An inclusive and diverse governance structure of the strategy for patient-oriented research (SPOR) Evidence Alliance
Abstract
The Strategy for Patient Oriented Research (SPOR) Evidence Alliance is a research initiative in Canada whose mission is to promote the synthesis, dissemination, and integration of research results into health care and public health decision-making and clinical practice. The aim of this paper is to (i) outline the governance and committee structure of the SPOR Evidence Alliance, (ii) outline the procedures for patient and health system decision-maker engagement, and (iii) present the capacity-building strategy for governance members. The governance structure includes the following six standing committees: the International Advisory Committee, Steering Committee, Executive Committee, Knowledge Translation Committee, Partnerships Committee, and Training and Capacity Development Committee. The guiding principles embrace inclusiveness, support, mutual respect, transparency, and co-building. There are currently 64 committee members across the six committees, 13 patient and public partners, 8 health system decision-makers, 7 research trainees, and 36 researchers. A multi-disciplinary and diverse group of people in Canada are represented from all regions and at various levels of training in knowledge generation, exchange, and translation. This collaborative model makes the SPOR Evidence Alliance strong and sustainable by leveraging the knowledge, lived experiences, expertise, skills, and networks among its 342 members and 12 principal investigators.
Introduction
The Strategy for Patient Oriented Research (SPOR) Evidence Alliance is a research collaboration among diverse groups in Canada whose mission is to promote the synthesis, dissemination, integration, and translation of research results into health decision-making for the Canadian health system. The SPOR Evidence Alliance was established after a successful funding competition by the Canadian Institutes of Health Research (CIHR), Canada’s major federal funding agency for health research. The funding competition was a call to address challenges and inefficiencies identified in the 2011 CIHR SPOR strategy (Canadian Institutes of Health Research 2011). The 2011 CIHR SPOR strategy called for a multi-jurisdictional approach to address the challenge of limited capacity to synthesize, disseminate, and integrate research results more broadly into clinical and public health practices, as well as public health and health care decision-making. The main goal of the SPOR Evidence Alliance is to address these challenges and close the “gap” between the publication of research findings and their integration into health systems (Kush 2019).
As part of the funding deliverables, a governance structure was mandated by the CIHR to ensure efficient management, increased accountability, continuous evaluation of strategic goals, and sustainability of the CIHR SPOR strategy. The aim of this paper is to (i) outline the governance and committee structure of the SPOR Evidence Alliance and how its structure is organised around the values of equity, diversity, and inclusivity; (ii) outline the procedures for patient, public, and health system decision-maker engagement; and (iii) present the capacity-building strategy for governance members. This paper will provide guidance to other large research initiatives that require a transparent governance structure for their operations.
This report is part of a series of papers that focus on the SPOR Evidence Alliance’s partnership between researchers, patients, and health system decision-makers to support rapid-knowledge translation. Further information on the purpose and organization of this research initiative can be found in Tricco et al. (2022). Here, we describe the governance structure of the SPOR Evidence Alliance, the third paper describes the research request services of the SPOR Evidence Alliance (Zarin et al. 2022), and the final paper describes patient engagement in the SPOR Evidence Alliance (Li et al. 2022).
The SPOR Evidence Alliance is led by 12 principal investigators and comprised of 342 active members (full list of members is found in the Supplementary Material, Appendix S1), including researchers, trainees, patient and public partners, and health system decision-makers across Canada and globally. Collaboration is an important cornerstone for dissemination and uptake of research evidence. By coordinating efforts amongst its members and decision-makers, the SPOR Evidence Alliance accelerates access to high-quality scientific evidence for use in shaping Canadian health care policy and decisions.
Governance and committees
The SPOR Evidence Alliance leadership is comprised of a nominated principal investigator (ACT) and 11 principal investigators (JC, AL, SES, HC, DM, WI, CG, AA, FC, LL, PM). The four central coordinating office members (WZ, SC, ST, SM) are based out of the Knowledge Translation Program of St. Michael’s Hospital, Unity Health Toronto, and coordinate the key operations of the SPOR Evidence Alliance. The coordinating office is the main administrative team responsible for managing all logistical and administrative tasks and implementing the committee meetings with direction from the principal investigators.
The principal investigators established a governance structure that would fulfill the CIHR mandate based on a “results-based” model, which employs a small number of committees structured around overall governance, rather than day-to-day management and operational functions (e.g., finance, human resources, programming) (O’Flynn and Wanna 2008). The SPOR Evidence Alliance’s six results-based standing committees include the International Advisory Committee, Steering Committee, Executive Committee, Knowledge Translation Committee, Partnerships Committee, and the Training and Capacity Development Committee (Fig. 1).
