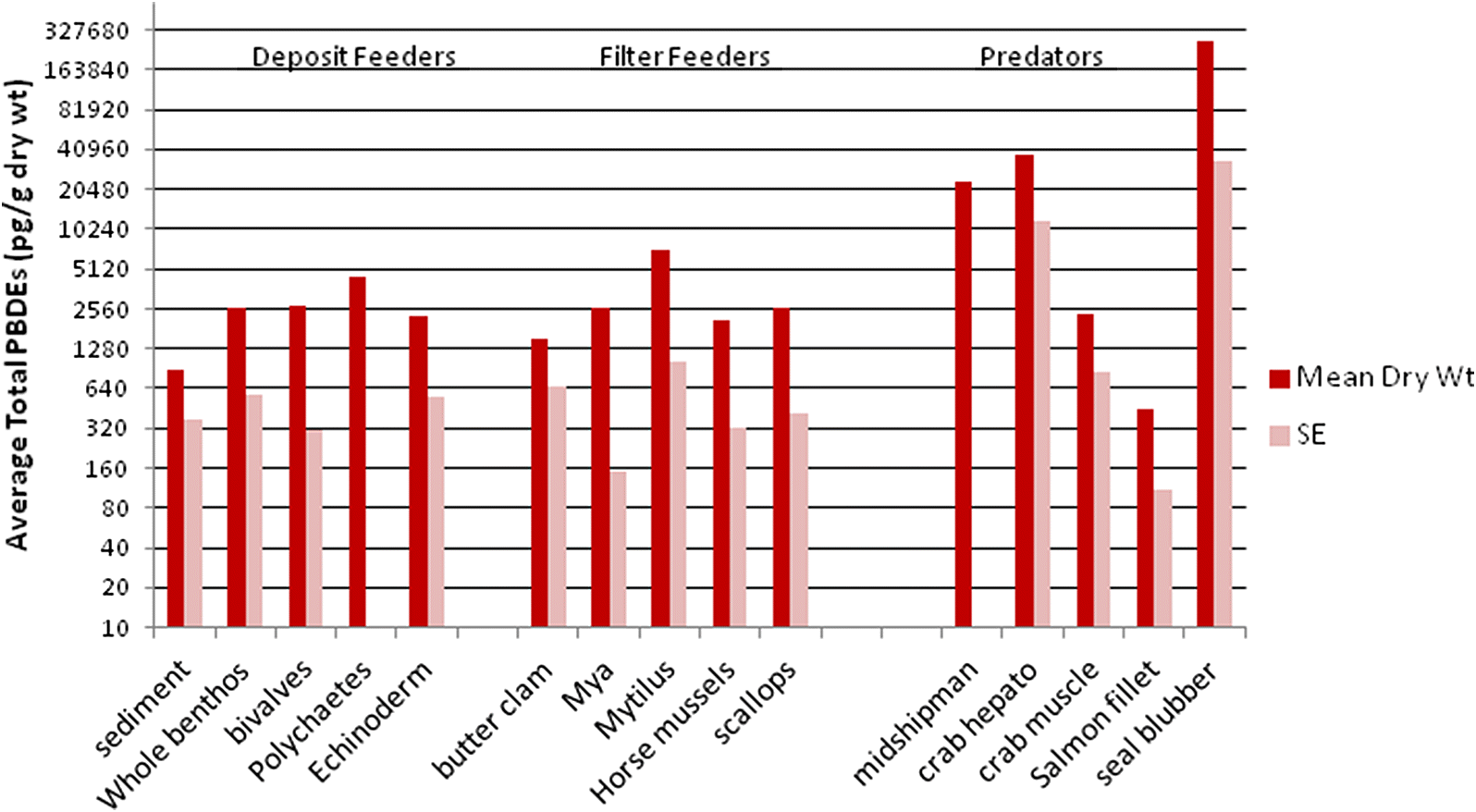
Shaw et al. 2012). In general, trophic transfer is dependent on the history of food consumption and individual mechanisms for contaminant loss. The higher total dry-weight PBDEs in benthic feeders (deposit or filter feeders) than in salmon fillets reflects the strongly particle active nature of these contaminants and, thus, their likelihood to accumulate most within organisms associated with sediments.
