Introduction
Amphibian species around the globe are facing alarming population declines that also present much broader ecological consequences for the delicate ecosystems they inhabit (
Halliday 2008). Recent estimates report close to 40% of known species are experiencing ongoing declines (
Scheele et al. 2019) due in large part to emerging infectious agents such as the chytrid fungus
Batrachochytrium dendrobatidis (
Bd) and ranaviruses (
Stuart et al. 2004;
Hayes et al. 2010). Because
Bd invades keratinized skin, it predominantly affects the adult anuran frogs, whereas anuran tadpole infections are typically nonlethal and are limited to keratinized mouthparts (
Berger et al. 1998;
Rachowicz and Vredenburg 2004). Conversely, while both tadpole and adult anurans may be infected by ranaviruses like Frog Virus 3 (FV3), tadpoles and metamorphic animals are particularly susceptible and often succumb to these infections (
Green et al. 2002;
Duffus and Cunningham 2010). Animal contact is presently thought to be the predominant means of ranavirus transmission (
Brunner and Yarber 2018), and we have recently shown that the tadpole skin is an important point of ranaviral entry and a site for viral replication (
Wendel et al. 2017).
Amphibian skin mucosa undoubtedly serves as a crucial barrier against, and a major point of contact with, their pathogen-rich environments (
Colombo et al. 2015). Moreover, it is indispensable to amphibian water, electrolyte, and gas exchange (
Feder and Burggren 1992). Although the integrity of the amphibian skin is intimately linked to the health of these animals, the immune cell composition of amphibian cutaneous tissues remains relatively unexplored. Some frog species possess cells resembling mammalian dendritic and Langerhans cells (
Carrillo-Farga et al. 1990;
Castell-Rodríguez et al. 2001;
Mescher et al. 2007), macrophages, and lymphocytes (
Mescher et al. 2007). Granulocytes have also been found in amphibian skin tissues but typically only during times of heightened immune response like infection and injury (reviewed in
Grogan et al. 2018).
Granulocyte-lineage mast cells are unique in that they are found within mammalian skin in nonpathological contexts (
Galli and Wershil 1996;
Baccari et al. 2011). They are also known to populate avian, reptilian, and amphibian skin tissues, although in far fewer numbers (
Pelli et al. 2007;
Baccari et al. 2011). In more recently diverged vertebrates such as mammals, mast cells are particularly important to immune defenses at primary sites of contact with the environment or pathogens, such as mucosal tissues and skin (
Piliponsky et al. 2019). Both their prominent antiviral (
Kurane et al. 1984) and antifungal (
Nieto-Patlán et al. 2015) capacities are facilitated by numerous compounds stored within their granules. These include heparin and histamine, which are responsible for the characteristic metachromatic granule staining, along with substance P, serotonin, and lectins, which contribute to these cells’ ability to destroy pathogens and recruit other immune effectors (
Baccari et al. 2011;
Sridharan and Shankar 2012). However, much less is known about the precise function of amphibian mast cells in this context and rather, the majority of research has instead focused on mast cells localized in the amphibian tongue and nervous system (
Baccari et al. 2011).
We recently characterized several aspects of amphibian granulopoiesis, including roles of the granulocyte chemokine CXCL8 (also known as interleukin-8; IL-8) isoforms in the recruitment of tadpole and adult frog granulocytes (
Yaparla et al. 2016;
Koubourli et al. 2017,
2018). During those studies, we noted prominent expression of several granulocyte-specific genes, including the
cxcl8a and
cxcl8b chemokines as well as the granulocyte growth factor, granulocyte colony-stimulating factor (
gcsf, also known as
csf3) and its receptor and granulocyte marker (
gcsfr, also known as
csf3r) in the skin of healthy tadpole and adult
Xenopus laevis. We examined the presence of mast cells as well as a nonmast cell resident granulocyte population within the skin of
X. laevis tadpoles and adult frogs. Based on our previous findings and given the substantial gaps in the literature, we further sought to address possible roles of these skin immune purveyors in antimicrobial defenses of these animals. The present work contributes to the understanding of the cells that maintain amphibian skin homeostasis and protect these animals from invading pathogens, an imperative endeavor as we look for new conservation approaches.
Materials and methods
Animals
Nieuwkoop and Faber (NF) (
Nieuwkoop and Faber 1975) stage 54 outbred tadpoles and approximately 1-year-old, mixed-sex adult
X. laevis were purchased from Xenopus 1 (Dexter, Michigan, USA). All animals were housed and handled under strict laboratory regulations of the Animal Research Facility at the George Washington University (GWU) and as per the GWU Institutional Animal Care and Use Committee regulations (Approval number 15-024).
Cell culture maintenance
All cell cultures were established in amphibian serum-free medium supplemented with 10% fetal bovine serum, 0.25%
X. laevis serum, 10 μg/mL gentamicin (Thermo Fisher Scientific, Waltham, Massachusetts, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco, Thermo Fisher Scientific). Amphibian phosphate buffered saline (A-PBS) has been previously described (
Koubourli et al. 2017).
Production of recombinant frog cytokines and chemokines
The production of recombinant granulocyte colony-stimulating factor (rG-CSF), rCXCL8a, rCXCL8b, and the recombinant control (r-ctrl) was described by
Koubourli et al. (2018) and
Yaparla et al. (2018). In brief, the recombinant cytokines were produced by generating expression constructs containing the signal peptide-cleaved representations of the respective cytokine cDNAs in the pMIB/V5 His A insect expression vector (Invitrogen, Carlsbad, California, USA) backbone and transfecting these into Sf9 insect cells (Cellfectin II, Invitrogen). Positive transfectants were scaled up to 500 mL cultures, grown for 5 d, and the recombinant proteins isolated from the supernatants of these cultures using Ni-NTA agarose (Qiagen, Hilden, Germany) columns as described by
Koubourli et al. (2018) and
Yaparla et al. (2018). The r-ctrl was produced by transfecting Sf9 cells with an empty pMIB/V5 His A insect expression vector (Invitrogen) and processing the supernatants from the resulting transfected cultures using the same methodology as described for the above cytokines.
Anti-X. laevis granulocyte colony-stimulating factor receptor (G-CSFR) polyclonal antibody
The production of the polyclonal rabbit IgG against the recombinant
X. laevis G-CSFR was described by
Koubourli et al. (2018). In brief, a recombinant
X. laevis G-CSFR (1 mg total) was generated and submitted for rabbit immunization protocols (ProSci Inc., Fort Collins, Colorado, USA). The resulting rabbit immune serum was assessed by western blot against the rG-CSFR and applied to a HiTrap Protein A HP column (GE HealthCare Life Sciences, Marlborough, Massachusetts, USA) to isolate the IgG fraction. A Sulfo-Link Protein Kit (Thermo Fisher Scientific) was then used according to the manufacturer’s instructions to purify the IgG fraction that cross-reacted with the rG-CSFR.
Granulocyte enrichment, isolation, and pharmacological depletion in X. laevis
Tadpole and adult frog granulocytes were generated as previously described by
Koubourli et al. (2017). Briefly, rG-CSF in A-PBS (10 μL final volume) was pipetted onto parafilm, taken up with finely pulled glass needles and injected into the peritonea of tadpoles (1 μg/tadpole) and adult frogs (5 μg/frog). Twenty-four hours following injection, total peritoneal leukocytes were lavaged with 2 × 25 μL volumes of A-PBS and subsequently enumerated via a hemocytometer using a 1:1 dilution of cell suspension to 0.4% Trypan Blue (Sigma-Aldrich, St. Louis, Missouri, USA) to account for dead and dying cells.
