Introduction
Harmful algal blooms (HABs) have increased in intensity, frequency, and distribution across Canada and the world (
Orihel et al. 2012;
Pick 2016;
Ho et al. 2019). In Ontario alone, the number of reported HAB events between 1994 and 2009 increased by 22-fold and, by 2009, nearly half of those reported were dominated by cyanobacteria (
Winter et al. 2011). This increase in cyanobacterial growth and dominance has been strongly attributed to increased nitrogen (N) and phosphorus (P) availability (
Paerl and Huisman 2009;
O’Neil et al. 2012;
Sukenik et al. 2015;
Paerl et al. 2016) and, more recently, climatic shifts. For example, thermal regime changes have resulted in earlier and warmer water temperatures (
O’Reilly et al. 2015;
Richardson et al. 2017), increasing the competitive advantage of certain bloom-forming cyanobacteria over other organisms (
Jöhnk et al. 2008;
Paerl and Huisman 2009). Despite predicted climatic changes in both temperature and precipitation patterns (
IPCC 2007;
McDermid et al. 2015), less attention has been given to the importance of precipitation regime changes, which may have an even greater impact on cyanobacteria bloom dynamics due to mixing and nutrient cycles (reviewed in
Reichwaldt and Ghadouani 2012).
Reservoirs vary in physical, chemical, and biological properties along a spatial gradient as a result of their design and geographic placement. Impoundment systems, constructed along river reaches, are often narrow and channelized where a river enters the reservoir (riverine zone). This shallow, well-mixed zone transitions in width and depth (transitional zone) to a broader and deeper, lake-like system (lacustrine zone) (
Thornton et al. 1996). Primary production and phytoplankton phenology along the riverine–transitional–lacustrine gradient is primarily mediated by light and nutrient availability, among other interacting factors (
Kimmel and Groeger 1984). However, large precipitation events could potentially disrupt characteristic primary production patterns in these zones, especially if the event introduces sizable nutrient loads, increases flushing rates, or affects other water-column properties prior to or during a bloom period. For example,
Wood et al. (2017) reported decreased cyanobacterial biomass following an extreme rainfall event that led to water column cooling and destratification in a shallow, eutrophic New Zealand lake. Varying responses to extreme rainfall should be anticipated given the importance of site and event-specific factors.
Changes to seasonal phytoplankton phenology, especially in terms of cyanobacterial bloom dynamics and biomass, are a great concern to water managers. Several bloom-forming cyanobacterial species such as
Aphanizomenon flos-aquae,
Microcystis aeriginosa, and
Anabaena (Dolichospermum) flos-aquae synthesize an array of bioactive compounds that pose acute, chronic, and even potentially fatal health risks to humans and other organisms through dermal contact, inhalation, and (or) ingestion of contaminated waters (
Chorus and Bartram 1999;
Chorus et al. 2000;
Codd 2000). Metabolite production as well as the composition of toxins released are complex and tied not only to the cyanobacterial species, successional patterns, and biomass, but also to various environmental parameters including N, P, temperature, light, pH, salinity, and micronutrients (
Chorus and Bartram 1999), which may all be altered by intense rainfall events (
Reichwaldt and Ghadouani 2012). To date, only limited information exists regarding how toxicity may change as a result of precipitation events.
