Introduction
Ranaviruses are pathogens of ectothermic vertebrates like reptiles. Because reptile physiology is strongly influenced by the temperature of their surrounding environment, so is the pathogenesis of ranaviral infections. The environmental temperature-dependent physiology includes many aspects of the ectothermic immune system (both adaptive and innate) and, while immune function is often severely reduced at lower temperatures, many immune components have optimal temperatures beyond which the efficacy diminishes (
Zimmerman et al. 2010).
As with the ectothermic immune response, the replication rate of ranaviruses is also linked to temperature (
Ariel et al. 2009). It has been concluded that the decrease in mortality at higher temperatures (>25 °C) is associated with ranaviruses’ inability to replicate effectively above 32 °C in cell culture (
Chinchar et al. 2009;
Allender et al. 2013). However, it is difficult to differentiate the degree to which temperature-dependent pathogenesis is a result of the effect of temperature on the replication of the virus or on the immune system or other physiological components of the ectothermic host (
Brunner et al. 2015).
As temperature can influence ranaviral disease, temperature therapy (exposing animals to environmental temperatures that are associated with reduced disease) may be a cheap and effective method for treating acute ranaviral disease in captive animals if the optimal temperature for survival is known. High environmental temperatures may effectively force the animals into behavioural fever, increasing their immune response and reducing viral replication, which subsequently results in reduced mortality. Behavioural fever is the process by which a diseased ectotherm (e.g., reptile) actively brings about a febrile state through the behavioural selection of higher environmental temperatures (
Monagas and Gatten 1983;
do Amaral et al. 2002;
Merchant et al. 2007). Infected or antigen-challenged ectotherms will generally select an environmental temperature a few degrees higher than control groups, and it is thought that this temperature is correlated with the optimal immune response of the animal (
Spellerberg 1972;
Monagas and Gatten 1983;
Merchant et al. 2007). There is evidence that toads perform behavioural fever to fight ranaviral infections (
Sauer et al. 2019). Thus, knowing the optimal temperature of infection, it will be possible to make recommendations of the optimal temperature for temperature therapy and the best temperatures for basking sites in enclosures to ensure behavioural fever is possible.
Understanding the effect of temperature on infection also has important ramifications for understating wild disease dynamics. Knowing the rage of temperatures that infection can occur at and the optimal temperature for infection, it may be possible to identify at-risk populations or predict temperature-driven epizootics. As the climate continues to change it is important to understand how variation in temperature may affect aspects of ranaviral disease.
Australia is home to ranaviruses,
frog virus 3 (FV3) and
epizootic hematopoietic necrosis virus, and susceptible ectothermic vertebrates such as the Krefft’s river turtle (
Emydura macquarii krefftii) (
Ariel et al. 2015;
Chinchar et al. 2018). Krefft’s river turtles belong to the Pleurodira suborder of turtles, which are distinct from the native turtles of Asia, Europe, and North America and may be a useful model for other Pleurodirid turtles (of Africa, Australasia, and South America). The effect of ranaviral dose at a single temperature has been reported for Krefft’s hatchlings (
Wirth et al. 2019). This study investigates how infection rate with a local ranaviral isolate (Bohle iridovirus; FV3) varies within a range of environmental temperatures representative of topical Australia (16–34 °C).
Discussion
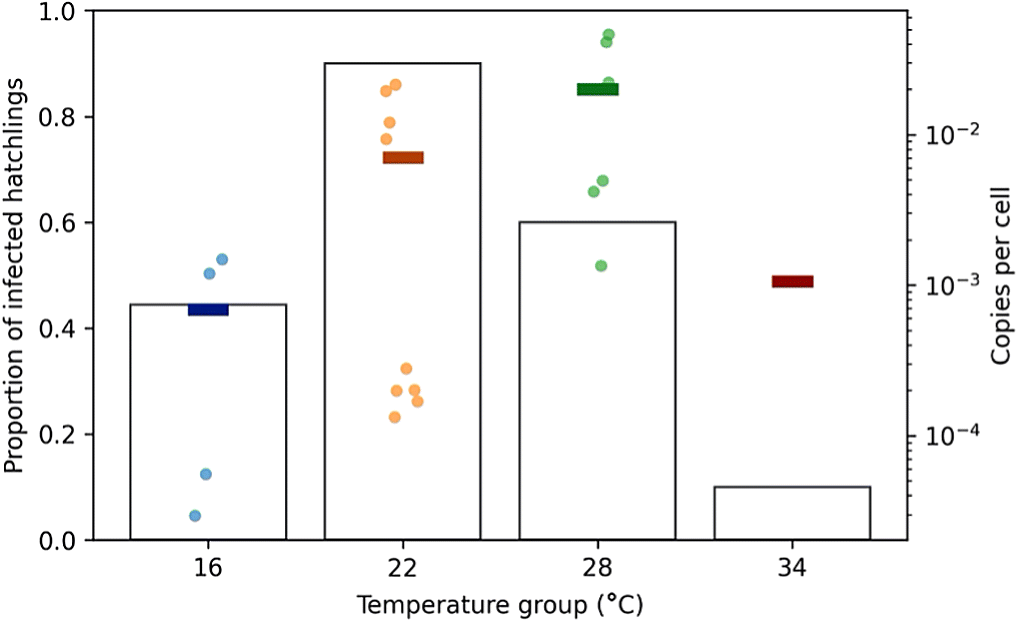
Based on the distribution of infection rates (
Fig. 1) and temperature logger data (
Table 2), the predicted optimal temperature for ranaviral infection (i.e., the maximum number of infected) in Krefft’s river turtles is 23.2 °C. Assuming that uninfected turtles would not later develop a detectable infection (i.e., turtles that did not react in the qPCR assay have cleared the infection), we can say that temperature has a significant effect on the rate and length of infection. This will have impacts on the transmission of the virus. The more animals infected and the longer an animal is infected, the greater the chance of viral transmission.