To enrich the frog skin granulocytes, tadpoles and adult frogs were respectively injected subcutaneously with 0.5 or 2.5 μg of rCXCL8a, rCXCL8b or equal volumes of r-ctrl in 5 μL of A-PBS using finely pulled glass needles. Skins were collected for further analysis 24 h postinjection.
The tadpole and adult skin granulocyte numbers were pharmacologically reduced by subcutaneously injecting animals with 50 mg/kg of reparixin (CXCR1/2 inhibitor; MedChemExpress, Monmouth Junction, New Jersey, USA) in 5 μL of A-PBS using finely pulled glass needles and a 6 h time of treatment, established during preliminary experiments.
Frog Virus 3 stocks and infections
Frog Virus 3 (FV3; wild type, ATCC VR-567) production was previously described by
Morales et al. (2010). In brief, baby hamster kidney (BHK-21) cells were infected with FV3 (multiplicity of infection; MOI: 0.1), grown at 30 °C and 5% CO
2 for 5 d. The FV3-containing supernatants were collected over 30% sucrose by ultracentrifugation, resuspended in saline and the viral titers were determined by plaque assay over BHK-21 cells.
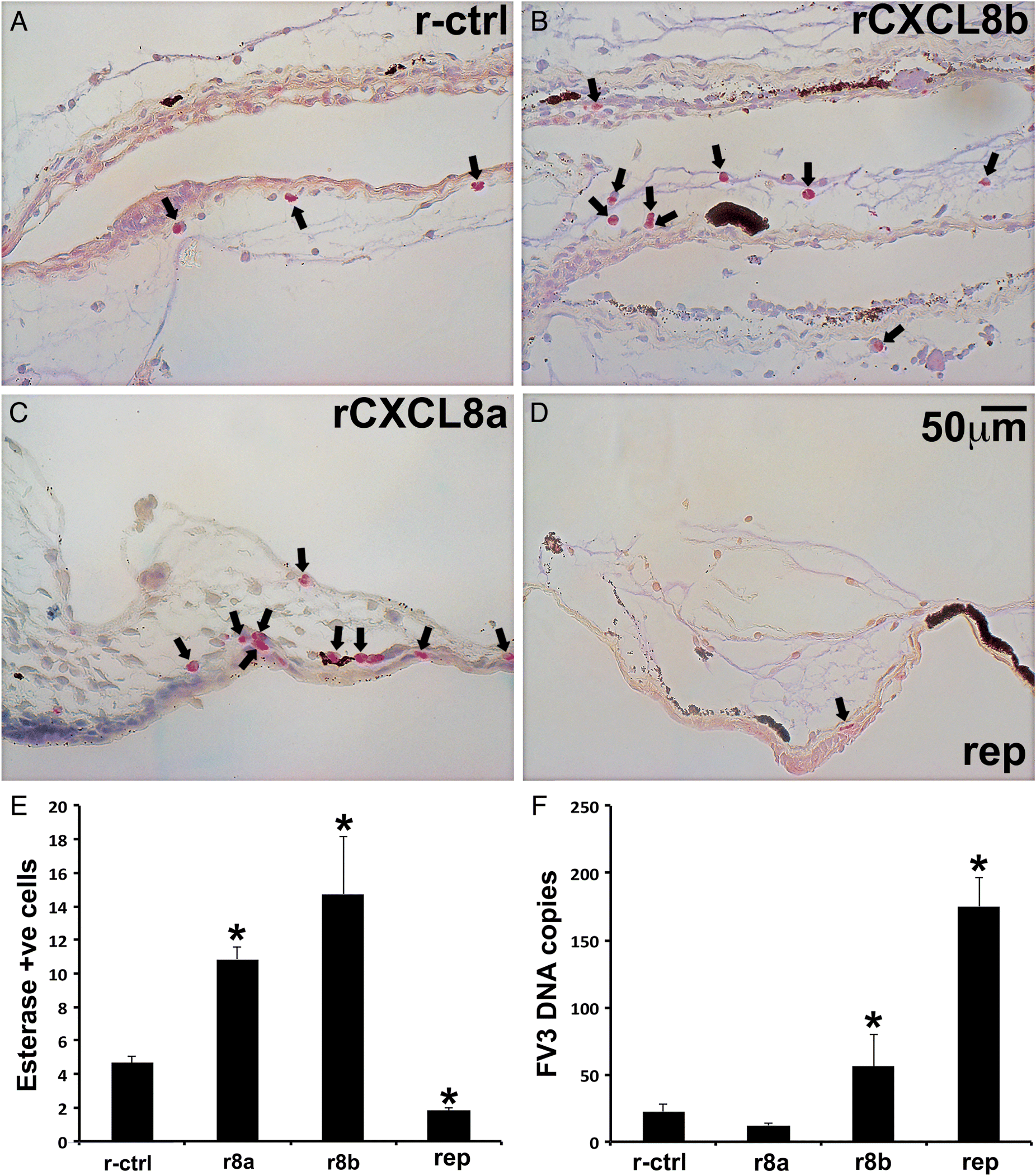
Tadpole (stage NF 54–56) skin granulocytes were manipulated as described above, at which time (24 h post-CXCL8a/b injection or 6 h postreparixin injection) the animals were challenged for 1 h in a 100 mL water bath containing 1 × 106 plaque forming units (PFU) (i.e., 1 × 104 PFU FV3/mL water). Animals were then washed in FV3-free water and housed for an additional 24 h, euthanized by tricaine mesylate (TMS) overdose and the dorsal skin tissues collected for histology or RNA/DNA isolation, as described below.
Batrachochytrium dendrobatidis stocks and infections
Batrachochytrium dendrobatidis (
Bd isolate JEL 197; a kind gift from Dr. Louise Rollins-Smith) was grown in 1% tryptone broth or on 1% tryptone agar plates (Difco Laboratories, Detroit, Michigan, USA;
Flechas et al. 2019), supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco) at 21 °C.