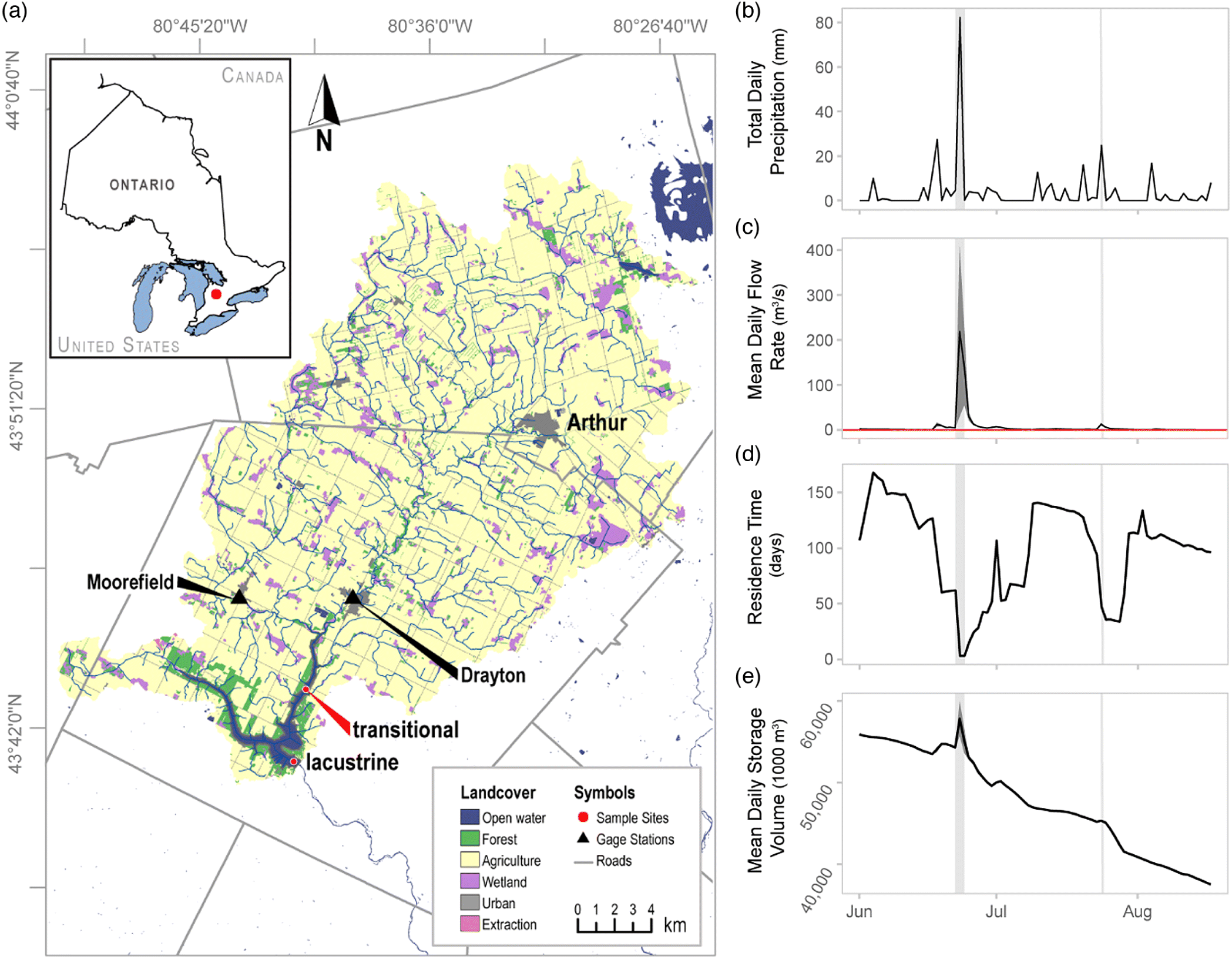
Here, we document the effects of an extreme early summer rainfall event on cyanobacterial biomass and toxin dynamics in a eutrophic reservoir and contrast these effects to a second, smaller mid-summer rainfall event. Conestogo Lake (Ontario, Canada;
Fig. 1), has experienced recurring, late-summer cyanobacterial blooms dominated by
A. flos-aquae since 2004 (
Guildford 2006; S. Cooke, personal communication 2018). On 23 June 2017, the upper Conestogo watershed experienced a record rainfall event (daily total 78 mm measured at Conestogo Dam (
Grand River Conservation Authority (GRCA) 2017,
2018)) that resulted in severe flooding and replaced ∼80% of the water in Conestogo Lake during this 4.96-d event (
GRCA 2018). We first evaluate how this extreme event affected the reservoir by characterizing the physical and chemical changes across reservoir spatial zones. We then follow the trajectory of bloom development and toxicity through the season to better understand how hydrological events can alter bloom occurrence and potential health risks.
Discussion and conclusions
Globally, increases in extreme rainfall events are predicted to outpace changes in total precipitation (
Allen and Ingram 2002;
IPCC 2007;
Soulis et al. 2016). The impacts of extreme rainfall on cyanobacterial blooms are inherently complex (
Reichwaldt and Ghadouani 2012;
Wood et al. 2017) with the overall effect regulated by parameters including intensity, volume of water inflow, and timing with respect to seasonality (reviewed in
Reichwaldt and Ghadouani 2012). The limited studies investigating such extreme rainfall events on cyanobacterial bloom development have identified changes to flushing rates, water column mixing, and nutrient inputs from rainfall events as the main abiotic factors affecting cyanobacteria and phytoplankton communities (
Bouvy et al. 2003;
Reichwaldt and Ghadouani 2012)—all factors that appeared to impact the bloom dynamics in Conestogo Lake.