Li et al. (2010) also found that lagoon fish offshore of Xiamen Island in southeastern China had lower BDE (particularly deca-BDEs) concentrations than clams and crabs.
Determining trophic transfer is complicated by the logistical difficulties in the calculation of whole-body burden in large crustaceans, fish, and marine mammals. The difference in matched crab hepatopancreas and muscle PBDE concentrations (higher in dry weight but lower in lipid weight for hepatopancreas) in the current study illustrates this problem. There is very limited information on the relative concentration of PBDEs in different tissue types for higher trophic level marine animals (
Wolkers et al. 2009;
Yordy et al. 2010;
Shaw et al. 2012;
Trumble et al. 2012).
The general consensus in the literature is that PBDE accumulation is primarily lipid-mediated (
Raach et al. 2011). For that reason, trophic transfer studies typically focus on lipid-weight contaminant comparisons. Samples taken from living mammals are typically from the lipid-rich subcutaneous fat layer, as they were for seals in the current study.
Yordy et al. (2010) measured persistent organic pollutant (POP) distributions in a range of tissue types in stranded bottlenose dolphin and showed that blubber, the primary site of metabolic lipid storage, is also the primary site for POP accumulation, contributing >90% to the whole-body burden. However, they note that as lipid mobilizes from blubber, contaminants may redistribute to other tissues.
Shaw et al. (2012) showed no differences between hepatic and blubber tissue total PBDEs in stranded seals in the northwestern Atlantic, although BDE 209 was much more concentrated in liver than in blubber.
Boon et al. (2002) found generally higher PBDE concentrations in liver than blubber in North Sea marine mammals, although BDE 209 was not included in the analysis. In contrast,
Moon et al. (2010) found higher overall concentrations of PBDEs in blubber than in liver for Korean marine mammals. Based on the above examples from the literature, it is clear that PBDE distribution in different body tissues is not simply related to lipid distribution. Therefore, there is a lack of understanding of how PBDEs sequester into different tissue types in apex predators, particularly mammals.