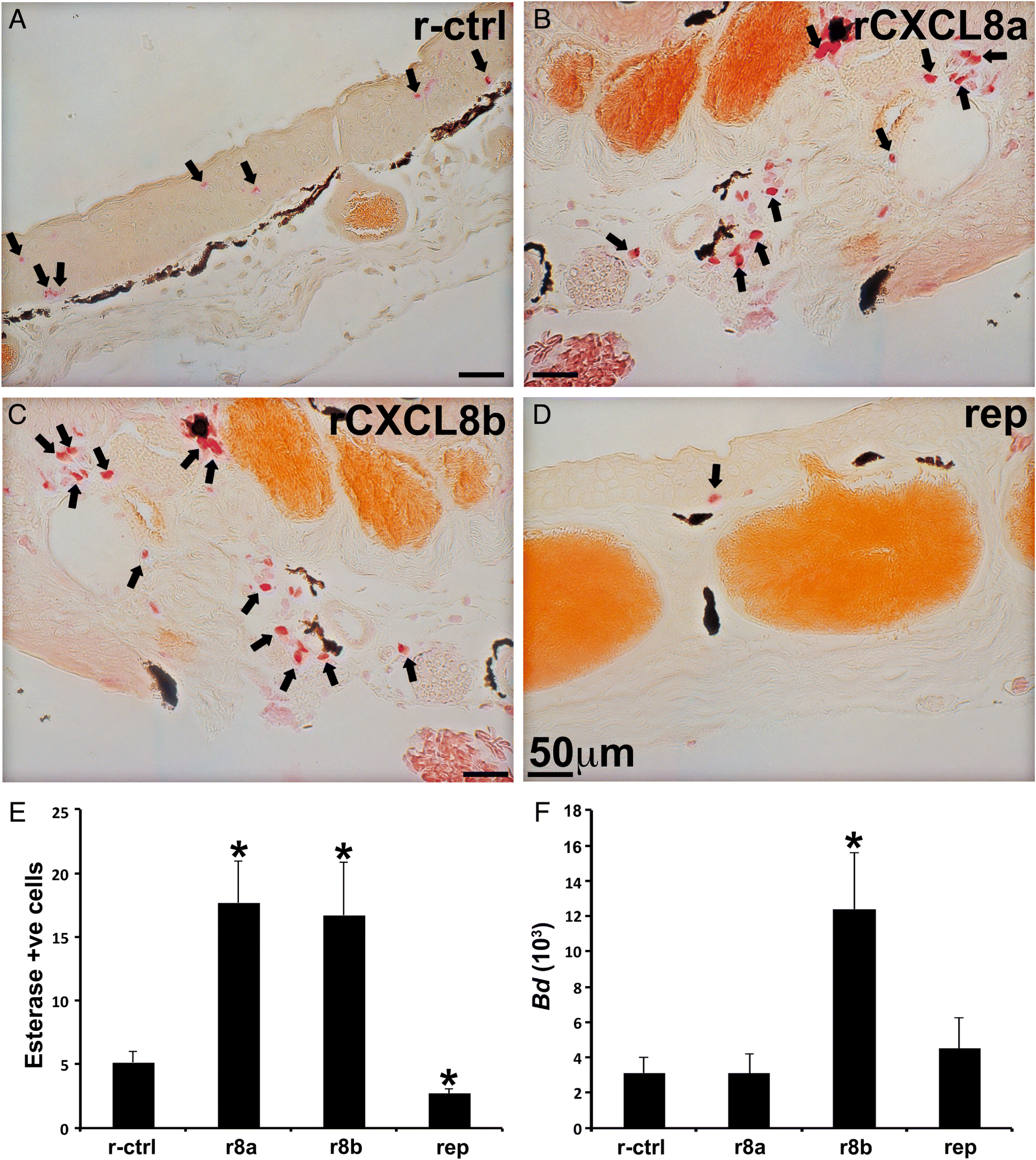
For Bd infection studies, confluent Bd-tryptone plates were flooded with water to release zoospores, which were enumerated and used for water bath infections. Adult frog (approximately 1 year old) skin granulocytes were manipulated as described above, and the animals were challenged with 107 zoospores of Bd in 100 mL of water. After 3 h, an additional 400 mL of water was added to frog tanks and the animals were housed for an additional 7 d before being euthanized and their skins processed for histology or RNA/DNA isolation, as described below.
Acquisition of X. laevis skin tissue for chemotaxis assay and gene expression
Tadpole and adult X. laevis were euthanized by TMS overdose and 5 mm2 sections were removed from their dorsal skin surfaces using sterilized forceps and scissors for chemotaxis and gene expression assays. Dorsal skin was removed in the same manner for histology. Skin samples were used immediately after transection for chemotaxis assays, placed in Trizol reagent (Invitrogen) for gene expression analyses, or placed in 10% neutral buffered formalin (VWR, Radnor, Pennsylvania, USA) for histology fixation.
Chemotaxis assays
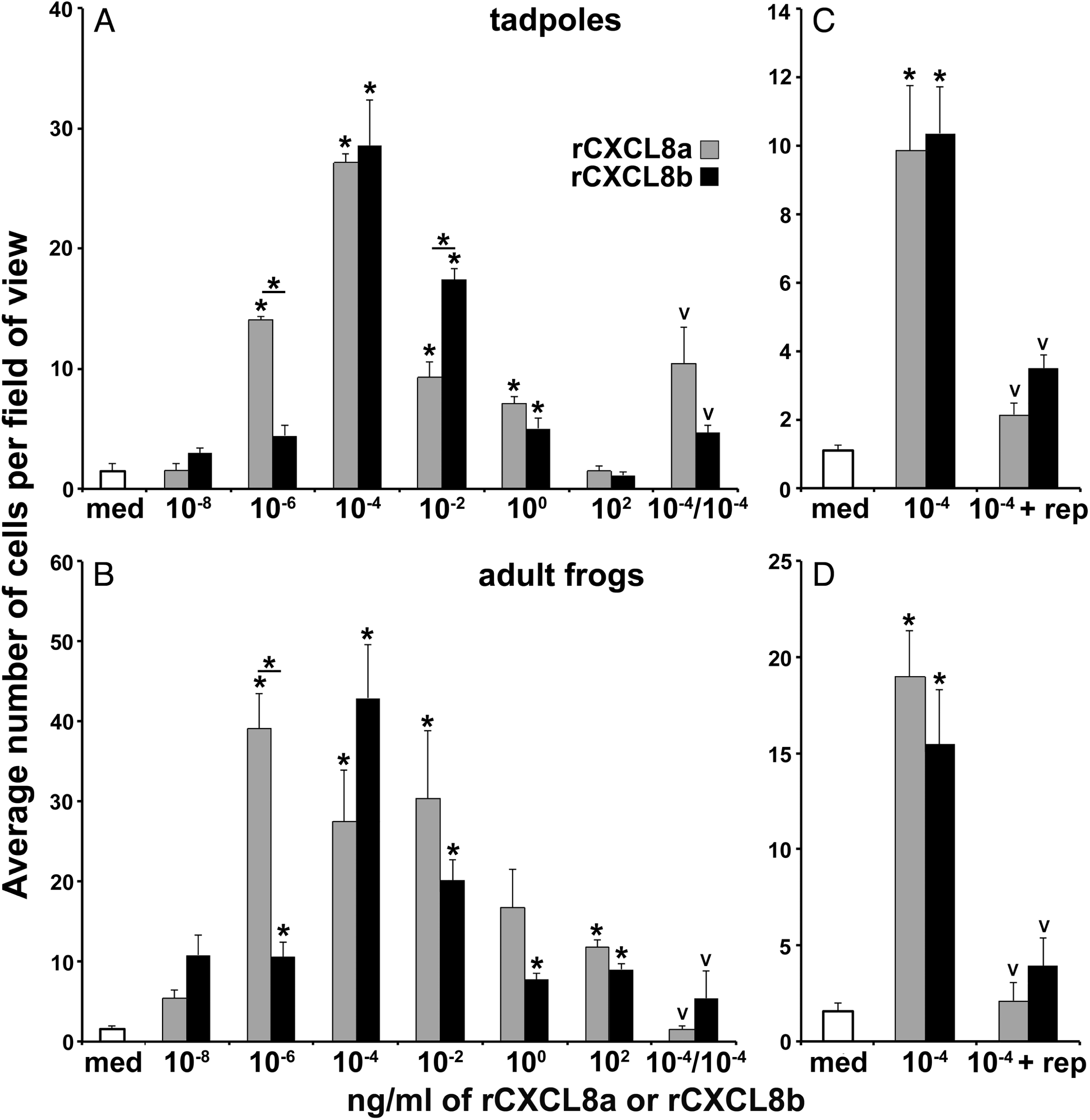
All chemotaxis assays were performed using blind well Boyden chambers (Neuro Probe, Gaithersburg, Maryland, USA). The bottom wells were loaded with culture medium alone or medium containing 102–10−8 ng/mL of rCXCL8a or rCXCL8b in 100 μL of medium. The wells were then overlaid with 13 mm chemotaxis filters (5 μm pore size; Neuro Probe) and tadpole (N = 3–6 per chemokine concentration) or adult frog (N = 3–6 per chemokine concentration) skin sections (obtained as detailed above) were oriented in the top wells such that the dermal layer was closest to the filter in 100 μL medium. The tadpole and adult frog skin granulocyte chemotaxis towards the most chemo-attractive concentrations of rCXCL8a and rCXCL8b was confirmed three times, independently.