Fig. 1.
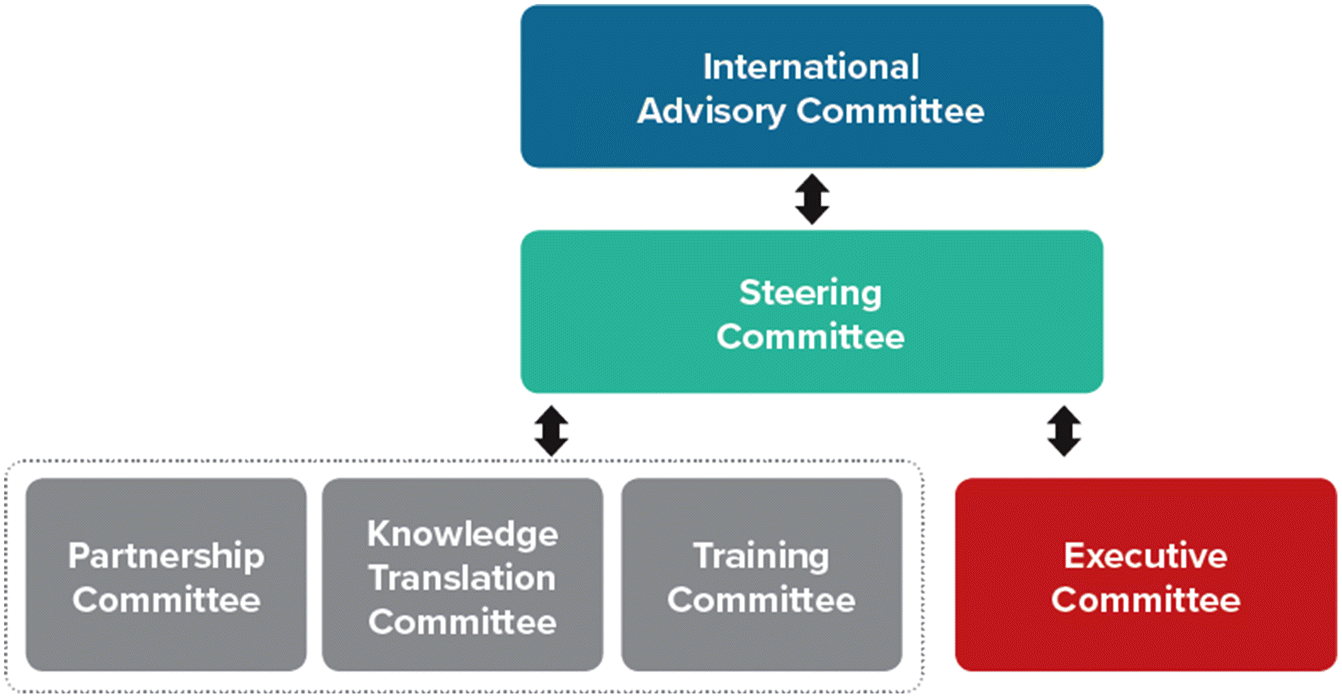
Each of the committees plays a unique role in advising the strategic direction and sustainability of the SPOR Evidence Alliance by providing advice to the principal investigators. All final decisions rest with the principal investigators, which is consistent with the structure of all research funding provided by the CIHR. The committees serve the following functions:
1)
The International Advisory Committee has an overarching function to provide input on activities (e.g., quality and productivity of core functions), future directions, and strategic opportunities (e.g., exploration of sustainability strategies, potential partnerships with international organizations with similar objectives).
2)
The Steering Committee is devoted to aligning all components of the initiative so that committee members contribute to achieving defined strategic goals and objectives. The Steering Committee provides strategic leadership on the overall direction of the SPOR Evidence Alliance, advises on budget, as well as potential funding partnerships. It is comprised of a representative from all other committees to allow sharing of communications across all committees.
3)
The Executive Committee advises on the query services outlined in Zarin et al. (2022), in this series, providing recommendations to the query research teams on budget, timeline, deliverables, and patient and stakeholder engagement. This committee also provides advice on the day-to-day operations of the SPOR Evidence Alliance and supports development of operational policies and procedures.
4)
The Partnerships Committee provides advice on how best to nurture existing partnerships and identify new linkages to support activities. They monitor engagement to ensure that patients’ perspectives are appropriately integrated in all activities and serve as champions for the SPOR Evidence Alliance within their respective networks. A key focus of the Partnerships Committee is to promote ethical and respectful research partnerships with Indigenous communities, ensure research projects promote Indigenous sovereignty and self-determination, and be respectful and inclusive of Indigenous knowledges and ways of knowing, being, and doing.