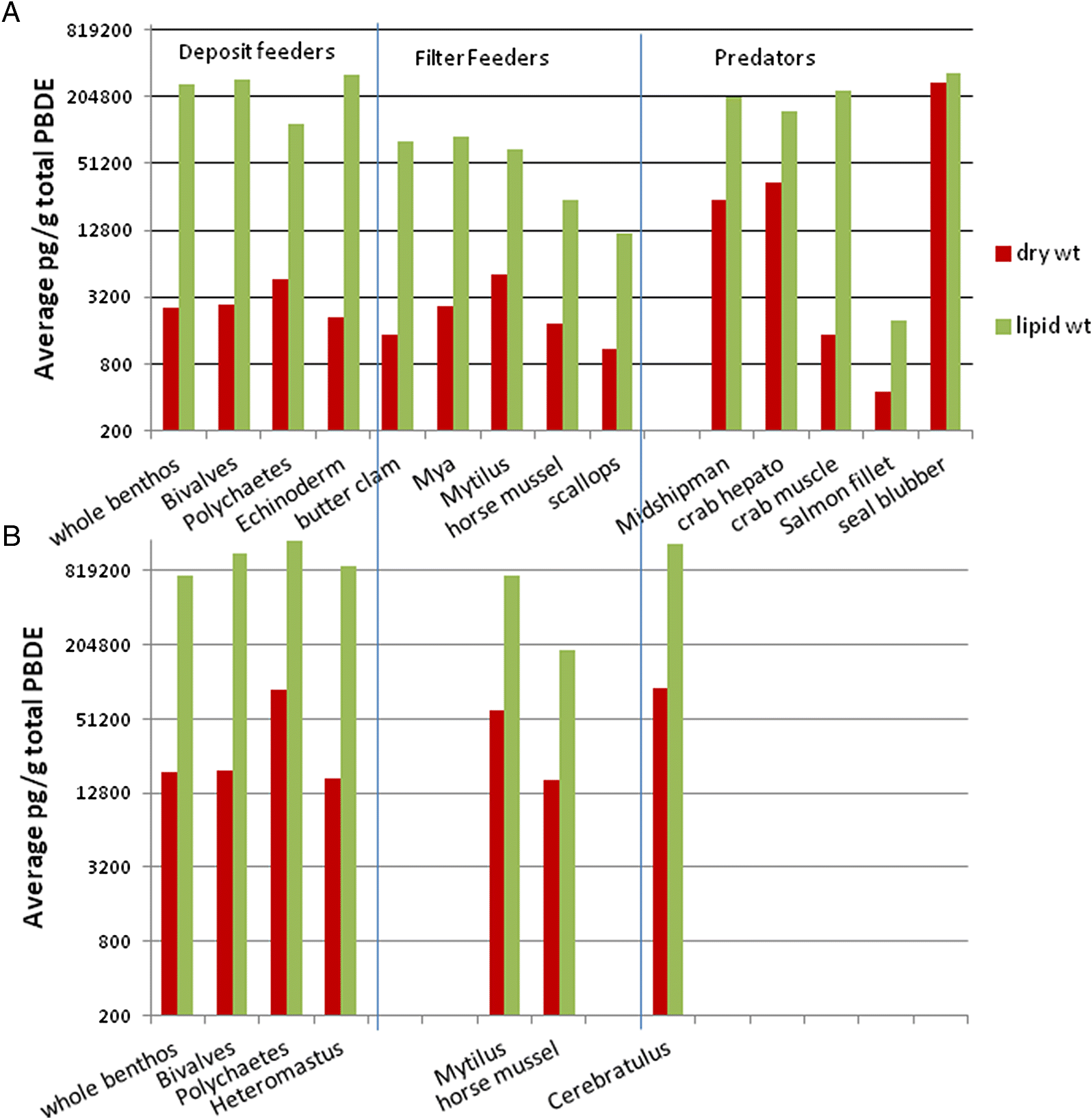
The much higher accumulation of total PBDEs (107×) on a dry-weight basis from background infaunal benthos to seal blubber was not borne out by lipid-weight PBDE accumulation (~1.3×). It is probable that the PBDE lipid accumulation rate would be still lower for total body burden in seals. This suggests a lack of lipid-normalized accumulation of total PBDEs from primary sediment feeders to higher trophic levels, but that PBDE uptake is remarkably high in the low-lipid primary sediment feeders, particularly near organic enrichment sources. The assumption of PBDE storage primarily in lipids is therefore questionable for primary consumers. It seems unlikely that the contaminants would be so concentrated in the very limited lipid reserves of these direct sediment feeders.
The filter-feeding bivalves (rock scallops, horse, and blue mussels) showed a much lower lipid-weight PBDE concentration than direct sediment feeders. If these organisms are more important in the diet of seals than bottom fish or crabs, then lipid-weight accumulation (about 4×) of PBDEs in seal blubber was clearly evident. All other taxa showed biodilution of lipid-weight PBDEs relative to whole benthic infauna. This low or negative trophic accumulation of PBDEs has also been noted in food webs for the Canadian Arctic (
Kelly et al. 2008). Biodilution of selected PBDE congeners has also been reported off the coast of China (
Zhang et al. 2012) for marine fish, and in mesocosm studies of benthos and carp (
Tian et al. 2010;
Tian et al. 2012). Thus, a clear understanding of food resources for higher trophic fauna is required to understand bioaccumulation of persistent contaminants. The results of the current study suggest that biomagnification of total PBDEs may not be occurring in the organisms tested in this study, but rather, lipids become an increasingly prominent PBDE storage component at higher trophic levels.
