Introduction
Global ocean temperatures have been rising for decades, including in the Northwest Atlantic, where temperature increases have been observed throughout the water column (
Kavanaugh et al. 2017;
Rheuban et al. 2017). The biological response to these changing conditions on marine populations can be profound, altering physiology, phenology, species interactions, and the availability of suitable habitat (
Doney et al. 2012;
Miller et al. 2018;
Maxwell et al. 2019), which may, in turn, influence the abundance, distribution, and overall success of marine species (
Shackell et al. 2014;
Stortini et al. 2015;
Kavanaugh et al. 2017). Particularly, warming temperatures can lead to geographical shifts in suitable habitat and corresponding shifts in marine taxa distributions (
Shackell et al. 2014;
Hale et al. 2017;
Kavanaugh et al. 2017;
Rheuban et al. 2017;
Pinsky et al. 2018;
Greenan et al. 2019;
Timbs et al. 2019)
. Changes in climate can induce habitat loss, decrease fecundity, and increase mortality, resulting in an overall decline in population abundance (e.g.,
Maxwell et al. 2019). Generally, these changes have been associated with overfishing (
Pauly et al. 2002). The influence of climate change and overfishing on abundance and stock structure has been well studied for species experiencing abundance declines (
Ciannelli et al. 2013;
Reuchlin-Hugenholtz et al. 2015,
2016), whereas the stock structure of species experiencing abundance increases, including species recently recovered from known historically low abundances, has been much less examined.
In the Northwest Atlantic, Atlantic halibut
Hippoglossus hippoglossus (Linnaeus, 1758) has experienced a monotonic increase in biomass and regional landings since the early 2000s in Canadian waters (
Fig. 1;
Shackell et al. 2022). Atlantic halibut is the largest groundfish in the Atlantic Ocean, can grow to more than 2.5 m long with weights exceeding 300 kg, and can reach ages up to 50 years (
Cargnelli et al. 1999;
Armsworthy et al. 2014). Atlantic halibut occupy a broad geographic range in the Northwest Atlantic, from the coast of Virginia in the south to the waters off Disko Bay, Greenland, in the north. It occupies a temperature range from −1.5 to 15 °C and depths from 20 to 1640 m (
Cargnelli et al. 1999;
Armsworthy et al. 2014;
Murphy et al. 2017). Though Atlantic halibut occupies a wide geographic range, its distribution is not uniform (
Shackell et al. 2022). Juvenile Atlantic halibut, in particular, forms persistent aggregations (
Boudreau et al. 2017). The extent of juvenile Atlantic halibut habitat has been shown to be proportional to adult landings within Northwest Atlantic Fishery Organization (NAFO) management divisions (
French et al. 2018).
Prior to the 1830s, Atlantic halibut was routinely discarded by fishers as a nuisance catch because its thick flesh made salt preservation unreliable (
Grasso 2008). As the demand for fresh fish grew in the 1840s, the Atlantic halibut fishery expanded in U.S. waters, leading to a collapse by 1880 (
Moore 1999;
Kanwit 2007;