5)
The Training and Capacity Development committee advises on all capacity-building initiatives, providing advice on how these initiatives and participant experiences can be improved. This includes advising on how to optimize trainee and patient learning to empower them to become meaningful partners in the research process.
6)
The Knowledge Translation Committee advises on dissemination strategies, providing advice on how to ensure research findings and knowledge outputs can reach target audiences.
Guiding principles
All committee members must respect the guiding principles of the SPOR Evidence Alliance, which are inclusiveness (i.e., diversity regarding type of member whether it is a researcher, trainee, or decision-maker), support (i.e., creating a brave space), mutual respect (i.e., ensuring that there is shared power on all committees between researchers and decision-makers), transparency (e.g., open science), and co-building (e.g., co-production of research in partnership with patient and public partners and other relevant health system decision-makers). These guiding principles are communicated on the SPOR Evidence Alliance website, as well as in the terms of reference for the committees (SPOR Evidence Alliance n.d.). The principal investigators, committee chairs, and central office ensure that these principles are upheld in the meeting proceedings, all policy or procedure development, and the day-to-day operation of the SPOR Evidence Alliance. More information on our guiding principles can be found in Tricco et al. (2022).
Each of the SPOR Evidence Alliance’s standing committees are responsible for advising on the evaluation and long-term sustainability of the initiative. Here are some examples that illustrate how the committees provide their recommendations. The Knowledge Translation Committee (previously co-chaired by HC and succeeded by PM) helped to undertake a network-wide online survey and a focus group using a convenience sample of participants at the 2019 Annual General Meeting to identify dissemination priorities (Supplementary Material, Appendix S3). These dissemination priorities were in turn implemented across the SPOR Evidence Alliance. The Partnerships Committee (co-chaired by TH and formally PO) guided the development of the client experience survey to evaluate the degree of satisfaction