PBDE congener homologue groups
The rate of debromination of PBDEs in sediments or water is positively correlated with level of bromination (
Keum and Li 2005; see also
Shaw et al. 2012), so that lower-brominated forms break down more slowly than higher ones. Although BDE 209 initially debrominates quickly with fresh deposits in sediments, this process slows down considerably (
Keum and Li 2005) and stops after a period of time (
Gerecke et al. 2005). Debromination in sediments seems to occur in a step-wise pattern culminating in the highly toxic tetra forms (
Lee and He 2010), and can be enhanced by reducing agents such as iron-bearing minerals and sufides (
Young et al. 2005), low redox (
Keum and Li 2005), anaerobic bacteria (
Gerecke et al. 2005;
He et al. 2006;
Lee and He 2010) and photolysis (
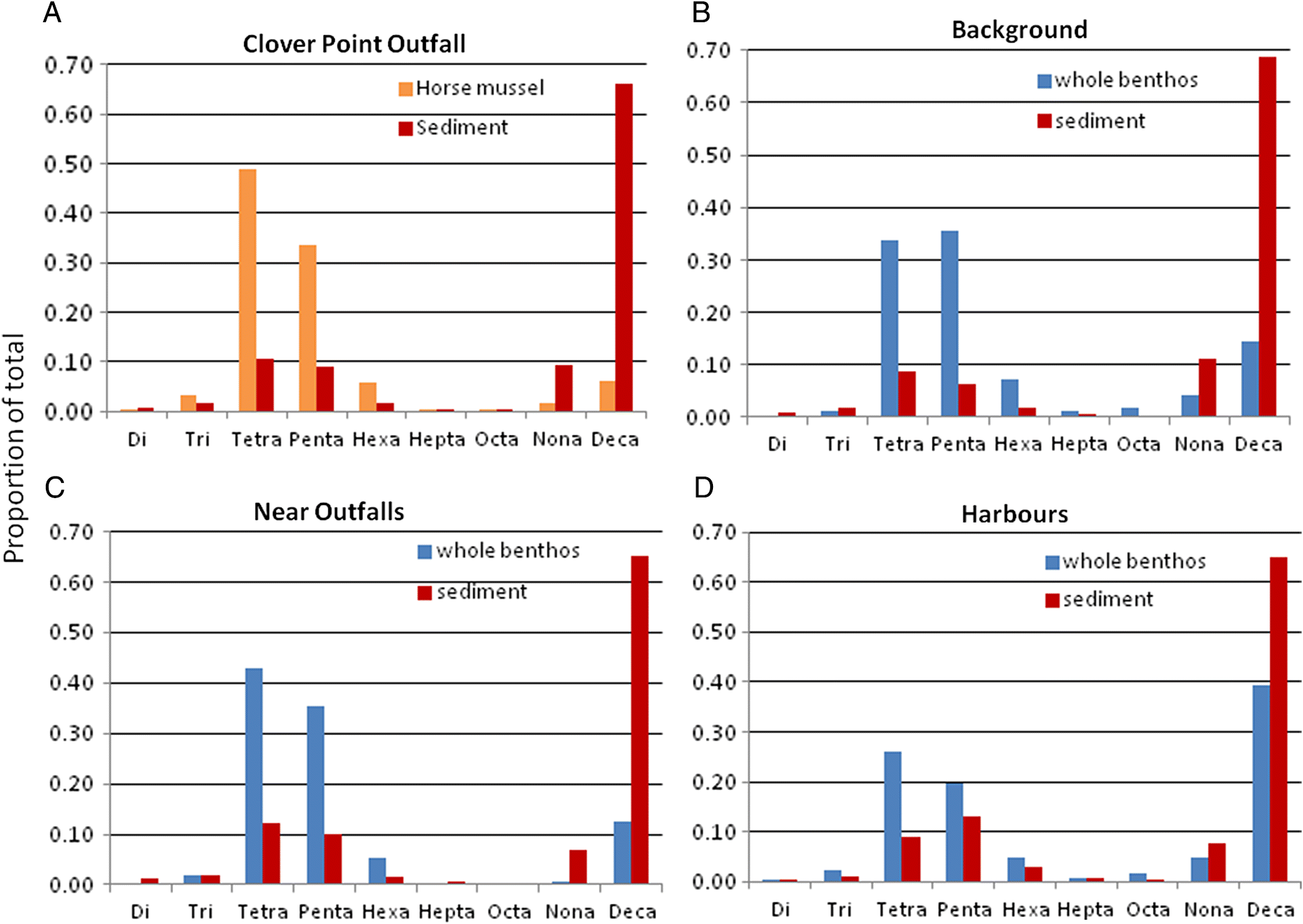
Wei et al. 2013). Thus BDE 209 tends to be fairly stable in sediments under aerobic, subtidal conditions (as in background samples in the current study). In support of this supposed stability, the proportional composition of sediment PBDE homologue groups in the current study was very similar near municipal wastewater outfalls, in harbours, and in background areas. This does not suggest that significant debromination occurred with distance from source in southern BC (see also
Johannessen et al. 2008;
Grant et al. 2011).