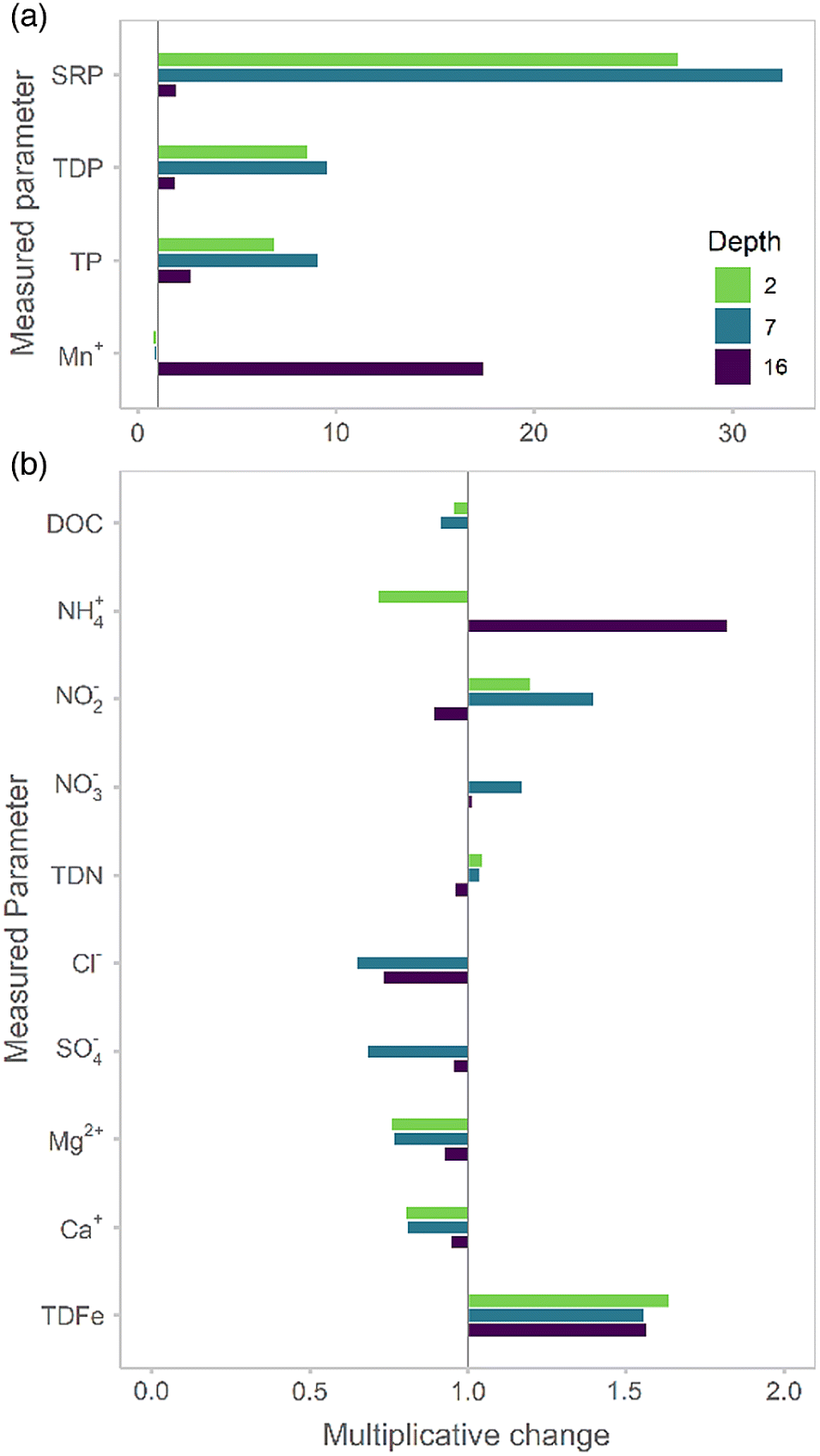
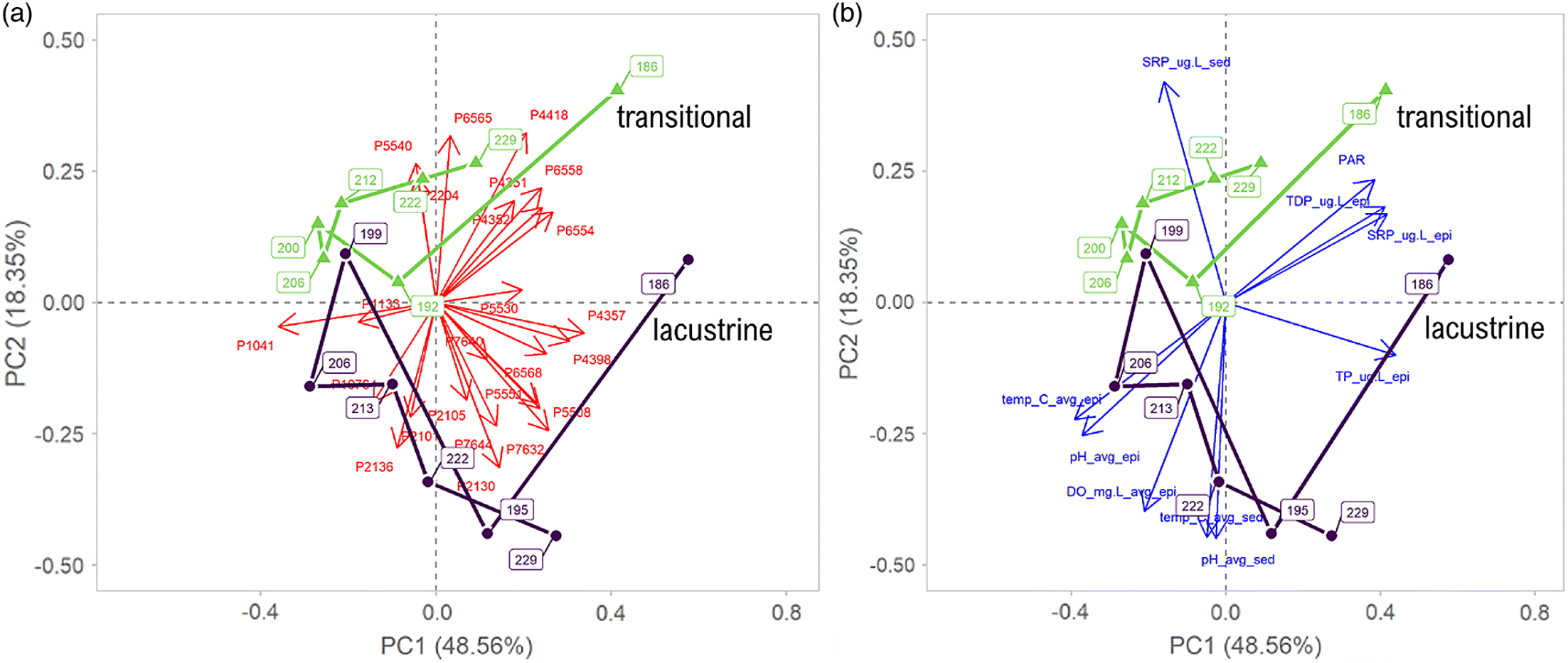
The record rainfall event effectively created a new, early-summer high-P regime as a result of the significant influx arriving from the Conestogo River and a near-complete replacement of the reservoir volume. Epilimnetic SRP concentrations in Conestogo Lake have ranged between 0 and 1.8 μg·L
−1, whereas TDP and TP range between 8 and 15 μg·L
−1 (
Guildford 2006). During this event, epilimnetic SRP concentrations increased by ∼7–27 times (from ∼2 to 65 μg·L
−1). Substantial increases in P loading, such as these, are common following heavy rainfall, particularly if they are preceded by warm, dry periods (
Jones and Poplawski 1998;
Lisboa et al. 2020). For example, in Australian reservoirs, a 440 mm rainfall in a 3-d period resulted in an input equivalent to 400% of the average annual in-lake P mass (
Jones and Poplawski 1998). In our study system, the upper Conestogo watershed is predominantly Tavistock Till, which is particularly prone to erosion and serves as a key mechanism for particulate P transport (
Loomer and Cooke 2003;
Macrae et al. 2007). High flow events can also be important to transport of soluble P species, as P is desorbed from particulate forms or released from soils (
Lisboa et al. 2020).