from stakeholders who used the research query services, which are outlined in Zarin et al. (2022). The client experience survey is now used for every query to obtain feedback on the process and suggestions for improvement. The Executive Committee (chaired by ACT) advised on the development of the conflicts of interest policy, and the Steering Committee (co-chaired by AB, PNS, and formally MD) provided guidance in developing the patient partner appreciation policy to appropriately show appreciation for patient partners’ contributions within the initiative. These policies were directly implemented by the SPOR Evidence Alliance and are available on the website (SPOR Evidence Alliance 2020).
Committee member numbers and recruitment
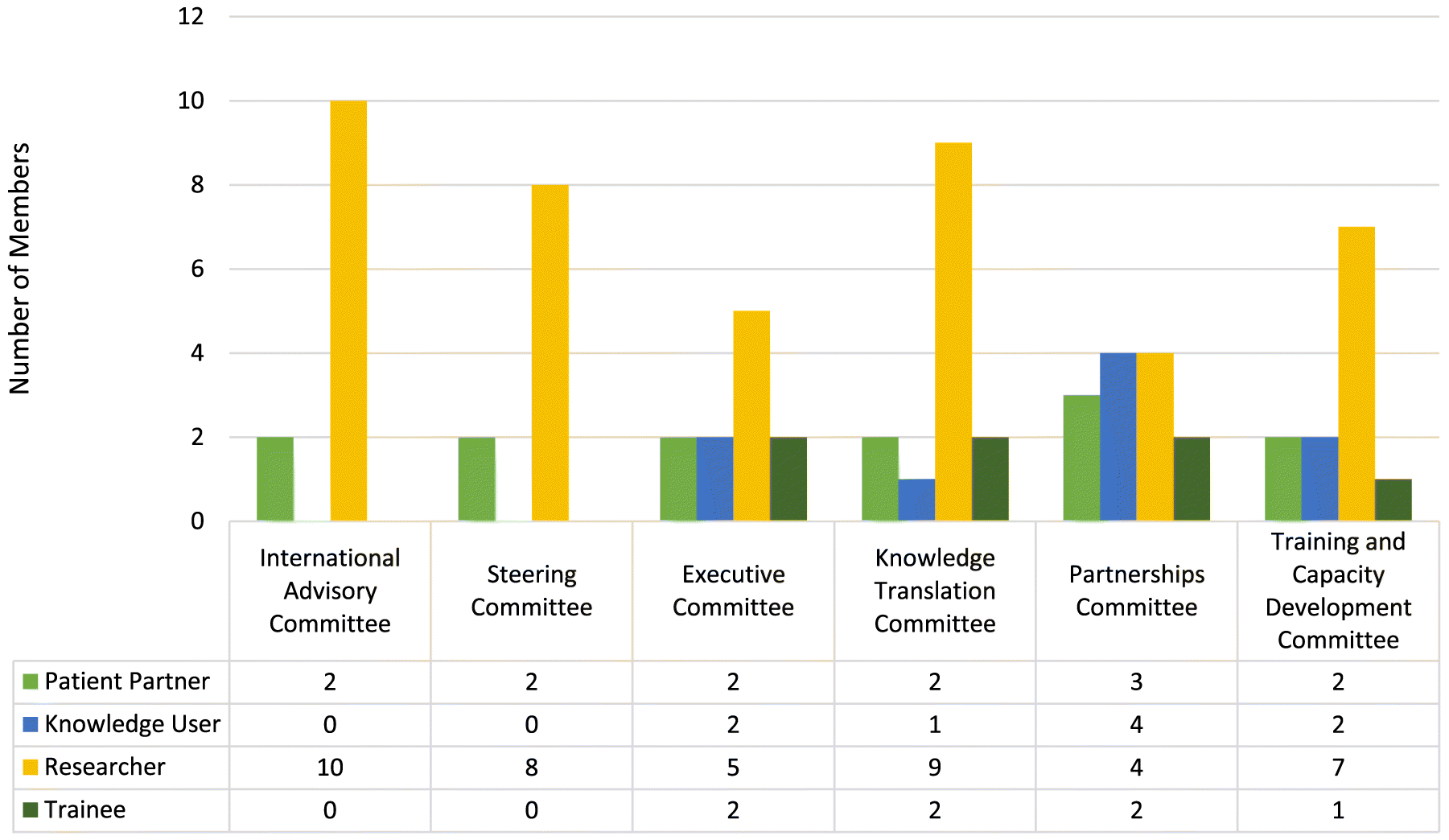
There are currently 64 committee members serving 72 seats across the six committees, 13 patient and public partners, 8 health system decision-makers, 7 research trainees, 36 researchers. Eight members are cross-appointed to the Steering Committee to facilitate continuity of communication across the governance structure (Figs. 2 and 3).
Fig. 2.
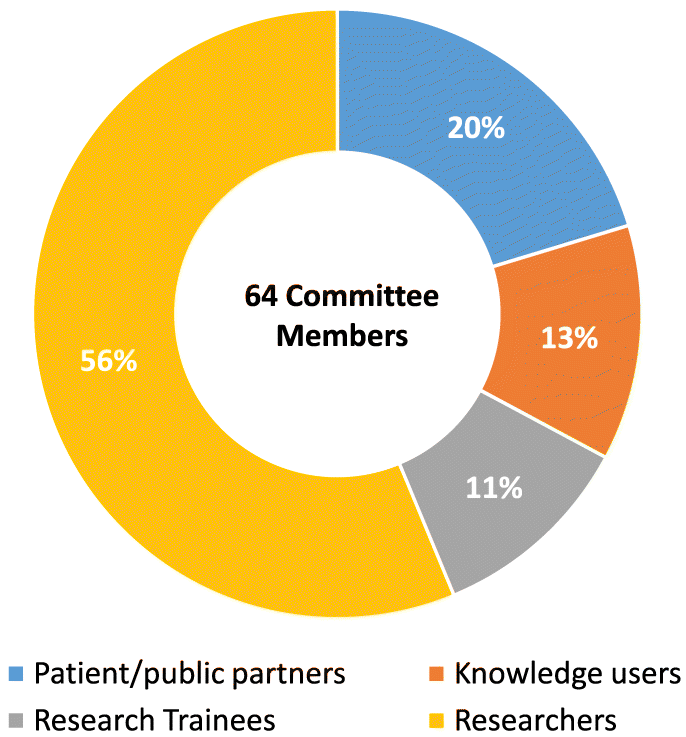
Fig. 3.
Members represent various organizations, academies, and institutes across Canada and beyond (Table 1). The original membership of the committees was strategically based on experience, expertise, geographic representation, gender diversity, and knowledge. Now, as seats become available, an open call to all SPOR Evidence Alliance members goes out for applications of interest based on their role and membership type (e.g., researcher, patient partner). Applicants apply through an online application process whereby they are asked to indicate their committee preference (first and second choice, depending on the number of committees with vacancies), provide a statement of intent, confirm availability for committee meetings, and submit a conflict of interest declaration. The central coordinating office then reviews all completed applications. The applications are put forward to the nominated principal investigator who reviews the applicants with consideration for the SPOR Evidence Alliance guiding principles, applicant experience, and interest. The proposed applications are presented to the applicable committee co-chairs for review, feedback, and final approval.