After 3 h of incubation at 27 °C with 5% CO2, the top layers were aspirated and the topsides of the filters were wiped with cotton swabs. The bottom faces of the filters were then stained with Giemsa (Gibco, Thermo Fisher Scientific) and migrating cells quantified by counting 10 random fields of view per filter (40× objective).
The chemokinesis (gradient-independent cell migration) experiments were performed by loading 10−4 ng/mL of rCXCL8a or rCXCL8b (the most chemo-attractive concentration) to both the bottom and the top wells of the chemotaxis chambers, in conjunction with tadpole or adult frog skin sections in medium in the top wells. After 3 h of incubation, the migrating cells were enumerated as above.
The CXCR1 and CXCR2 inhibition studies were carried out using reparixin (MedChemExpress). The optimal dose (10−4 ng/mL) of rCXCL8a or rCXCL8b was loaded to bottom chemotaxis chambers and tadpole or adult skin sections in conjunction with reparixin (1 nM final) in the upper chambers. After 3 h of incubation, the migrating cells were enumerated as above.
For all gene expression and pathogen exposure studies, 10−4 ng/mL of rCXCL8a or rCXCL8b was used to chemo-attract cells out of the tadpole or adult skin sections (N = 5–6 per chemokine, 5 mm2). The cells were collected from bottom wells of the Boyden chambers and enumerated by hemocytometer counts using a 1:1 dilution of cell suspension in A-PBS to 0.4% Trypan Blue (Sigma-Aldrich, St. Louis, Missouri, USA) to account for dead cells.
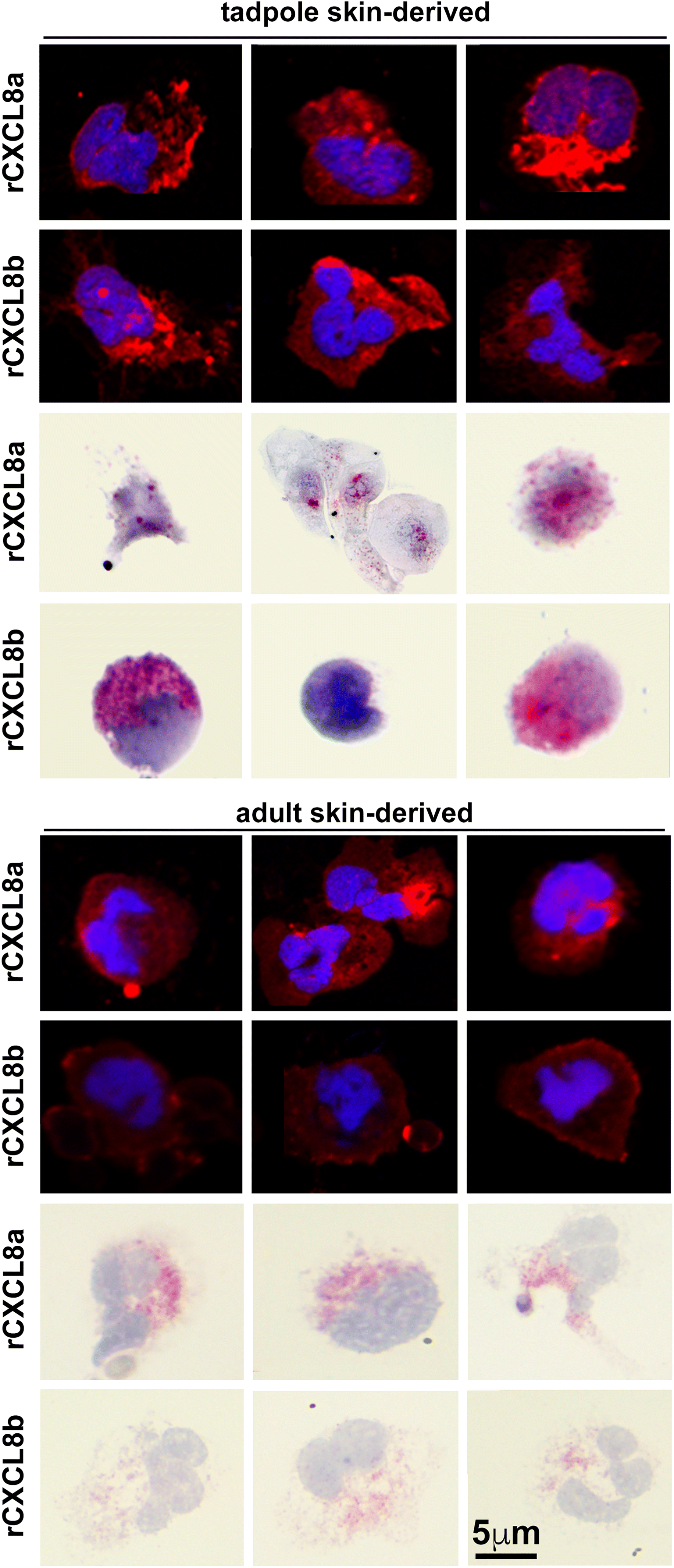
For anti-G-CSFR and esterase activity analyses of the tadpole and adult skin granulocytes, cells isolated from six individual tadpole or adult skins (from six bottom chemotaxis chambers) were combined and cyto-centrifuged onto glass slides. The cells were then stained with a polyclonal rabbit anti-rG-CSFR IgG (2.5 μg total) overnight at 4 °C and goat antirabbit IgG Dylight 488 (ThermoScientific) secondary antibodies and counterstained with Hoechst nuclear stain (ThermoScientific). Alternatively, the cells were stained using a naphthol AS-D chloroacetate specific esterase (NASDCl-esterase; Leder) kit (Sigma-Aldrich, St. Louis, Missouri, USA) according to the manufacturer’s directions.
RNA and DNA isolation, cDNA synthesis, and quantitative gene expression analyses
For all experiments, tadpole and adult frog cells or tissues were homogenized in Trizol reagent (Invitrogen), flash frozen on dry ice, and stored at −80 °C until RNA and DNA isolation in accordance with manufacturer’s directions. The isolated (phenol and chloroform extraction method) RNAs (500 ng total) were reverse transcribed into cDNAs using cDNA qscript supermix (Quantabio, Beverly, Massachusetts, USA), according to manufacturer’s instructions.