Most tissue accumulation studies for PBDEs focus on the kinetic properties of various-sized molecules and their hydrophobicity to explain uptake dynamics of different PBDE congeners into various faunal types and tissues. Tissue accumulation of different molecular weight (degree of bromination) PBDEs has typically been related to octanol/water partitioning (
Kow) in many taxa and ecosystems (
deBruyn et al. 2009;
Tian et al. 2012;
Frouin et al. 2013;
Alava et al. 2016).
Kelly et al. (2004,
2008) questioned the use of log
Kow for predicting uptake potential of hydrophobic organic compound groups in Arctic food chains, as error levels and variability in observations are often high, and other factors besides kinetic properties of the compounds are clearly important (see also
Magnusson and Tiselius 2010;
Klosterhaus et al. 2011). In a passive physical system, log
Kow clearly affects contaminant uptake from water into lipids (
Macdonald et al. 2002). However, we still don’t understand how these contaminants are processed in the guts of most marine organisms. For example, rapid debromination of higher brominated congeners to lower, more recalcitrant ones (likely in the gut) tends to give the mistaken impression that the larger compounds are less easy to assimilate than smaller ones (
Munschy et al. 2011). In addition, age (size) appears to affect congener balance in Dungeness crabs in BC (
Ikonomou et al. 2006), suggesting that further debromination may occur in tissues over time. Without a clear understanding of internal processes in all taxon groups, it is not possible to predict uptake rates of different congener groups from kinetic properties of molecules. We can only measure the ultimate balance of them in tissues and hope to empirically determine internal and external conditions in biota and tissues that mediate this balance.
In addition, studies that selectively examine only a few congeners are unlikely to determine the full bioaccumulation potential of PBDEs. Tissue PBDEs are a mixture of non-degradable congeners (especially penta 99 and tetra 47—see
He et al. 2006) which cannot be excreted, congeners that are more readily metabolized and excreted (low-brominated congeners—
Shaw et al. 2009), and congeners that debrominate during trophic transfer. Metabolic degradation of PBDEs in living tissue is expected to be due to oxidation or ether-bond cleavage that does not seem to occur in sediment-bound PBDEs (
Keum and Li 2005) and appears to be limited in marine taxa. Some tissue debromination has been found in freshwater and marine fish (
Shaw et al. 2009;
Munschy et al. 2011;
Zeng et al. 2013) and in crabs (
Ikonomou et al. 2006), and hepatic debromination of 6%–25% of BDE 209 has been found in marine mammals (
McKinney et al. 2011). The absence of BDE 209 in crab hepatopancreas but not muscle in the current study is suggestive of greater or more rapid debromination within the hepatopancreas than the muscle, and highlights the aforementioned problem of comparing contaminant levels in different tissue types for large taxa.