Table 1.
| Organisation, academy, or institute |
|---|
| Alberta Health Services |
| Alberta SPOR SUPPORT Unit |
| Arthritis Consumer Experts |
| Arthritis Research Canada |
| Aurora College |
| British Columbia Cancer Agency |
| British Columbia SUPPORT Unit |
| Canadian Arthritis Patient Alliance |
| Canadian Cardiovascular Society |
| Canadian Task Force on Preventive Health Care |
| Cancer Care Ontario |
| CHILD-BRIGHT Network |
| Cochrane Australia |
| Cochrane Methodology Review Group |
| Dalhousie University |
| Fraser Health Authority |
| Government of Prince Edward Island |
| Harvard Law School |
| Imperial College London |
| Kaiser Permanente Southern California |
| Laval University |
| Maritime SPOR SUPPORT Unit |
| McMaster University |
| Memorial University of Newfoundland |
| Michael Smith Foundation |
| Newfoundland SPOR SUPPORT Unit |
| Hotıì ts’eeda Northwest Territories SPOR SUPPORT Unit |
| Ontario SPOR SUPPORT Unit |
| Ottawa Hospital Research Institute |
| Public Health Agency of Canada |
| Quebec SPOR SUPPORT Unit |
| Queen’s University |
| Royal College of Physicians and Surgeons |
| Saskatchewan Centre for Patient-Oriented Research |
| Saskatoon Council on Aging |
| Simon Fraser University |
| Society for Academic Continuing Medical Education |
| SPOR Network CPN |
| St. Michael’s Hospital, Unity Health Toronto |
| Centre for Research in Evidence-based Practice |
| Joanna Briggs Institute |
| University of Adelaide |
| University of British Columbia |
| University of Calgary |
| University of Manitoba |
| University of Melbourne |
| University of New Brunswick |
| University of Northern British Columbia |
| University of Ottawa |
| University of Toronto |
Terms of reference
The terms of reference for each committee were originally drafted by the nominated principal investigator and the central coordinating office team. The terms of reference described each committee’s roles and responsibilities. Each committee independently reviewed their terms of reference against their committee mandates and updated the terms of reference, as deemed necessary to best support the activities of the committee. The terms of reference are reviewed on an annual basis and provided on the SPOR Evidence Alliance website (SPOR Evidence Alliance n.d.).
Meeting frequency
The meeting frequency for all the committees has varied over the years (Table 2).
Table 2.
| Committee | Meeting frequency year 1 | Meeting frequency year 2 and onwards |
|---|---|---|
| International Advisory Committee | Every six months Annual general meeting | Once a year Annual general meeting—optional |
| Steering Committee | Every month Annual general meeting | Every 3 months Annual general meeting |
| Executive Committee | Every month Annual general meeting | Every 4 months Annual general meeting |
| Knowledge Translation Committee Partnerships Committee Training and Capacity Development Committee | Every 3 months Annual general meeting | Every 4 months Annual general meeting |
The annual general meeting is an opportunity for all members of the governance structure to meet face-to-face every year. The intention is to foster networking and promote relationships and build trust between researchers, trainees, patient and public partners, and decision-makers. Every year there is a theme for the Annual General Meeting to enhance learning and capacity building. The agendas for the previous meetings can be found in Supplementary Material, Appendices S2, S3, and S4.
Inclusion of the patient and public partner voice in the SPOR Evidence Alliance governance structure
Patients and public are included in all the SPOR Evidence Alliance’s core research activities, leadership, and governance. The SPOR Evidence Alliance aims to engage with a wide range of patient and public partners and other decision-makers across Canada and supports the important roles that patients can serve in research.
Upon formation of the SPOR Evidence Alliance, a separate patient partner committee was originally conceptualized by the principal investigators. However, after hearing from the patient partners with extensive experience in governing research initiatives, it was decided that patients should be integrated into all committees as co-chairs and as members. Patient partners have equal voting and decision-making privileges as other members who sit on committees. Further information on patient engagement in the SPOR Evidence Alliance can be found in Li et al. (2022).