DNA was isolated from the Trizol following RNA isolation. In brief, following phase separation and extraction of RNA, the remaining Trizol layer was mixed with back extraction buffer (4 M guanidinethiocyanate, 50 mM sodium citrate, 1 M Tris pH 8.0) and centrifuged to isolate the DNA-containing aqueous phase. The DNA was precipitated overnight with isopropanol, pelleted by centrifugation, washed with 80% ethanol, and resuspended in TE buffer (10 mM Tris pH 8.0, 1 mM EDTA). DNA was then purified by phenol–chloroform extraction and resuspended in molecular grade water.
All quantitative analyses of X. laevis gene expression were performed using the CFX96 Real-Time System (Bio-Rad Laboratories, Hercules, California, USA) and iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories). The Bio-Rad CFX Manager software (SDS) was employed for all expression analysis. All expression analyses were conducted using the ΔΔCt method relative to the gapdh endogenous control gene.
The FV3 and Bd loads were determined using absolute qPCR, performed on isolated DNA and using serially diluted FV3 or Bd standard curves. For FV3 DNA load analyses, an FV3 vDNA Pol (ORF 60R) PCR fragment, cloned into the pGEM-T vector (Promega, Madison, Wisconsin, USA), and serially diluted to yield 108–101 vDNA Pol fragment-containing copies was used for FV3 DNA quantification. Amplification was performed using primers designed against the FV3 vDNA Pol gene. For Bd load analyses, the DNA isolated from enumerated Bd (107 zoospores) was serially diluted (107–102) and used as a standard curve for Bd quantification, using primers designed against the Bd ribosomal RNA internal transcribed spacer 1 (ITS1).
All primers were designed using the Integrated DNA Technologies (Research Triangle Park, North Carolina, USA) PrimerQuest Tool and validated in-house prior to use. Sequences of all employed primers are listed in
Table 1 and denoted according to their respective functional roles.
Histology
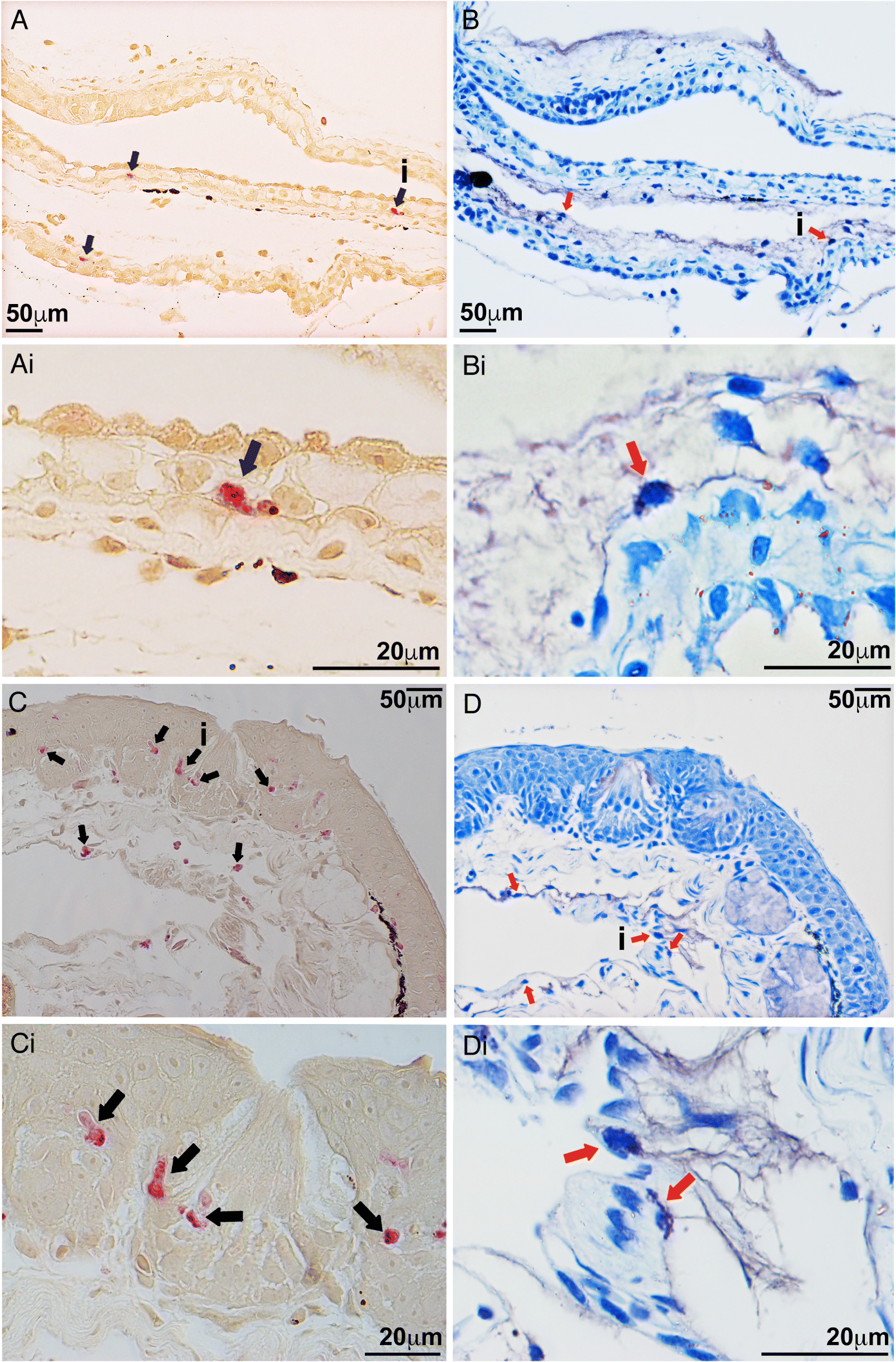
All tadpole and adult frog tissues were immediately fixed in 10% neutral buffered formalin (VWR) upon excision. Tissues were processed and embedded in paraffin, sectioned (5 μm) and stained with hematoxylin and eosin, (NASDCl; specific)-esterase (Leder, Sigma-Aldrich), periodic acid-Schiff stain (PAS, Abcam, Cambridge, UK) or Toluidine blue stains (Sigma-Aldrich). All histology preparations and sectioning were performed by the GWU Pathology Core Lab. All histology stains were performed according to the respective manufacturers’ instructions and optimized to frog or tadpole skin tissues when needed.
For staining of rCXCL8a-elicited skin granulocytes, the recovered cells were cyto-centrifuged onto glass slides and stained using a NASDCl-specific esterase kit (Sigma-Aldrich; according to manufacturer’s directions) or with a HiTrap Protein A HP column (GE Health) and A Sulfo-Link Protein Kit (ThermoScientific) purified anti-rG-CSFR primary rabbit antibody (2.5 μg total) overnight at 4 °C. The following day the cells were stained with a goat antirabbit IgG Dylight 488 (ThermoScientific) secondary antibodies (1 h) and counterstained with Hoechst nuclear stain (ThermoScientific).
Statistical analyses
Statistical analyses of the gene expression and NASDCl-esterase and metachromatic-granule (Toluidine blue) cell counts data were conducted using paired Student’s t-tests. Chemotaxis data were examined using ANOVA and Tukey’s post hoc tests via GraphPad Prism 7.0 software. Alpha level was set at 0.05; p values <0.05 were considered statistically significant.