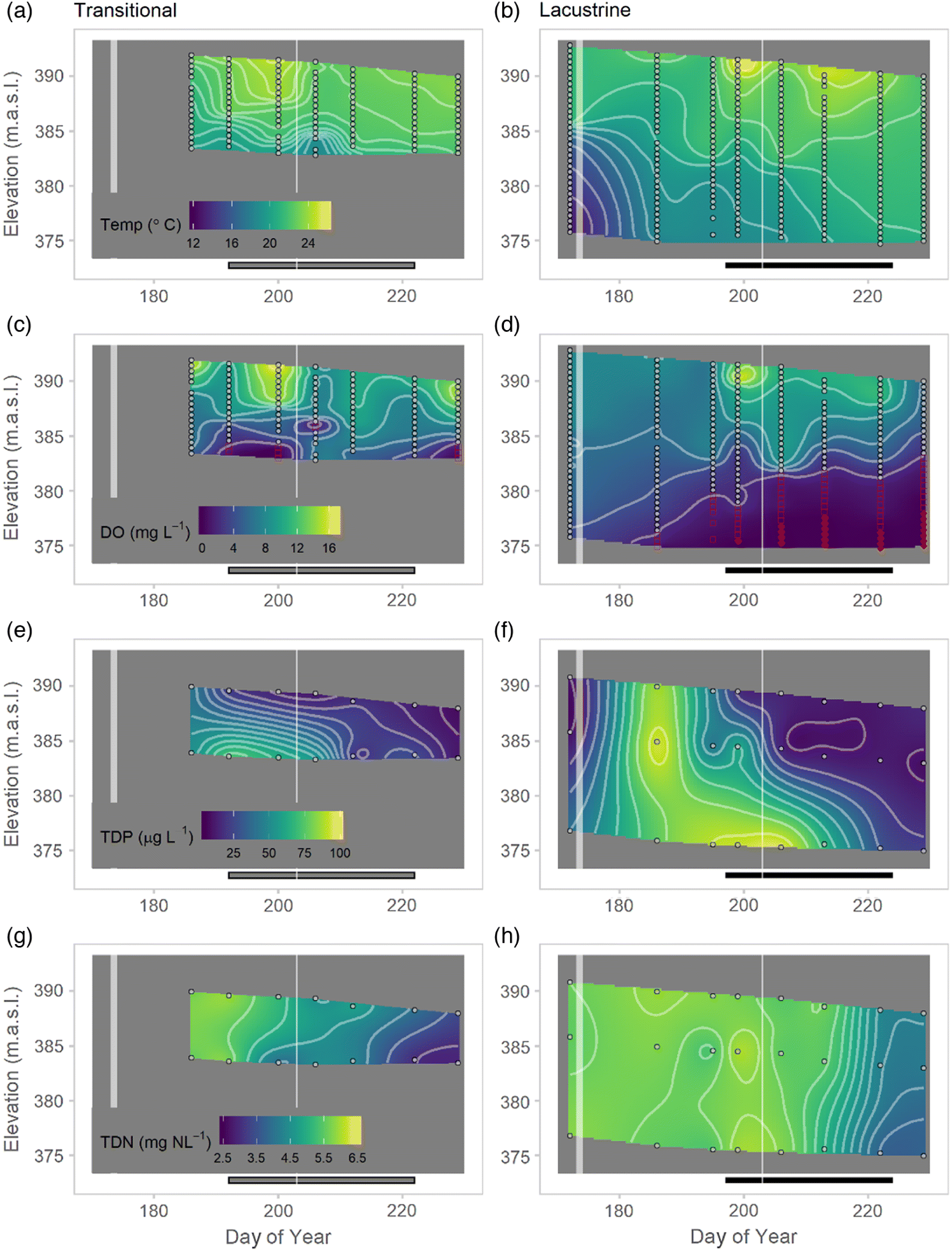
Though surface water temperatures remained similar following the June extreme rainfall event, the bottom sediments warmed from 13 to 18 °C. While we cannot isolate the effects of thermal and biogeochemical changes in bloom initiation, we note that in-lake nitrogen concentrations appear to have changed little as a result of the flooding despite the low concentrations (<1 mg NO
3-N·L
−1) in the upper Conestogo River preceding the event. This catchment has chronic high N concentrations (3–5 mg NO
3-N·L
−1), associated with long-term intensive agricultural usage within the catchment (
Guildford 2006;
Irvine et al. 2019). The lack of change in N suggests that it was not likely to be the key factor leading to early bloom initiation as previously noted (
Guildford 2006). Iron too has been postulated as a key nutrient to support cyanobacterial growth and bloom induction (
Molot et al. 2014). Although iron speciation was not monitored here, changes in total dissolved iron are likely to have been more associated with the traditional bloom period rather than the extreme event.