Inclusion of Indigenous Peoples’ voices in the SPOR Evidence Alliance governance structure
The original governance structure for the SPOR Evidence Alliance proposed the inclusion of an independent Indigenous Peoples’ sub-committee within the partnerships committee. This sub-committee was to be responsible for outreach to Indigenous communities to identify research projects that could be conducted by researchers affiliated with the SPOR Evidence Alliance. To establish this sub-committee, the SPOR Evidence Alliance leadership engaged in consultation with two individuals (JJ, JW) who have extensive knowledge of partnership-based, community-initiated health research with Indigenous Peoples. They advised a thoughtful approach to partnerships with Indigenous Peoples due to the history of research being conducted in an exploitive way against Indigenous communities in Canada (Ball and Janyst 2008; Smith 2012). A decision was made and agreed upon, to have these individuals join the partnerships committee so that their activities could be shared and contribute to capacity building within the SPOR Evidence Alliance membership.
As there is currently no guidance for the conduct of ethical, equitable knowledge synthesis activities, additional time and funding was required to begin the work to establish and support ethical and equitable knowledge synthesis strategies inclusive of Indigenous Peoples. Their work is currently in the early stages to build networks and materials to support activities that (i) ensure all knowledge synthesis and related activities promote Indigenous sovereignty and self-determination; (ii) promote the development of knowledge synthesis strategies that are respectful and inclusive of Indigenous knowledge and ways of knowing, being, and doing; and (iii) ensure Indigenous Peoples are ethically engaged on projects (Walker et al. 2021).
Capacity building of the committee members
The SPOR Evidence Alliance builds capacity towards developing and disseminating knowledge synthesis, clinical practice guideline development, knowledge translation, and patient-oriented research. Using the SPOR Capacity Development Framework (Canadian Institutes of Health Research 2015), the SPOR Evidence Alliance offers multidisciplinary mentorship opportunities in various settings with a focus on professional skills development, best practices, effective resource sharing, and career support. These principles are used to involve trainees and early career investigators as participants across all committees. It is hoped that their participation will allow the development and refinement of their leadership skills, as well as provide learnings on how to conduct and lead research projects.
Canadian agencies with similar governance structures
Research initiatives similar to the Evidence Alliance exist across Canada, as outlined in Tricco et al. (2022). Although the governance structures of these initiatives vary, they all include committees with varying responsibilities. Here we highlight the governance structures for two similar initiatives and compare theirs with the governance model that was established for the SPOR Evidence Alliance.
Canadian agency for drugs and technologies in health
The Canadian Agency for Drugs and Technologies in Health (CADTH) is an independent, not-for-profit organization established in 1989 (Canadian Agency for Drugs and Technologies in Health n.d.). Similar to the SPOR Evidence Alliance, CADTH’s role is to deliver evidence, analysis, advice, and recommendations to health care decision-makers so that they can make informed decisions (Rawson and Adams 2017). However, CADTH is not considered an academic research endeavour and is not funded by CIHR like the SPOR Evidence Alliance is. Compared to the SPOR Evidence Alliance, CADTH’s governance structure is more formal because of its contract with the Government of Canada who “owns,” funds, and manages it. CADTH is managed by a board of 13 directors. More than three-quarters of CADTH’s board members are employed by, or directly associated with, federal, provincial, or territorial governments. The board comprises four types of directors: jurisdictional (seven individuals holding Deputy Minister, Assistant Deputy Minister, or Chief Clinical Advisor positions in federal, provincial, or territorial governments of which six are directly responsible for drug plans), health authorities (a provincial health network administrator and a practicing physician), academic (an individual with a relationship with the Government of British Columbia) and public (two individuals who are members of various companies and institutional boards).
Drug safety and effectiveness network
The Drug Safety and Effectiveness Network (DSEN) was established by CIHR, Health Canada, and other stakeholders in 2011. Similar to CADTH and the SPOR Evidence Alliance, DSEN is responsible for conducting all types of reviews (e.g., systematic reviews, rapid reviews, scoping reviews, network meta-analysis) for CADTH (Drug Safety and Effectiveness Network n.d.), Health Canada, the Public Health Agency of Canada (PHAC), the ministries of health, and the World Health Organization, among others. However, the focus is solely on drugs. DSEN is a pan-Canadian team of investigators with world-renowned expertise in knowledge synthesis, biostatistics, knowledge translation, clinical epidemiology, sex and gender analysis, and health economics. Similar to the SPOR Evidence Alliance’s organizational structure, their governance structure comprises a coordinating office, an executive working group, a steering committee, and a scientific committee. Three main coordinating centres conduct research activities based on their specific directives (e.g., the Methods and Applications Group for Indirect Comparisons conducts network meta-analyses) (Drug Safety and Effectiveness Network Methods and Applications Group for Indirect Comparisons n.d.).