Grasso 2008). Atlantic halibut was not as heavily fished in Canadian waters, although there were declines prior to the establishment of the Exclusive Economic Zone in 1977, after which Atlantic halibut began to recover. In 1988, Canada implemented regulations on the Canadian Atlantic halibut fishery (
den Heyer et al. 2013;
DFO 2015a). Despite the establishment of regulatory measures, Atlantic halibut and many other groundfish species experienced a period of decline in the early 1990s, leading to stock collapses and fishing moratoria in some areas (
Bundy et al. 2019;
Shackell et al. 2021). At present, most groundfish species have yet to fully recover (
Pedersen et al. 2017;
Shackell et al. 2021). However, Atlantic halibut is an exception, and has recovered to levels greater than those prior to its most recent collapse. This increased abundance is reflected in increased landings in recent years (
Cox et al. 2016;
Trzcinski and Bowen 2016;
DFO 2018). Currently, Atlantic halibut is one of the most commercially valuable groundfish species in the Northwest Atlantic (
DFO 2019). Atlantic halibut is assessed and managed as three stocks in the U.S. and Canada. These stocks include two in Canada, the Gulf of St. Lawrence corresponding to NAFO Divisions 4R, 4S, and 4T (
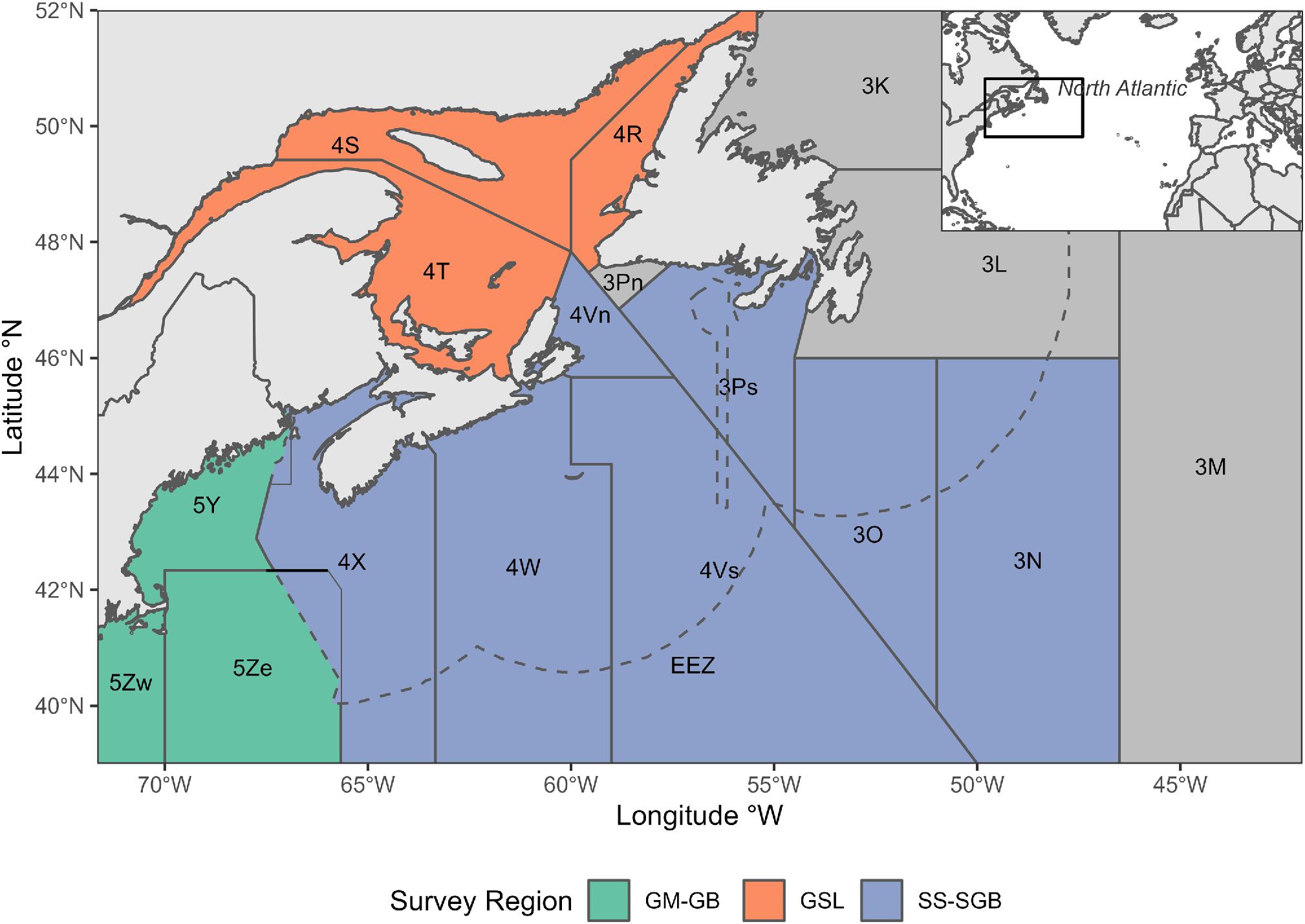
DFO 2015b) and the Scotian Shelf-Southern Grand Banks corresponding to NAFO Divisions 3N, 3O, 3Ps, 4V, 4W, 4X, and 5Zw (
DFO 2015a), and one in the U.S., the Gulf of Maine-Georges Bank stock corresponding to NAFO Divisions 5Y and 5Ze (
Fig. 1).
The current economic importance of Atlantic halibut has led to increased research efforts, especially with respect to halibut spatial ecology. Through a combination of genomic studies, migration patterns observed from electronic and conventional tagging, and geographic variation of life history traits, the emerging population structure shows two subtly genetically distinct populations (
Kess et al. 2021), within which there are subpopulations comprised of multiple migratory contingents (
Shackell et al. 2022). It is unknown how climate change will influence the ecology or biology of Atlantic halibut or how either will influence stock dynamics in the study region. Temperature is a key variable constraining the available habitat of juvenile Atlantic halibut (
French et al. 2018) and has also been linked to variation in growth and maturity schedules across the Northwest Atlantic (
Shackell et al. 2019). The clear links between temperature and the physiology and ecology of Atlantic halibut motivate an exploration of the relationship between recent demographic changes in halibut and warming in the Northwest Atlantic (
Brickman et al. 2021). The characterization of the historical spatial-temporal relationship between temperature, habitat conditions, and abundance will provide a foundation to predict how Atlantic halibut stocks will respond to continued warming, providing critical information to fisheries’ managers to anticipate how the managed stocks of Atlantic halibut could respond to continued warming in the Northwest Atlantic.