In the current study, it appears that most of the debromination and (or) differential concentration of the highly brominated congener groups occurs with initial uptake of sediment PBDE in the guts of sediment feeders. This process is likely mediated by anaerobic microbes (
Gerecke et al. 2005;
He et al. 2006;
Lee and He 2010). Debromination of BDE 209 in aquatic organisms to congeners not present in the habitat matrix has been demonstrated (
La Guardia et al. 2007), and shown to be strongest in primary consumers (
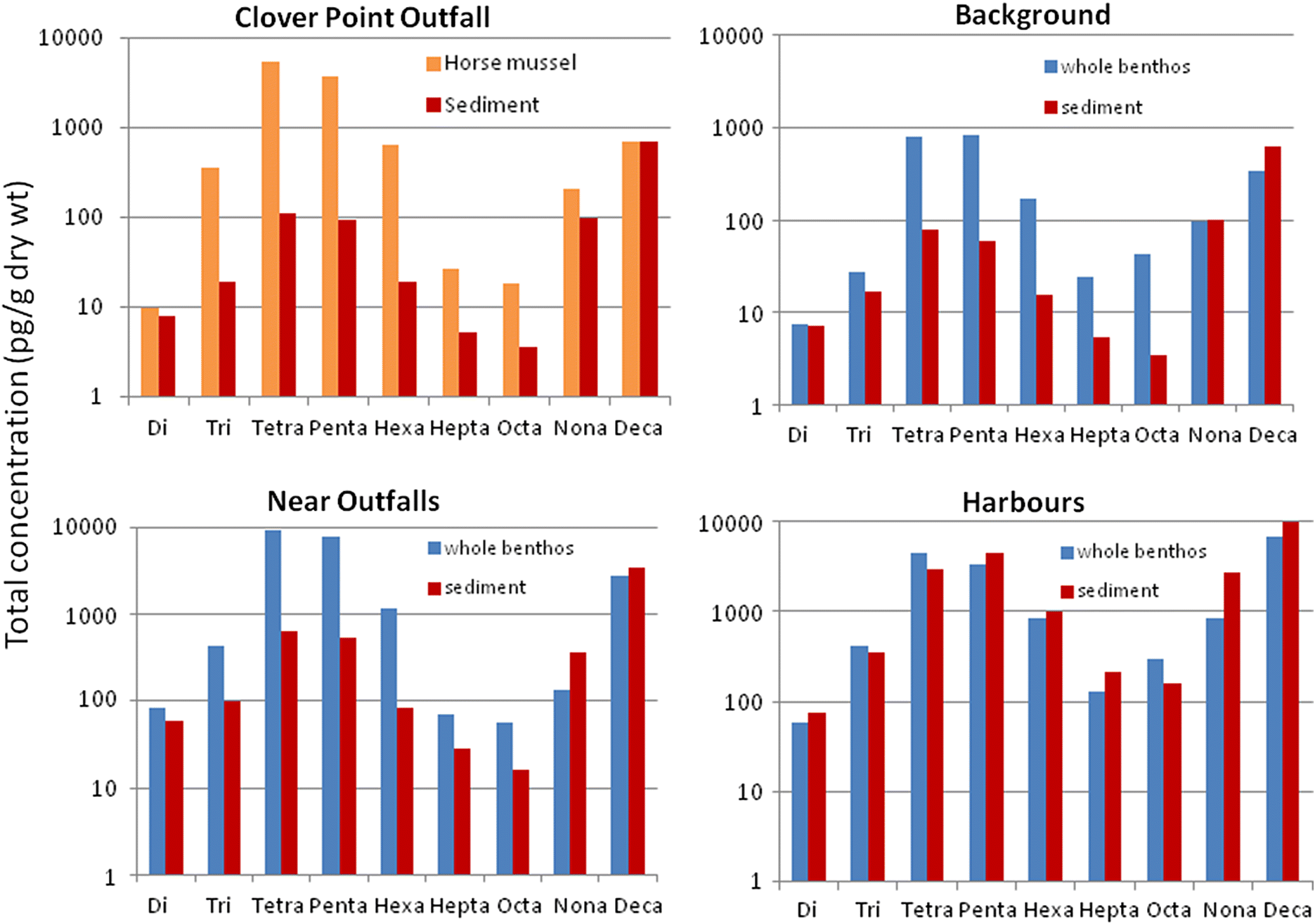
Bartrons et al. 2012). In the current study, concentrations of deca-BDE and nona-BDE were very similar between sediments and sediment feeder tissues regardless of the location; however, the relative proportion of these congener groups was markedly lower in sediment feeders than matched sediment samples, in conjunction with a 50- to >200-fold increase in proportion of other congener groups (tetra-, penta-, and hexa-BDEs).