Clinical signs observed in this study were similar to those that were reported for ranaviral infection in other turtles, although not as extensive as previous reports (
Wirth et al. 2019). Given more time these animals may have either recovered or developed more clinical signs; however, terminating the experiment at 21 d allowed us to prevent suffering of the animals (they did not have time to develop clinical signs) while still being able to study the effect of temperature on infection.
There was a large variation in the viral loads observed within temperature groups. Interestingly, the copies per cell of ranaviral DNA within each temperature group appears to divide equally into “high” and “low” reactors with at least a 10-fold difference between the mean high and low copy numbers. Such a difference in individuals that received the same treatment may be explained by some dichotomous biological trait, such as those associated with sex, that increases or decreases viral replication. Unfortunately, the sex of these hatchlings cannot be easily determined via physical exam and was not recorded at necropsy. Krefft’s river turtles do not have temperature-dependent sex determination, so the sex distributions within the temperature groups are assumed to be 50/50, which would explain the equal numbers of high and low viral loads in the temperature groups if this is the result of the sex of the animals. Future work should investigate the possible effects of sex (or other dichotomous variables) on ranaviral infection in turtles.
Post hoc analysis revealed that there was a significant difference in viral loads only between the 16 and 28 °C groups (
p = 0.027). At lower temperatures, viral replication is likely reduced, thus resulting in reduced viral load. Adult red-eared sliders (
Trachemys scripta elegans, suborder Cryptodira) exposed to a ranavirus and held at either 22 or 28 °C had significantly reduced ranaviral loads and halved morbidity in the 28 °C group (
Allender et al. 2013). Similarly, we found that the infection rate was higher in the 22 °C group compared with the 28 °C group. However, we observed no significant difference in the viral loads between the 22 and 28 °C groups.
In a similar experiment, four Cryptodirid species of juvenile turtles (including red-eared sliders) did not have reduced mortality in the higher temperature group, both groups (22 and 27 °C) had 100% mortality (
Allender et al. 2018). Krefft’s river turtles exhibit age-dependent ranaviral pathogenesis, with hatchlings being more susceptible than adult turtles (
Ariel et al. 2015). The same may be true for red-eared sliders; the dose given to the juvenile turtles may have overwhelmed the turtles and thus negated the effect of temperature (
Allender et al. 2013,
2018). Because adult red-eared sliders are less susceptible, the effect of temperature on viral replication/the immune system is still prominent (
Ariel et al. 2015;
Allender et al. 2018;
Wirth et al. 2018).
In our study, we chose to use a median infectious dose (ID
50), as determined by
Wirth et al. (2019) for Krefft’s turtle hatchlings at 28 °C. As expected, the infection rate in the 28 °C group in this study was close to 50% (60%;
Fig. 1). Using a median dose allows for easy detection of a change in the dependent variable (infection, death, etc.) as a result of changing different factors (e.g., environmental temperature). We chose the ID
50 over the median lethal dose (LD
50; 10
4.43 TCID
50 mL
−1) for this study for welfare reasons. The results of an infectious dose study are still valuable, although more applicable to a disease transmission than pathogenesis. We are not sure how these temperatures will affect Krefft’s river turtles exposed to a higher dose (such as an LD
50). It may be that when Krefft’s turtle hatchlings are exposed to higher doses, the amount of virus will overwhelm any prophylactic effects of temperature, as seen with red eared-sliders. In any case, we still do not understand the natural transmissions dynamics, so it is difficult to determine what dose would be useful to accurately reflect wild disease.
Temperature therapy >34 °C may also be useful for treating acute ranaviral disease, although further study will be required to determine the effects of temperature on pathogenesis. While 34 °C appears effective at reducing infection rates, BIV itself is not inactivated at 34 °C (
La Fauce et al. 2012); thus, once an animal returns to cooler temperature the infection may re-establish if the virus is not completely cleared. To reduce the chances of ranaviral infection in captive animals, we would recommend that turtles are provided with a basking area of at least 34 °C, thus allowing the animals to perform behavioural fever at temperatures high enough to reduce infection. Lower temperatures (e.g., ≤16 °C) may also be effective at reducing infection rates; however, these temperatures are not optimal for turtle health and result in reduced appetite and activity.
The optimal temperature for ranavirus isolate propagation in a range of cell lines is 24 °C (10, 15, 20, 24, and 28 °C tested;
Ariel et al. 2009). The average annual temperature in Townsville (where this study was conducted) is 24.1 °C. Both the optimal temperature for ranaviral propagation and the average annual temperature in Townsville are close to the temperature of maximum infection rate (23.2 °C) estimated in this study. Australian freshwater turtles (like Krefft’s turtles) are semi-aquatic and spend the majority of their time in the water. When in the water, the turtles’ body temperatures are in thermal equilibrium with water temperature (
Manning and Grigg 1997). Ranaviral infection rates would be expected to be highest in months when water temperature is around 23 °C, i.e., May–June and September–October. However, the temperature most effective at reducing infection rate used in this study (34 °C) is not uncommon in Northern Australia where these turtles are native. Thus, ranaviral-infected Krefft’s river turtles could have the opportunity to use behavioural fever to reduce infection rates, although finer-scale studies of thermal and basking site availability are required to quantify this opportunity.
In this study we, used reaction in a qPCR assay of the liver as a proxy for infection. While the liver is a major target of ranaviral replication, all conclusions reported in this study should be interpreted in light of the fact that reaction in a qPCR assay does not equal infectious virus.