Species distribution models (SDMs) are widely used, powerful, and practical for understanding species-specific niches, predicting abundances, and/or mapping distributions (
Robinson et al. 2017). The correlative SDM approach models environmental variables that correlate with abundance or presence/absence data. Generalized additive models (GAMs) are increasingly used to model species distributions because they can capture nuanced, nonlinear relationships between environmental covariates (e.g., temperature, depth, and space) and the presence of a target taxon (
Murase et al. 2009;
Grüss et al. 2018). GAMs are often used to identify optimal environmental conditions and/or to predict the probability of the presence or abundance of a taxon based on a set of observed conditions (e.g.,
Wood 2006). GAMs are particularly well suited to model novel environmental conditions associated with climate change (
Chaudhary et al. 2021;
Champion et al. 2022).
Here we explore the influence of climate change on the distribution of Atlantic halibut in the Northwest Atlantic. We first looked retrospectively, evaluating whether recent changes in Atlantic halibut distribution in the Northwest Atlantic were related to contemporaneous spatial-temporal trends in thermal habitat availability and growing degree days (GDDs). GDD is a measure of thermal time, or the cumulative temperature over a given period (
Neuheimer and MacKenzie 2014). We then build on these functional relationships and predict, using a correlative SDM approach, the distribution of Atlantic halibut in the Northwest Atlantic based on a regionally downscaled oceanographic model and climate forecasts modelled under two emission scenarios: representative concentration pathway (RCP) 4.5 (reduced emissions) and RCP 8.5 (high emissions).
Methods
Study area
The domain for this study encompasses most of the area occupied by Atlantic halibut in the Northwest Atlantic. This area ranges, from north to south, from Labrador to Maryland, and from the inner Gulf of St. Lawrence to the edge of the Flemish cap (total area range covers about 38–52° N, and about 40–70° W) encompassing three spatial management units in Canadian (Gulf of St. Lawrence and the Scotian Shelf-Southern Grand Banks) and U.S. waters (Gulf of Maine–Georges Bank) and the corresponding NAFO Divisions (
Fig. 2).
Survey data
In Canada, the Fisheries and Oceans Canada (DFO) Research Vessel (RV) trawl surveys have been conducted annually since the 1970s (
Ricard and Shackell 2013). Random stratified surveys are conducted within spatially distinct regions, with vessel timing and gear type varying depending on management needs. For Atlantic halibut, these surveys provide information on abundance and recruitment, which are used for stock assessment (
DFO 2015b;
Cox et al. 2016;
Trzcinski and Bowen 2016). Data from regional surveys in the Scotian Shelf, Newfoundland and Southern Grand Banks, the Northern Gulf of St. Lawrence, and the Southern Gulf of St. Lawrence were used to model the distribution of Atlantic halibut. Atlantic halibut captured during these trawl surveys were predominantly smaller individuals (30–80 cm).
Trawl surveys conducted in U.S. waters by the National Oceanic and Atmospheric Administration (NOAA) and National Marine Fisheries Service (NMFS) provide a similar index to the Canadian surveys of abundance and recruitment (
Sigourney et al. 2006;
Col and Legault 2009). The gear type for both the fall and spring surveys changed over the time series. Various conversions have been used to correct abundance indices for groundfish due to gear changes; however, conversion factors are not available for Atlantic halibut. Most Atlantic halibut caught in the U.S. research bottom trawl surveys from 1977 to 2000 were <80 cm based on length–frequency distributions (
Sigourney et al. 2006). Atlantic halibut under 80 cm in Canadian and U.S. waters are assumed to be juveniles based on known length at maturity relationships (
Sigourney et al. 2006;
Shackell et al. 2019). Combined data from DFO and NOAA surveys spanned from 1965 to 2019 and included variables such as latitude, longitude, temperature, depth, region, year, abundance, biomass, and weight (
Table 1).
All groundfish trawl survey data were curated, and variables were modified to form a constant naming convention between surveys. A binomial presence/absence approach was adopted for SDMs to make the data more comparable, given the catchability differences inherent among surveys with different vessels, gear types, and seasons. Models were assumed to represent juvenile Atlantic halibut because most fish in both the Canadian and U.S. surveys were <80 cm long.
High-resolution ocean model
Thermal habitat and growing degree days
To characterize changes in the thermal habitat suitability of Atlantic halibut in the study range, we opted for thermal averages and GDDs. This decision was based on the availability of high-quality temperature data and the recognized impact of temperature on the duration and intensity of the growing season. Thermal averages and GDD have widespread use, physiological relevance, data availability, and comparability across studies, making them suitable indicators for this purpose. Other indicators, such as minimum and maximum temperatures or thermal variability, are not as commonly used and may have limited utility for Atlantic halibut due to its broad thermal tolerance.