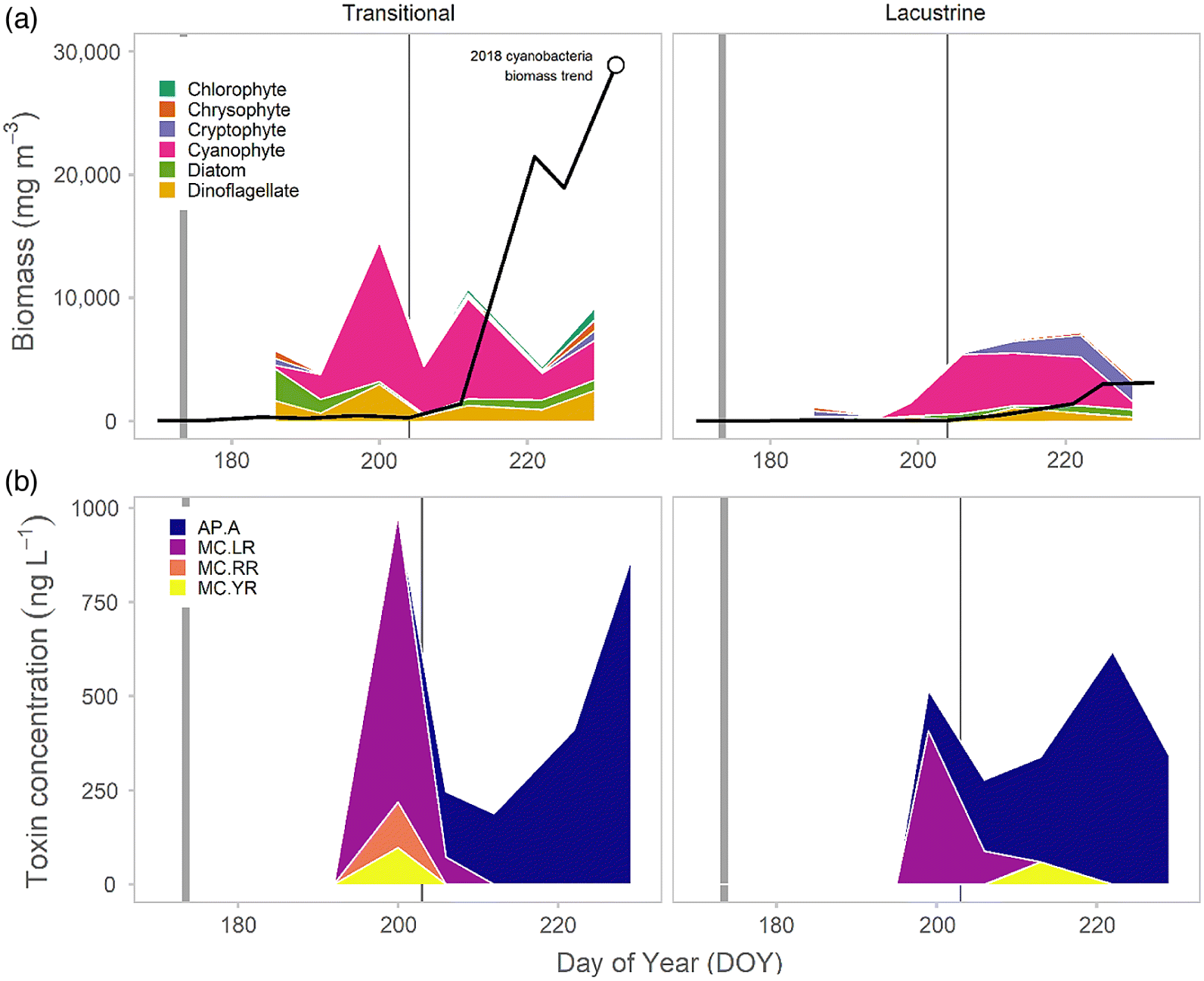
It appears that the excessive supply of P initiated the development of the observed weakly toxic early-summer cyanobacterial bloom (
Figs. 5 and
6). Typically, N-fixing
A. flos-aquae dominated blooms do not develop in the Conestogo Reservoir until late summer (S. Cooke, personal communication;
Guildford 2006), ∼35–40 d after our documented extreme event when typical in-lake N concentrations are much lower (