Discussion
As an aquatic species,
X. laevis skin must support gas, water, and ion exchange as well as serve as a physical barrier against myriad pathogens present in their environments (
Feder and Burggren 1992). In turn, the global amphibian declines highlight the urgency to better understand their cutaneous immune components. To this end and through the present work, we identified resident granulocyte populations in the skin tissues of tadpole and adult
X. laevis, bearing some but not all hallmark characteristics of mast cells. We further demonstrated that these cells have a role in the anti-viral and anti-bacterial properties of
X. laevis skin.
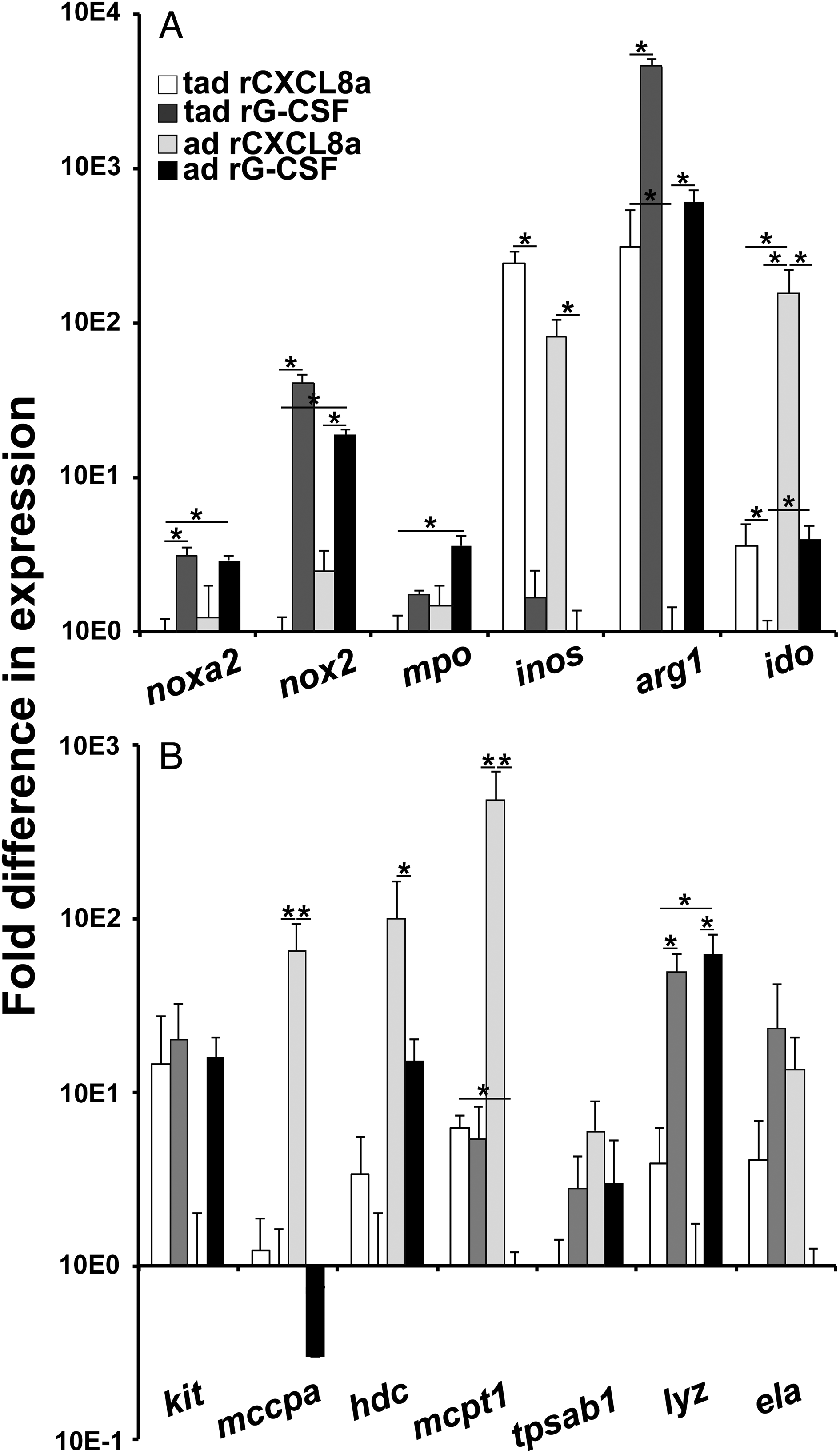
The
X. laevis skin-resident polymorphonuclear granulocytes, confirmed by their esterase activity (
Braun et al. 1996), described here do not possess the defining metachromatic staining, a defining feature of mast cells and occupy distinct layers of the skin from the what appear to be more conventional mast cells. Nonetheless, these skin granulocytes did express notable levels of several mast cell markers compared with more conventional (i.e., peritoneum-derived) granulocytes. Indeed, both tadpole and adult
X. laevis skin granulocytes expressed significant mast cell carboxypeptidase, used as a marker of zebrafish mast cells (
Dobson et al. 2008) and histidine decarboxylase. To our knowledge, the only granulocyte-lineage cells thought to reside in the tissues of healthy vertebrates are mast cells, although other granulocyte-lineage cells, namely neutrophils and basophils, readily infiltrate infected and (or) inflamed skin tissues of more recently diverged vertebrates (
Minnicozzi et al. 2011;
Rigby and DeLeo 2012). It seems probable that amphibian skin-resident immune populations, as dictated by their unique physiology and environments, may be distinct to those that are characteristic of terrestrial vertebrates. In fact, the aquatic vertebrates studied to date have considerably fewer mast cells (possessing metachromatic granules) as compared with animals that exist mostly or entirely on land (
Baccari et al. 2011). The presence of specialized, skin-resident amphibian immune cell population(s) is especially appealing given the paucity of knowledge surrounding aquatic vertebrate hematology and immunity combined with the fact that these animals are in constant contact with millions of viruses, bacteria, fungi, and protozoans via their skin. Both tadpole and adult
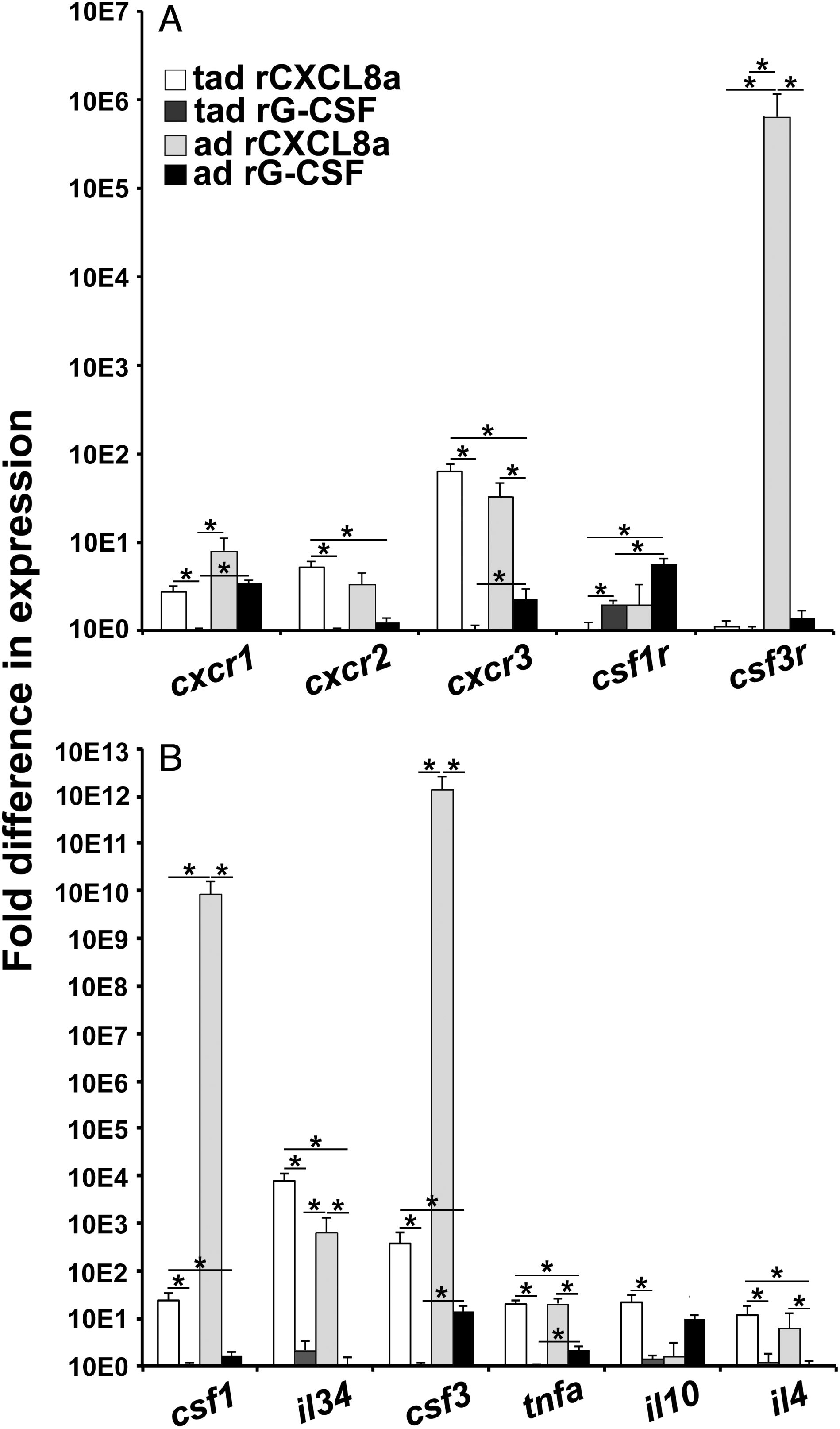
X. laevis skin granulocytes express significant levels of
mhc1a and
mhc2b compared with the more conventional tadpole and adult frog peritoneum-derived G-CSF-granulocytes, which supports the notion that these skin-resident granulocytes may serve as immune sentinels of these tissues.
In light of their unique habitats and physiologies, it is entirely fathomable that amphibians would possess disparate granulopoietic strategies to those of terrestrial vertebrates. Notably, the differentiation of mammalian mast cells as well as the developmentally related basophils is attributed to the function of interleukin-3 (IL-3) and stem cell factor (SCF; Kit ligand) cytokines (
Varricchi et al. 2018).
Xenopudinae amphibians are thought not to encode any of the IL-5 family members, including IL-3, IL-5, or granulocyte/macrophage colony-stimulating factor (GM-CSF, CSF-2;
Wang and Secombes 2015). And while IL-3 and SCF appear to be more important to the development of basophil and mast cells, respectively (