The sediment feeders near municipal wastewater outfalls and in background locations tended to have the same general proportions of all congener groups. However, in the harbour sediment feeders, there were higher proportions of deca-BDE in tissues and an overall similar proportional configuration to sediments. This suggests that uptake of PBDEs may be much more rapid in the harbours than in other habitats because of very high sediment PBDE concentrations; thus, intake may overtake the rate of elimination and debromination. Considering the biodilution evident in the total PBDE uptake in the harbour sediment feeders, it appears that the PBDEs in intensely contaminated harbour sediments mostly pass through the guts of the sediment feeders. Laboratory studies of deca- and nona-BDE uptake and elimination in a marine oligochaete show that these congeners accumulate in tissues initially, then stop accumulating at a much lower concentration than in the sediments after a few weeks, and rapidly decline further during depuration (
Tian and Zhu 2011).
The proportional loss of nona- and deca-BDEs relative to sediment occurs in all organisms. This pattern becomes more extreme, along with increasing tetra–hexa congener proportions in crab, some salmon, and seal blubber samples. This could be due to ongoing debromination in the gut at each trophic level, as well as excretion or metabolization of nona- and deca-BDEs.
Deca-BDE is assumed to be poorly absorbed in the gut of marine mammals, but this was not found during a specific food uptake study by
Thomas et al. (2005). In addition, once it migrates to blubber it tends to be persistent (
Thomas et al. 2005), as found in some seal blubber samples in the present study. Certainly, the seal blubber, crab muscle, and salmon samples had the broadest range of deca-BDE, as well as the most pronounced and variable accumulation of tetra- and penta-BDEs, whereas the crab hepatopancreas samples were consistently lacking in deca-BDE. The high deca-BDE in seal, crab, and salmon group 2 samples (
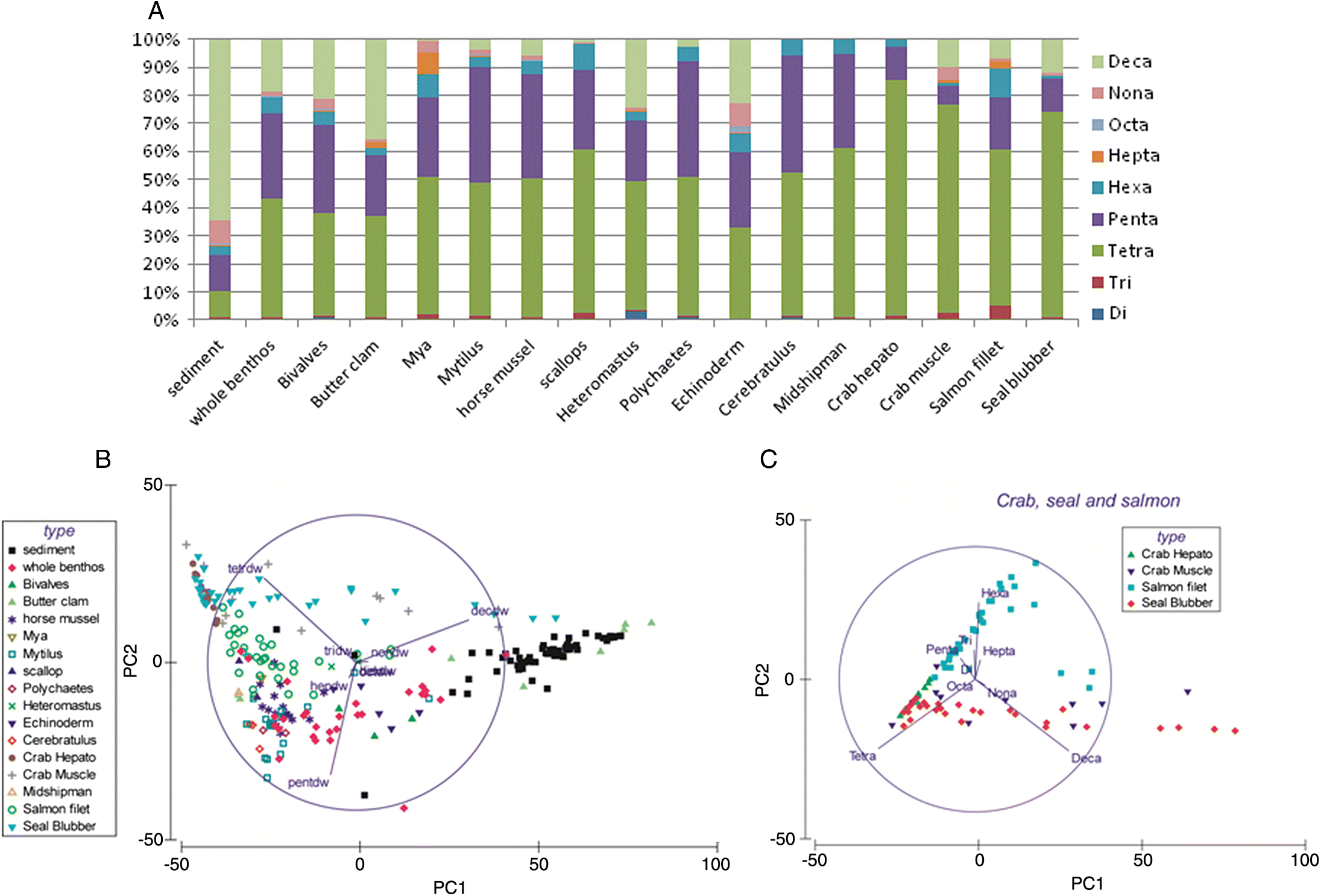
Fig. 9) and strong between-species correlations for congener accumulation patterns (
Table 5) suggests that these higher trophic organisms share common food sources, which are likely benthic.
Shaw et al. (2009,
2012) also suggested that the measureable BDE 209 levels in Atlantic seals and fish reflect a strong benthic connection in the food web.