Guildford 2006). The significant growth response by
A. flos-aquae, as compared with other cyanobacteria, may have been in part driven by cooler water temperatures.
Aphanizomenon species have lower temperature optima than other cyanobacteria (
Konopka and Brock 1978) thereby potentially providing a competitive advantage over other species. Additionally, substantial
Aphanizomenon akinete seeding from the sediments could have been flushed into the transitional zone water column by the increased river flows. Akinete populations have been postulated to act as an inoculum for summer blooms (
Cirés et al. 2013). The bloom originating in the transitional zone was not only initiated a week earlier than in the lacustrine, but it was also sustained through the typical bloom period in September (S. Cooke, personal communication 2018;
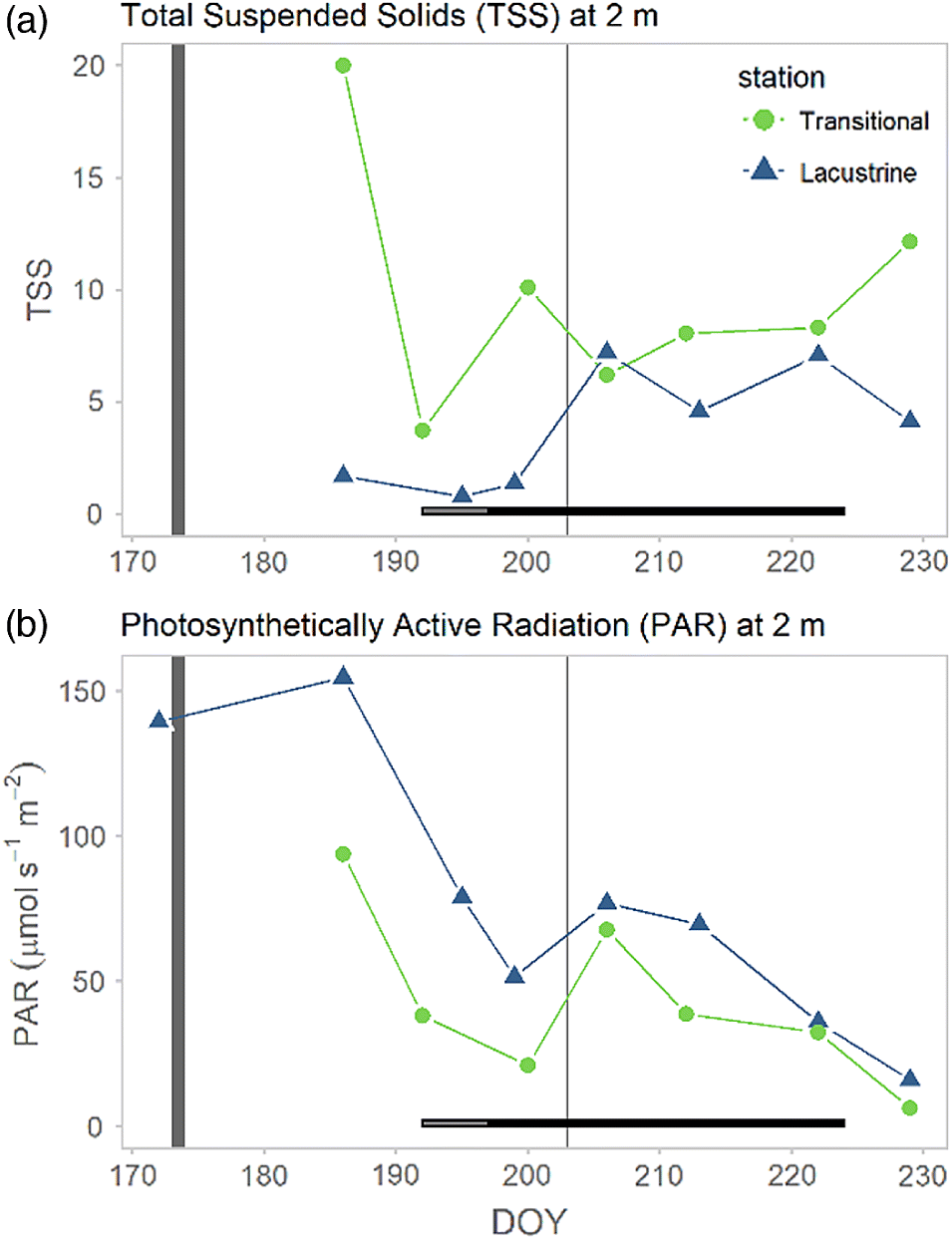
Guildford 2006), highlighting the potential for elongation of the bloom season and risk associated with extreme rainfall events. However, the magnitude of the transitional bloom was directly influenced by the mid-summer rainfall event, which flushed both biomass and toxins. The event was not of sufficient volume to have similar effects on the deeper, larger volume lacustrine zone.