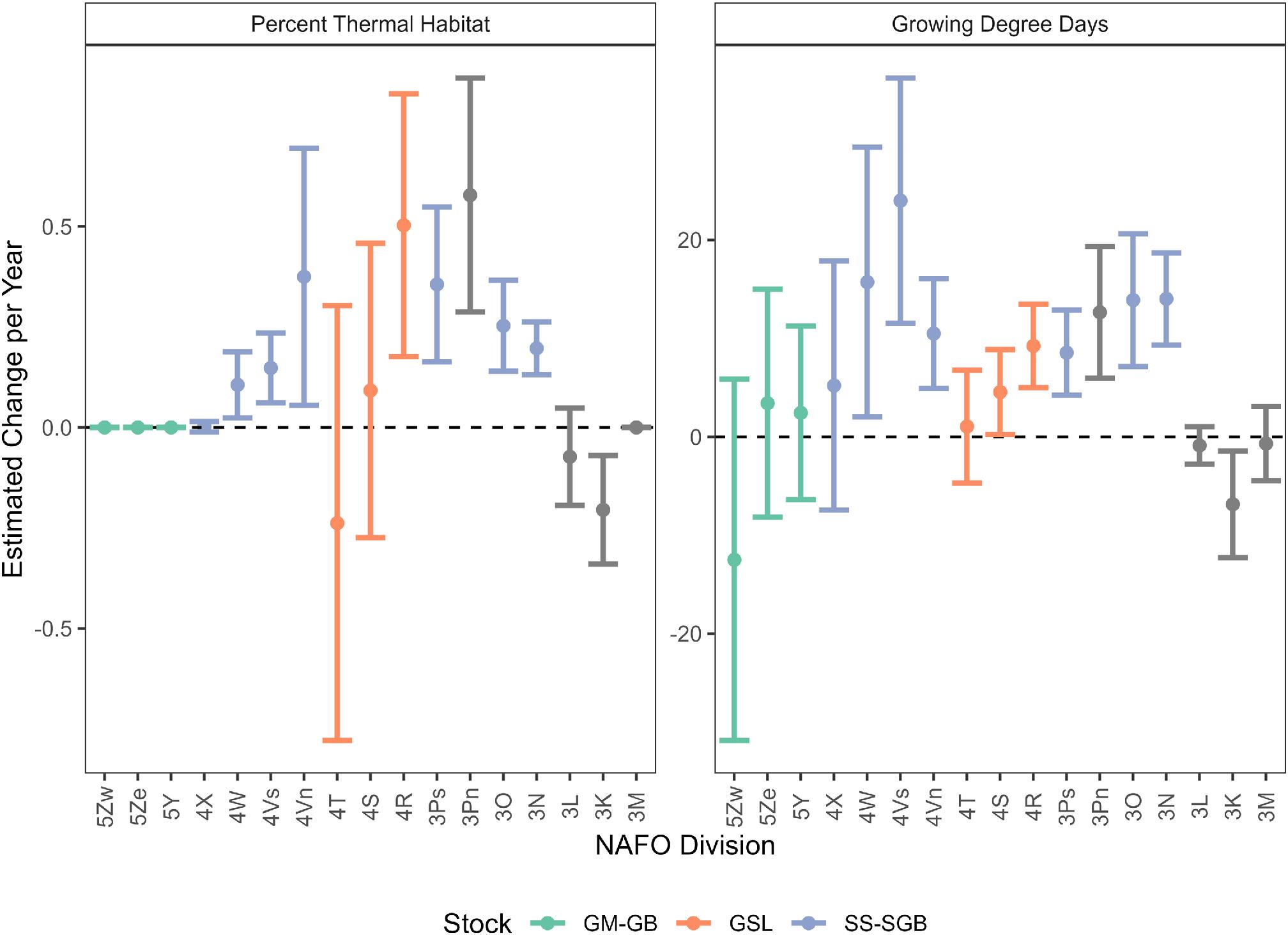
For thermal averages, a niche-threshold approach was applied, whereby changes in the available habitat were documented through time based on thermal and depth ranges. Here, the thermal habitat range of Atlantic halibut was derived using conservative percentiles of 0.10 and 0.90 for bottom temperatures in halibut occurrence data. The depth range was calculated using a slightly more relaxed set of percentiles of 0.5 and 0.95, as depth remains constant over time and to account for the steeper slope and greater depths found off the coast of Newfoundland. Using these niche-based thresholds, ratios describing the extent of depth-constrained thermal habitat relative to the total area within each NAFO Division (expressed as a percentage) were evaluated using annual averages, longitudinally from 1990 to 2018. A linear model (LM) was applied to evaluate temporal trends, assuming a Gaussian error distribution, where % thermal habitat was modelled as a function of the interaction between year and NAFO Division. The mean slope and confidence intervals were extracted from the LM per NAFO Division.