Challenges in the governance structure
Many challenges were faced by the SPOR Evidence Alliance when forming the committees. As with any iterative process, members of the committee felt that the terms of reference lacked clarity and requested to have clear roles outlined for chairs and members. As such, each committee also refined their committee mandates. In the original terms of reference, the difference between an advisory role (making recommendations) and decision-making role of the committees was not clearly defined and articulated. Clearly understanding and making this distinction in the terms of reference was an important lesson learned as committee members were uncertain on the decision-making chain at the outset. Once the decision-making authority was made clear, the committees felt more confident in discussing action items and collectively making key recommendations to the principal investigators.
One important challenge that has been encountered is the recruitment and retention of patient partners as members and co-chairs of the committees. Previously, patient partners have been recruited through word of mouth and an open call for applications. A review of recruitment strategies for patient engagement in health research identified four recruitment strategies for patient engagement: social marketing, community outreach, health system recruitment, and partnering recruitment (Vat et al. 2017). For the SPOR Evidence Alliance, health system, community outreach, and social marketing recruitment strategies would be the most effective. To identify patients with a particular condition or experience, the health system recruitment strategy may be the most effective, whereas community outreach might be more helpful to reach specific communities, such as diverse cultural groups. These strategies will be considered in the future to attract patient partners to engage in the SPOR Evidence Alliance.
Conclusion
Organizations with comprehensive governance frameworks are enabled to make effective decisions and advance their objectives. Established rules of governance identify clear roles for committee members, executives, and staff, and how they play into the overall organizational structure. Governance structures solidify each person’s position so that they will not stray away from the organization’s mission.
The SPOR Evidence Alliance’s inclusive governance model and committee structure supports patients and other stakeholders in the governance across all operations. A multi-disciplinary and diverse array of Canadians are represented from across Canada and at various levels of training in knowledge generation and translation. The reader is directed to the other papers in this series that outline other structural and organisational components of the SPOR Evidence Alliance as a Canadian model to build a rapid-learning health system (Li et al. 2022; Tricco et al. 2022; Zarin et al. 2022). This collaborative model makes the SPOR Evidence Alliance strong and sustainable by leveraging the knowledge, expertise, evidence, and skills of its 354 members and 12 principal investigators.
Funding
The Strategy for Patient-Oriented Research Evidence Alliance (SPOR EA) is supported by the Canadian Institutes of Health Research (CIHR) under the Strategy for Patient-Oriented Research (SPOR) initiative (grant number GSR-154442), and the generosity of partners from 41 public and not-for-profit agencies across Canada. SES is funded by a Tier 1 Canada Research Chair in Knowledge Translation. ACT is funded by a Tier 2 Canada Research Chair in Knowledge Synthesis. CL is funded by a CIHR project grant (grant number 451190). DM is funded by a University of Ottawa Research Chair. LCL is funded by a Tier 2 Canada Research Chair in Patient-oriented Knowledge Translation.
Acknowledgements
Our sincere thanks to Dr. Brian Mittman and Mr. Peter Oxland for serving in the governance structure and co-chairing the International Advisory Committee and the Partnerships Committee, respectively. We gratefully acknowledge Sinit Michael for referencing and providing logistical support in the preparation of this manuscript.
References
Ball J and Janyst P. 2008. Enacting research ethics in partnerships with indigenous communities in Canada: “Do it in a good way”. Journal of Empirical Research on Human Research Ethics, 3(2): 33–51.
Canadian Agency for Drugs and Technologies in Health. n.d. CADTH FAQs [online]: Available from cadth.ca/about-cadth/who-we-are/faqs.
Canadian Institutes of Health Research. 2011. Canada’s strategy for patient-oriented research: improving guideline development, dissemination and uptake [online]: Available from cihr-irsc.gc.ca/e/44000.html#a4.4.6.
Canadian Institutes of Health Research. 2015. Capacity development framework [online]: Available from cihr-irsc.gc.ca/e/49307.html.
Drug Safety and Effectiveness Network. n.d. Our governance [online]: Available from dsenmagic.com/our-governance/.
Drug Safety and Effectiveness Network Methods and Applications Group for Indirect Comparisons. n.d. DSEN MAGIC [online]: Available from dsenmagic.com/.
Kush R. 2019. Learning health systems: connecting research to practice worldwide: opening guest commentary. Learning Health Systems, 3(1): e10078.
Li LC, Hoens AM, Wilhelm L, Bubber V, PausJenssen E, McKinnon A, et al. 2022. Patient engagement in the SPOR Evidence Alliance: Reflection and learnings. FACETS 7(1): 126–138.