The environmental factors driving toxin production and their seasonal dynamics are quite complex. We detected low quantities (<1000 ng·L
−1) of three MC variants, MC-LR, -YR, and -RR, in the early summer cyanobacterial bloom that dissipated following the mid-summer event. The total concentration of microcystins never exceeded the recreational limit (20 000 ng·L
−1;
Health Canada 2012). Of these, only MC-LR was significantly correlated with the low biomass of a single taxa (
M. aeruginosa). However, because it difficult to assign strong relationships between the toxins and cyanobacterial species without direct molecular studies (
Vezie et al. 1998), it remains possible that there could be the presence of a rare, toxin-producing species or strain or multiple taxa involved in toxin production.
MC variants are not the only cyanobacterial compounds capable of impacting human health. Several studies using cyanobacterial extracts have reported harmful and (or) toxic effects that could not be explained solely by microcystin concentration or presence, suggesting the possibility of other toxic compounds (
Keil et al. 2002;
Teneva et al. 2005;
Baumann and Jüttner 2008;
Smutná et al. 2014;
Lenz et al. 2019). Although not often reported, improved analytical techniques have identified numerous other bioactive compounds such as cyanopeptolins and anabaenopeptins that are both detectable in freshwaters and often produced simultaneously with microcystin variants (
Harada et al. 1995;
Welker and Von Döhren 2006;
Gkelis et al. 2015;
Beversdorf et al. 2017,
2018) at similar or greater levels (
Janssen 2019). For example,
Beversdorf et al. (2017) reported an average of 0.65 μg·L
−1 total microcystin in Lake Koshkonong, Wisconsin, while anabaenopeptin-B and -F were measured at 6.56 μg·L
−1 combined. Though there are no case studies of human toxicity caused by anabaenopeptins, the compound inhibits carboxypeptidase A, and like microcystin, also inhibits protein phosphatases with slightly overlapping inhibitory concentration ranges (
Honkanen et al. 1990;
Sano et al. 2001;
Spoof et al. 2016). The concentrations of these toxins that could affect human health are unknown, which has prevented the development of recreational and drinking water regulations and (or) advisories for these potentially toxic compounds. Like some microcystins, the ecological effects of anabaenopeptins may be observed at relatively low concentrations. A recent study by Lenz et al. (2019) reported induced toxicity by low concentrations (10 μg·L
−1) of anabaenopeptins, including AP-A, on the nematode
Caenorhabditis elegans resulting in reduced reproduction, reduced lifespan, and delayed hatching. Though compounds like AP-A have been previously considered nontoxic, they may represent a new class of emerging toxins, whose potential impacts to human health and toxicity to aquatic organisms require immediate attention and, therefore, inclusion in risk assessment for lake mitigation and monitoring programs. Though the present study concluded prior to the peak bloom season in late August to September, GRCA managers took note of the sizable cyanobacteria biomass and issued public health alerts (S. Cooke, personal communication 2018,
CTV News 2017). We recognize this is as a limitation in our study as the additional information from the traditional bloom period could have aided in further understanding to the effects of extreme events.
Global bloom incidence has been associated with elevated nutrient loads and with warming (
Paerl and Otten 2016;
Ho and Michalak 2020). However, more attention is needed to understand the role of extreme events in altering bloom risk, how these events may differ in their seasonal impacts, and on spatially complex but crucial water resources, such as reservoirs. The environmental factors driving cyanobacterial blooms are inherently complex and dynamic, making predictions about toxicity and impact on end-user health and safety difficult to obtain across diverse ecosystems. Extreme disturbance events, like the early summer event described here, will only make that task more difficult since they are not only predicted to increase but will also likely mask historical drivers of changes in bloom risk. Managers cannot effectively manage a future ecosystem based on averages of the past. Instead, a holistic understanding of multiple drivers of cyanobacterial bloom risk, accounting for both extreme events, and reservoir structure is required. Within this region, we hypothesize that early season events have the greatest potential to worsen bloom risk by extending the bloom season as shown here. Later season events may lead to increased annual nutrient loads but may also flush existing blooms, especially in the narrow sections of the transitional zones.