GDDs are the number of days (per year) multiplied by a daily temperature above a threshold (°C·day). The concept of GDD was derived in agriculture to reflect that growth rates depend on temperature and the period above a critical threshold temperature (
Neuheimer and Taggart 2007;
Neuheimer et al. 2008;
Neuheimer and Grønkjær 2012) and can be used as an index of growing season length. For this analysis, the lower bound of the thermal range (2.46–9.25 °C) was used as the threshold, and depth (36–415 m) limits were applied as described above. Projections available from BNAM were aggregated monthly. The number of days in each month was multiplied by the average bottom temperature at each grid cell for months with a temperature above the thermal threshold; otherwise, values were set to zero. This returned the monthly GDD for each grid cell, which was then averaged over NAFO Divisions by month. To correct for the size of each NAFO Division, the product of the monthly average GDD and the fraction of grid cells above the threshold of 2.46 °C over the total number of grid cells in the corresponding NAFO Division was used to calculate the scaled monthly GDD:
where sGDD represents the scaled monthly GDD for a respective division (
d) and month (
m) and Cells
GDD represents the number of cells that are above the threshold of 2.46 °C. The scaled monthly GDD were then summed for each year to obtain the GDD for each region by year. Temporal trends of GDD were evaluated using a LM, assuming a Gaussian error distribution, for each NAFO Division over a 28-year period from 1990 to 2018. The mean slope and confidence intervals were extracted from the LM per NAFO Division for comparison.
LMs were used to estimate the rate of change of percentage thermal habitat and GDD for each NAFO Division. The response variable, thermal habitat, was estimated using year as a covariate and NAFO Division as a factorial grouping variable. Modelling began with a full model, and if the interaction term was significant, differences in the covariate (year) among NAFO Divisions were tested. LMs provided the difference between NAFO Divisions relative to division 3M, which was chosen as a point of reference because its rate of change was closest to zero in all models. The same procedure was followed to extract the slope coefficients and p-values for GDD. Net directional change was assessed as NAFO Divisions, which had confidence intervals excluding zero.
Species distribution modelling
We chose species distribution modelling to represent the realized niche of Atlantic halibut, in terms of temperature, depth, and space combined. This approach is likely more restrictive than a potential niche approach. Binomial GAMs were used to associate spatial information and environmental covariates with the presence of Atlantic halibut. GAMs included two continuous environmental covariates, bottom depth (m) and bottom temperature (°C), chosen as the primary environmental determinates of habitat suitability for Atlantic halibut (
French et al. 2018); two categorical variables, season to account for differences in bottom temperatures for surveys undertaken at different times of the year, and region to account for other differences such as catchability among regions associated with variation in bottom type, vessel, and gear type; and two geographical covariates, longitude and latitude to account for spatial autocorrelation that would not otherwise be resolved by the regional covariates. Environmental covariates were used from the DFO RV survey trawl data or NOAA NMFS surveys, where any survey set (trawl) with observed Atlantic halibut abundance was categorized as a presence and any set without any observed abundance as an absence. The SDM model approach was structured based on the conventions outlined in
Zurell et al. (2020) and
Pedersen et al. (2019), including model selection, model diagnostics, and assumptions for SDMs. Models were fit using the mgcv package (
Wood 2011) in R (
R Core Team 2021), following model statements in mgcv such as:
where
p is the probability of occurrence when given binomial response data,
g is the link function (logit link) between
p and each additive predictor,
s is a thin-plate regression spline fitted to a given predictor, and
te is a tensor product smooth fitted to the interaction between longitude and latitude that accounts for the spherical distance of longitude units because they scale differently depending on latitude, meaning that isotropy is not an appropriate assumption for this smooth (
Wood 2006). The “by” argument specifies the group-specific smoother for the covariate.
The number of bases (
k) for each smooth function was reviewed for each covariate, and their default values were retained with
k = 25 for latitude and longitude, 10 for temperature and 10 for depth as any less would result in clear underfitting. In mgcv, the argument method = “REML” (restricted maximum likelihood) was used to penalize the model for being too wiggly, which helps to reduce the likelihood of overfitting (
Wood 2017).
GAM evaluation and validation
The presence of concurvity in the SDM was investigated using functions within the mgcv package. Additionally, receiver operator characteristic (ROC) curve cross-validation methods were applied, using several data formulations to evaluate model performance. First,
k-fold cross-validation was done using 10 randomly separated data “folds” of the full dataset, where 10% of the data was held for validation. Second, a cross-validation using a “past and present” method was done, where the model was trained based on data from a historical period (1970–1999) and tested against data from a more contemporary period (2000–2018). Finally, hindcast monthly BNAM temperature and depth were extracted for each observation and used in place of the original temperature and depth readings to fit the model, and the trained model was then tested against the original data. Area under the curve (AUC) values of 0.5 indicated a model is incapable of predicting any better than random chance, while an AUC of 1 indicated a perfect ability to predict presence or absence. AUC values less than 0.7 were considered to have poor discrimination, whereas values from 0.7 to 0.9 indicate reasonably accurate discrimination, and values above 0.9 indicate exceptionally good discrimination (
Swets 1988).
Model selection was conducted based on evaluating which model had the optimal (best) balance between the greatest explanatory predictive power and the fewest necessary covariates. The full model was compared against reduced models using AUC values, AIC, and deviance explained. In fitting GAMs, the most important consideration is to ensure that the model is not overfitted to the data; otherwise, it may be unreliable for predictions. To avoid this issue, the number of basis functions for each smooth was assessed for fit through comparison to prior knowledge about Atlantic halibut distribution, with the assumption that enough basis functions should be allowed to represent the true relationship but not too few so that the relationship is restricted (
Wood 2006).