O’Flynn J, and Wanna J. 2008. Collaborative governance: a new era of public policy in Australia? ANU E Press, Canberra, Australia.
Rawson N, and Adams J. 2017. Do reimbursement recommendation processes used by government drug plans in Canada adhere to good governance principles? ClinicoEconomics and Outcomes Research, 9: 721–730.
Smith L. 2012. Decolonizing methodologies: research and indigenous peoples. 2nd ed. Zed Books Ltd., New York, USA. 4 p.
SPOR Evidence Alliance. 2020. Patient partner appreciation policy and protocol [online]: Available from sporevidencealliance.ca/wp-content/uploads/2020/10/SPOR-EA_Patient-Partner-Appreciation-Policy-and-Procedure_2020.pdf
SPOR Evidence Alliance. n.d. Our governance [online]: Available from sporevidencealliance.ca/about/governance-structure/.
Tricco A, Zarin W, Clement F, Abou-Setta A, Curran J, LeBlanc A, et al. 2022. Introducing the strategy for patient oriented research (SPOR) evidence alliance: A partnership between researchers, patients and health system decision-makers to support Rapid-learning and responsive health systems in Canada and beyond. FACETS 7(1).
Vat E, Ryan D, and Etchegary H. 2017. Recruiting patients as partners in health research: a qualitative descriptive study. Research Involvement and Engagement, 3: 15.
Walker J, Jull J, Forbes A, and Mongague H. 2021. Centering indigenous knowledges: engaging with indigenous ways of knowing, being and doing in knowledge synthesis, SPOR evidence alliance annual general meeting.
Zarin W, Lunny C, Chaudhry S, Thomas SM, LeBlanc A, Clement F, et al. 2022. A Canadian model for providing high-quality, timely and relevant evidence to meet health system decision-maker needs: the SPOR Evidence Alliance. FACETS 7(1):
Supplementary Material
Supplementary Material 1
- Download
- 932.14 KB
Information & Authors
Information
Published In

FACETS
Volume 7 • Number 1 • January 2022
Pages: 435 - 447
Editor: Debra Clendinneng
History
Received: 26 August 2021
Accepted: 9 December 2021
Version of record online: 24 March 2022
Notes
This paper is part of a collection titled “Strategy for Patient Oriented Research (SPOR) Evidence Alliance: A Canadian Model to Build Rapid-learning Health Systems”.
Copyright
© 2022 Lunny et al. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Data Availability Statement
The data and materials used and (or) analysed during this study are available from the corresponding author on reasonable request.
Key Words
Sections
Subjects
Authors
Author Contributions
CL developed the initial outline and wrote the manuscript.
WZ contributed to the development of all operational plans and wrote sections of the manuscript.
ACT edited the initial outline, wrote sections of the manuscript, and revised the manuscript.
AL, HC, LCL, FC, JAC, AMA-S, CG, DM, PM, WI, SES, and ACT obtained funding, conceptualized the research initiative, provided guidance on the operation and direction of the initiative, and reviewed the manuscript.
All are involved with the governance structure, contributed to the interpretation and narrative of the paper, reviewed, and revised the content, approved the final version, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work.
Competing Interests
All authors have nothing to declare.
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Carole Lunny, Wasifa Zarin, Sabrina Chaudhry, Sonia M. Thomas, Annie LeBlanc, Sophie Desroches, Tanya Horsley, Heather Colquhoun, Priscille-Nice Sanon, Minnie Downey, Zahra Goodarzi, Nancy N. Baxter, Kelly English, Elliot PausJenssen, Shanon McQuitty, Linda Wilhelm, Annette McKinnon, Alison M. Hoens, Linda C. Li, Fiona Clement, Janet A. Curran, Ahmed M. Abou-Setta, Christina Godfrey, David Moher, Pertice Moffitt, Jennifer Walker, Janet Jull, Cheryl Koehn, Wanrudee Isaranuwatchai, Sharon E. Straus, and Andrea C. Tricco. 2022. An inclusive and diverse governance structure of the strategy for patient-oriented research (SPOR) Evidence Alliance. FACETS.
7(): 435-447. https://doi.org/10.1139/facets-2021-0129
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
Cited by
1. Introducing the Strategy for Patient Oriented Research (SPOR) Evidence Alliance: a partnership between researchers, patients and health system decision-makers to support rapid-learning and responsive health systems in Canada and beyond
2. A Canadian model for providing high-quality, timely and relevant evidence to meet health system decision-maker needs: the SPOR Evidence Alliance