Varricchi et al. 2018), the absence of IL-5 family members in amphibians suggests their granulopoiesis may indeed be distinct from that of more recently diverged vertebrates.
Mast cells are thought to arise from common granulocyte or macrophage precursor (GMP) cells in both mammals and fish (
Dobson et al. 2008;
Qi et al. 2013) and thus, depend on G-CSF (CSF-3) as a growth factor at some point during their commitment, differentiation and (or) egress from their species-specific designated hematopoietic organs. Indeed, although SCF has been more prominently implicated in mast cell development, G-CSF has also been shown to promote the expansion of mammalian mast cells (
Ulich et al. 1991;
Yang et al. 1999). The expression of cell-surface G-CSFR on
X. laevis tadpole and adult skin granulocytes confirms their granulocyte lineage but does not permit us to clearly speculate on the relationships between these frog cells and the mammalian mast cells or other granulocyte subsets. However, when compared with more conventional, peritoneum-derived tadpole and adult frog granulocytes, these skin granulocytes exhibited gene expression more akin to, and characteristic of, mammalian mast cells. This includes greater skin granulocyte expression of interleukin-4 (IL-4), mast cell carboxypeptidase, and histidine decarboxylase, all of which are associated with mammalian mast cells (
Metcalfe et al. 1997;
Varricchi et al. 2018). Conversely, gene expression of the SCF receptor (
kit), a hallmark of mammalian mast cells (
Metcalfe et al. 1997), did not differ significantly between the tadpole skin and conventional granulocytes, but it was nearly absent in the adult frog skin-derived cells. Thus, the skin granulocytes described here may be distinct from mammalian mast cells, at least insofar as their low
kit expression and thus independence of SCF.
Whereas more recently diverged vertebrates encode a single CXCL8 (IL-8) chemokine, possessing the Glu-Leu-Arg (ELR) motif that is characteristic of mammalian proinflammatory chemokines (
Clark-Lewis et al. 1991,
1993), we recently demonstrated that amphibians possess two distinct CXCL8 forms, CXCL8a and CXCL8b. The former isoform participates in inflammatory responses, while the latter recruits granulocytes bearing immunosuppressive gene expression (
Koubourli et al. 2018). During those studies, we also observed prominent expression of both CXCL8 isoforms in the skins of tadpoles and adult frogs and demonstrated that both chemokine isoforms recruited tadpole and adult granulocytes by engaging CXC chemokine receptors, CXCR1 and CXCR2 (
Koubourli et al. 2018). Our present work indicates that the tadpole and adult skin granulocytes are chemoattracted towards CXCL8a and CXCL8b in vitro and in vivo, while pharmacological inhibition of the CXCR1/2 significantly diminishes the numbers of tadpole and adult skin granulocytes. Taken together, these data support the notion that CXCL8a and CXCL8b mediate the homing and (or) retention of these skin granulocyte populations. Notably, homing of mammalian mast cell precursors to the skin depends on CX
3CR1 (
Papadopoulos et al. 2000), whereas homing of mammalian mast cells to mucosal tissues depends on the activation of CXCR2 (
Gurish et al. 2001;
Abonia et al. 2005). To our knowledge, homologs of CX
3CR1 or its ligand (CX
3CL1) have not been identified or annotated in
Xenopudinae genomes. It is however interesting to consider that while the amphibian skin is also a mucosal tissue, the tadpole and adult frog skin granulocytes described here appear to be dependent on the CXCL8-CXCR1/2 axis for their presence in the skin tissues.