A comparison of sample locations does not suggest that deca-BDE accumulation in the crab, seal, or salmon samples was related to local discharge sources of PBDEs or lipid content of tissue samples. In other words, different samples of crab, salmon, or seal from the same location and sample batch had highly variable deca-BDE proportions. Other studies have shown that tissue BDE 209 concentrations in marine taxa can vary seasonally, with age, sex, and reproduction (
Ikonomou et al. 2006;
Wolkers et al. 2009;
Mongillo et al. 2012), resulting in a broad range in the proportion of BDE 209 between and within faunal types, particularly in the longer-lived higher trophic level taxa (crab, salmon, and seal). In addition, further variability may arise because there is a high probability that BDE 209 preferentially migrates to certain tissue types (
Munschy et al. 2011;
Shaw et al. 2012), where it may debrominate faster. A kinetic model of combined debromination, methoxylation, ether-bond breakage, excretion and reproductive transfer remains to be formulated for any marine taxon groups.
The large increase of tri- to hexa-BDE proportions and concentrations in sediment feeders relative to sediments is unlikely due simply to lipid concentration over time, as most of these sediment feeders are short-lived (usually annual or biannual) and have very low lipid content (see
Table S3). The dry weight tetra-BDEs accumulated in whole benthos at a far higher rate than all other congener groups (about 229×), which is about 3× higher than for total PBDEs. A simple summation suggests that potential debromination of all higher congeners can account for this gain in tetra-BDE. This tetra-BDE accumulation, closely followed by penta-BDE, has been described by many researchers for a wide variety of heterotrophs (e.g.,
Shaw et al. 2009;
Hallanger et al. 2011;
Bartrons et al. 2012;
Frouin et al. 2013), including bacteria (
Lee and He 2010).
The extreme proportional increase in tetra-BDEs and decline in penta-BDEs in crab and seal blubber samples relative to the sediment feeders may be related to selective debromination of the penta-BDEs (particularly BDE 99) and hexa-BDEs to tetra-BDE (particularly BDE-47) in the guts of these organisms (
Guo et al. 2007;
Ikonomou et al. 2011), and relative lack of debromination in tetra-BDEs in higher trophic level taxa.
Shaw et al. (2009) indicated that penta-BDE 99 debrominates to tetra BDE 47 in the guts of some fish (
Stapleton et al. 2004), or may be preferentially excreted (
Isosaari et al. 2005). This tetra/penta debromination dynamic is described for an Arctic food chain by
Kelly et al. (2008). These studies support the contention that the tetra-BDEs are a recalcitrant “dead-end” in the metabolic processing of PBDEs in marine organisms.