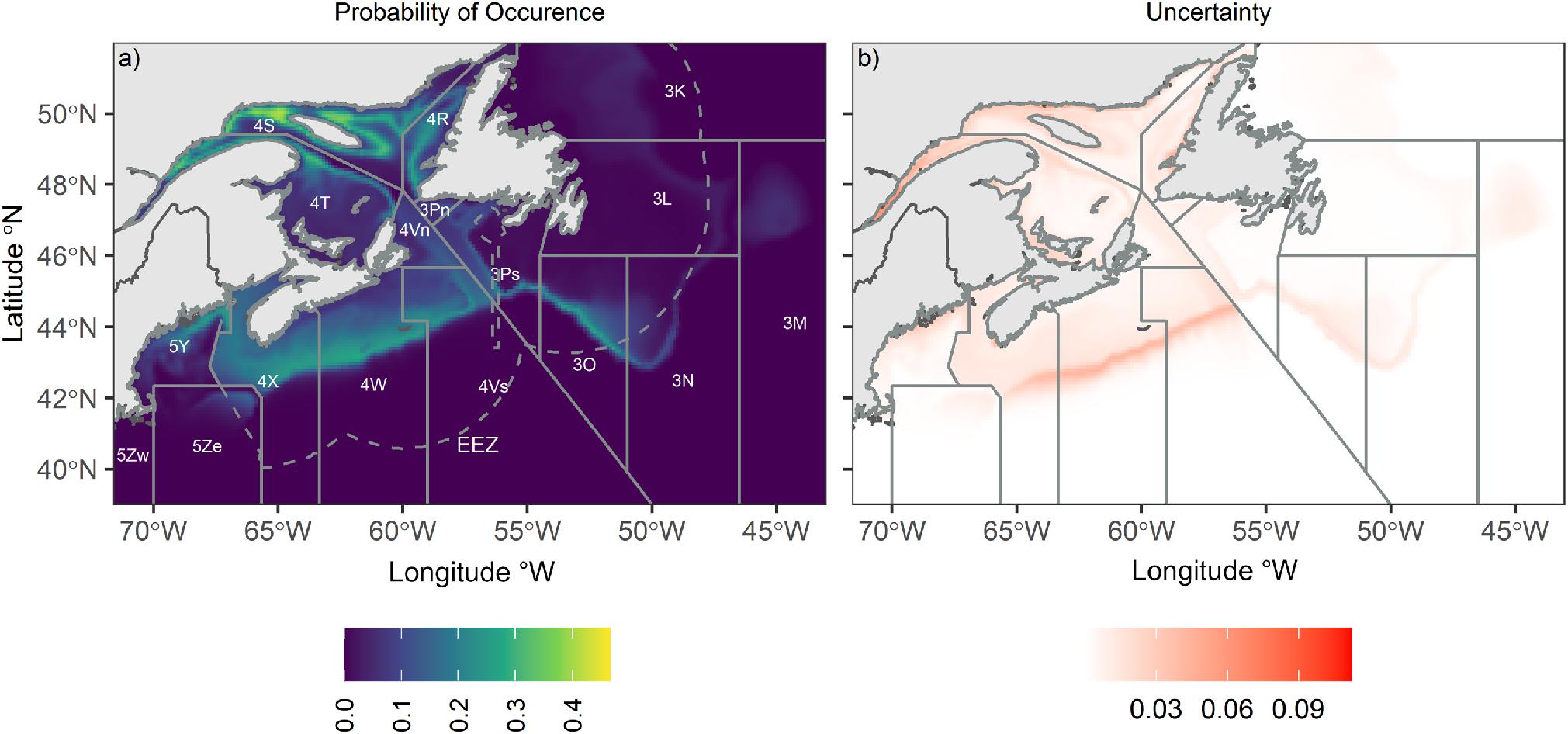
Species distribution projections
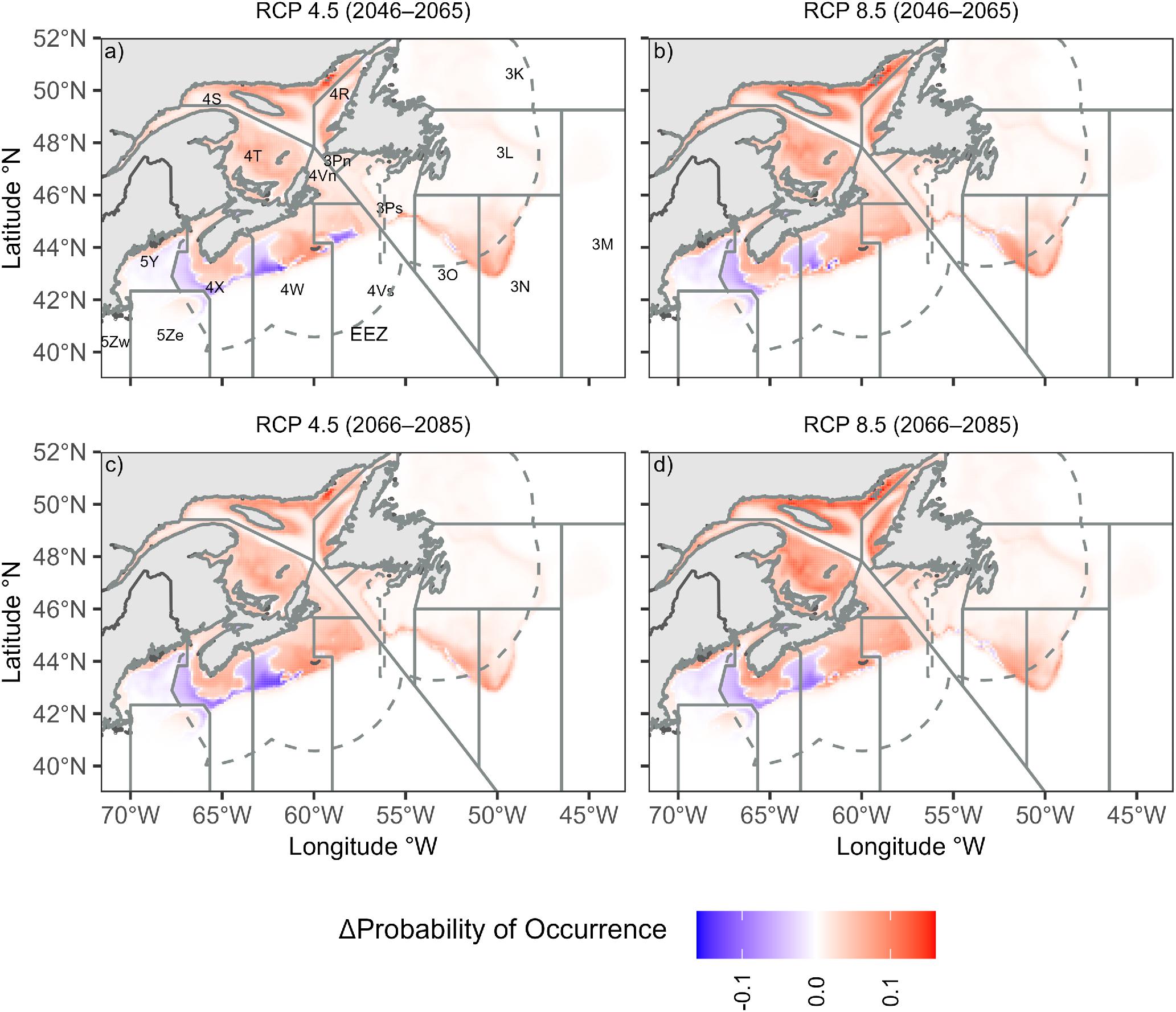
The final SDM was used to predict the probability of occurrence in the present climate conditions (1990–2015) and for future climate condition projections from the BNAM model. Future projections were evaluated for two periods, “near future” (2046–2065) and “far future” (2066–2085) and two emissions scenarios, RCP 4.5 and RCP 8.5, representing reduced and high-emission scenarios, respectively. The percentage change in probability of occurrence was calculated from the current and future projections for each scenario. The percentage change in probability of occurrence was then mapped to show the change in probability of occurrence spatially and temporally for each future period and RCP scenario. The percentage change in probability of occurrence was then compared against the initial probability of occurrence under the present climate scenario to visualize the predicted change of Atlantic halibut distributions across NAFO Divisions. The average percentage change in probability of occurrence was calculated for various areas for comparison (NAFO Divisions, management regions, north, south, and the full domain).
Discussion
Historically, since the 1850s, Atlantic halibut has been overfished in the U.S. (
Grasso 2008) and has yet to fully recover there (
Shackell et al. 2016,
2021). In Canada, since 1950, Atlantic halibut have undergone a cycle between periods of high and low abundance (
Grasso 2008;
Trzcinski and Bowen 2016;
DFO 2018). Since 2011, biomass in Canada has remained above the long-term mean (
DFO 2015a,
2018;
Cox et al. 2016;
Trzcinski and Bowen 2016), and Atlantic halibut has become one of the most commercially valuable groundfish species in the Atlantic region (
DFO 2019). As a result, with the increasing bottom temperatures in the northwest Atlantic, it is paramount to monitor and predict how changes in temperature will lead to changes in the distribution and recruitment of this important commercial species.