Our results indicate that enriching tadpole and adult skin granulocytes with rCXCL8b renders them more susceptible to FV3 and
Bd, respectively. This is consistent with our past results that suggest that CXCL8b chemoattracts granulocytes bearing immunosuppressive characteristics. Since both CXCL8a and CXCL8b are expressed in the skin tissues of tadpoles and adults, we speculate that these chemokines may mediate the homing of functionally distinct granulocyte subsets. Given our past work demonstrating
X. laevis CXCL8a and CXCL8b both function through CXCR1 and CXCR2 (
Koubourli et al. 2018), distinct tadpole and adult frog CXCL8a- and CXCL8b-recruited skin granulocyte subpopulations will also likely possess both CXCR1 and CXCR2 receptors. Because the ELR motif is present only on the
X. laevis CXCL8a isoform and is important to inflammatory chemokine-receptor interactions (
Cai et al. 2009), we postulate that the inflammatory and immunosuppressive frog granulocyte populations are recruited towards CXCL8a and CXCL8b, respectively, as a result of distinct ligand-CXCR1/2 receptor interactions. However, since both chemokines act through CXCR1 and CXCR2, if there are multiple granulocyte populations in the frog skin, they would express both receptors. Thus, without a greater understanding of the unique interactions of CXCL8 isoforms with their receptors, we cannot assume that the retrieval of tadpole and adult frog skin granulocytes using rCXCL8a and rCXCL8b, as described here, does not yield multiple skin granulocyte populations. Ultimately, it will be of great benefit to disentangle the unique interactions of CXCL8 isoforms with their receptors.
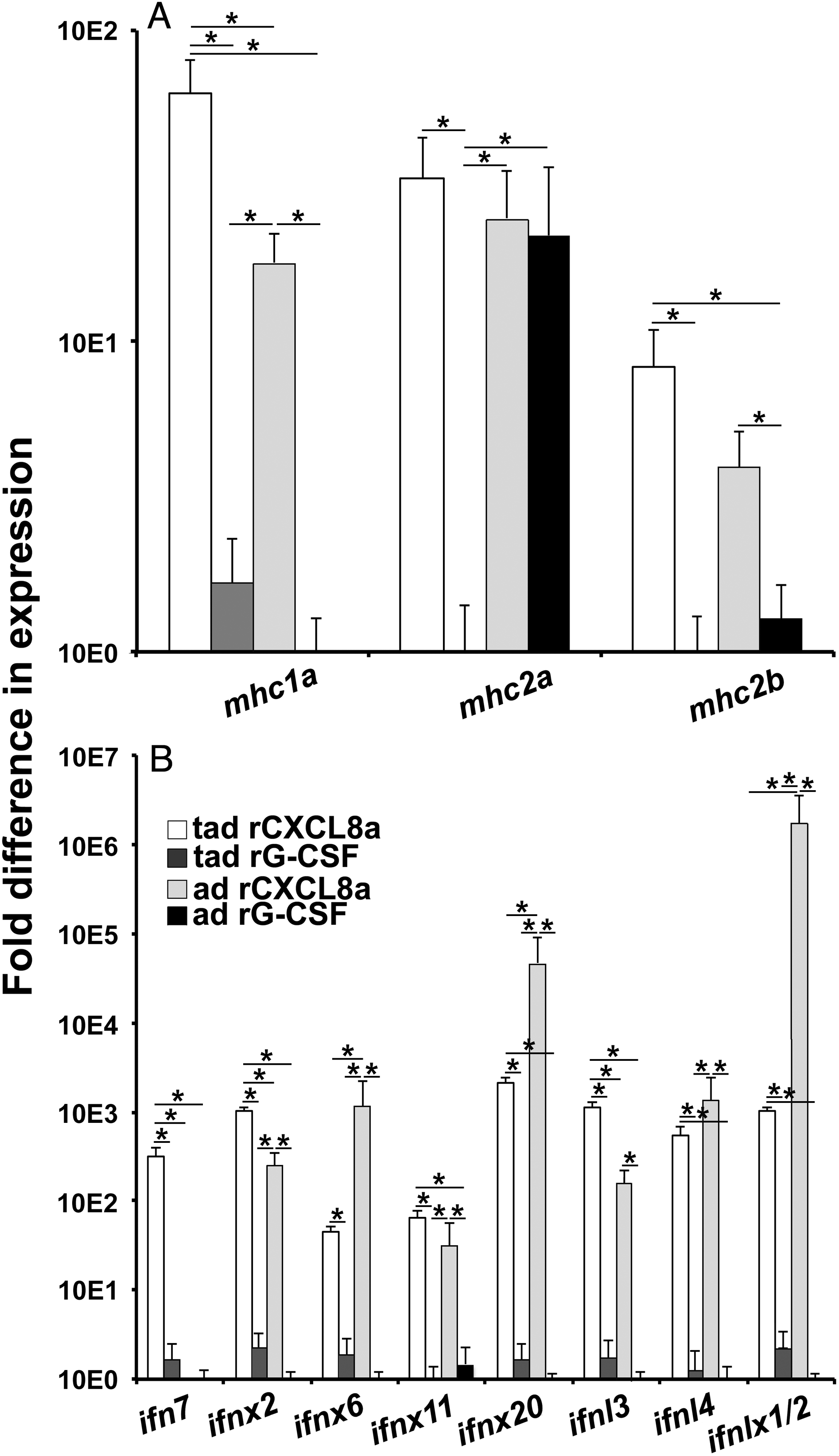
Despite the differences between mammalian mast cells and the
X. laevis esterase-positive skin granulocytes described here, the two cell types share in common their robust expression of antiviral interferon cytokines (
Lopušná et al. 2013). It is somewhat intuitive that resident skin immune cells of animals that are constantly bombarded by viral pathogens through their environments, would possess the capacity to orchestrate antiviral immune responses. When considered in the context of our recent work demonstrating the robust type I and type III interferon responses to skin FV3 infections of tadpole and adult
X. laevis (
Wendel et al. 2017,
2018), it is interesting that the resident skin granulocytes possessed relatively robust expression of both type I and III IFN genes while pharmacological depletion of these cells rendered tadpoles more susceptible to FV3. In line with these findings, the tadpole skin granulocytes significantly upregulated the majority of these
ifn transcripts upon challenge with FV3. Likewise, mammalian mast cells have been implicated in antiviral immunity (
Fukuda et al. 2013) and have been shown to produce antiviral IFNs following stimulation (
Kulka et al. 2004;
Dietrich et al. 2010). However, it is noteworthy that the repertoires of
ifn genes expressed by the tadpole and adult frog skin granulocytes do not entirely match the distinct whole skin
ifn gene expression patterns seen between tadpole and adult
X. laevis (
Wendel et al. 2017). Such a discrepancy indicates that these resident skin granulocytes are not the sole source(s) of tadpole and adult frog skin
ifn gene expression. It will be invaluable to learn through future studies the respective contribution of these skin granulocytes to amphibian antiviral IFN responses to FV3 and other relevant viruses.
Mast cells were first discovered in an amphibian species (
Ehrlich 1877;
Dines and Powell 1997) and perhaps not coincidentally, we report on the discovery of amphibian resident skin granulocyte population(s) exhibiting similarities to, as well as differences from mammalian mast cells. In turn, it is intriguing to consider just how unique the physiologies and habitats of amphibians are, as compared to those of more recently diverged terrestrial vertebrates against which we attempt to compare amphibian immune systems. As we face amphibian mass extinctions and concerning global declines (
Wake and Vredenburg 2008), it is essential to develop a comprehensive understanding of the immune cells and components that define amphibians in the context of their distinctive ecophysiologies and pathogenic pressures.