The amount of available thermal habitat and the number of GDD have increased since 1990 throughout much of the northwest Atlantic range of Atlantic halibut. There was minimal or no change in available thermal habitat in southern regions, including division 4X, where Atlantic halibut biomass and landings have consistently been the highest compared with other NAFO Divisions since 1970 (
French et al. 2018;
Shackell et al. 2022). The consistent pattern in available thermal habitat is likely due to the contemporary temperatures of these regions falling within the projected thermal window from 2.46 to 9.25 °C yet warming has not pushed temperatures above the projected upper limit. A negative rate of change of thermal habitat was observed in the most northeasterly areas of the model domain, roughly encompassing the Newfoundland shelves, reflecting the influence of the cold Labrador current on the region's thermal dynamics in an already relatively cold region compared to the rest of the domain (
Brickman et al. 2018). Relatively few Atlantic halibut were found in this area (
Boudreau et al. 2017;
French et al. 2018). The overall observed increase in the amount of available thermal habitat corresponds to increasing bottom temperature patterns in the northwest Atlantic (
Kavanaugh et al. 2017;
Rheuban et al. 2017;
Brickman et al. 2018). Our analysis approach builds on previous models of thermal habitat change (e.g.,
Shackell et al. 2022) by incorporating GDD and an improved approach to defining thermal habitat.
An increase in the amount of thermal habitat due to warming temperatures was also observed in other species such as summer flounder
Paralichthys dentatus, striped bass
Morone saxatilis, black sea bass
Centropristis striata, and Barents Sea cod
Gadus morhua (
Eriksen et al. 2017;
Henderson et al. 2017;
Kleisner et al. 2017;
McBride et al. 2018). Our results emphasize the relationship between warming bottom temperatures and recent expansions of Atlantic halibut. The general increase in biomass and abundance could be associated with the overall increase in both available thermal habitat and GDD from 2004 to 2018. The increased availability of thermal habitat may have allowed a greater dispersion of Atlantic halibut, which in turn could have reduced local intraspecific competition at a time of reduced interspecific competition due to a reduced biomass of many other groundfish species (
Shackell and Frank 2007;
Shackell et al. 2010). Greater abundances and dispersion may also be partially caused by changes in fishing mortality due to the establishment of a minimum legal size throughout much of the range (
Trzcinski and Bowen 2016;
Shackell et al. 2022). The increase in GDD likely also led to a longer growing season and correspondingly increased survival during the early life stages.
Overall, recent temperature increases have promoted longer growing seasons and the expansion of suitable habitat, which likely have contributed to increasing trends in Atlantic halibut abundances similar to those observed for other species in recent years (
Henderson et al. 2017;
Friedland et al. 2020). Increased landings may, in part, be due to this increased availability of suitable habitat, given that the magnitude of landings in each NAFO Division is proportional to the size of juvenile Atlantic halibut nursery areas (
French et al. 2018), which supports the nursery-size hypothesis that states the larger the area of juvenile Atlantic halibut habitat, the higher the adult production (
Iles and Sinclair 1982;
Rijnsdorp et al. 1992;
Gibson 1994;
Beverton 1995). The expansion of available juvenile habitat is directly linked to increased temperature, leading to quicker adult recruitment and correspondingly increased adult biomass that ultimately results in increased fishery landings (
sensu the nursery-size hypothesis).
The GDD index showed a significant positive rate of change in the majority of NAFO Divisions indicating a longer growing season for Atlantic halibut. These positive rates of change may accelerate both larval and juvenile growth and/or reduce mortality during these early life stages, thereby providing increased recruitment (
Lein et al. 1997;
Galloway et al. 1999). Across the domain, contemporary evidence shows Atlantic halibut in warmer regions grows faster and matures earlier than in cooler regions (
Shackell et al. 2019). Increased growth leads to a shorter time to maturation, thereby increasing recruitment rates and overall reproductive rates. Potentially increased abundances promote the expansion of Atlantic halibut into suitable habitats in the northwest Atlantic. Similar increases in biomass have been observed in several marine groundfish due to an increased growing season and increased reproductive rates in the Gulf of Maine (
Henderson et al. 2017). However, once temperatures exceed physiological limits, declining fitness is expected.
Based on congruent trends in abundance, GDD, and thermal habitat over time, the warming environment is likely a strong determinant of stock status for Atlantic halibut and possibly underpinned the 2004–2018 abundance increase. The probability of Atlantic halibut occurrence is predicted to increase in the northern regions and remain relatively unchanged in the southern regions in all future climate scenarios. In particular, the highest increase in the probability of occurrence was predicted in the Gulf of St. Lawrence, consistent with the concept that less currently occupied northern areas are warming and consequently increasing suitable habitat with ongoing climate change (
Jones and Cheung 2015;
Friedland et al. 2020). The Gulf of St. Lawrence has experienced oxygen depletion alongside climate change and currently holds a large persistent hypoxic zone, which would greatly affect stenothermal species such as Greenland halibut
Reinhardtius hippoglossoides (
Stortini et al. 2015). However, Atlantic halibut, being eurythermal and better able to avoid the hypoxic zone, may be relatively less affected than Greenland halibut. The southern areas of the Gulf of Maine are predicted to remain relatively unchanged or decrease slightly in probability of occurrence in future climate scenarios. Future decreases are most probable in southern regions and are to be expected if bottom temperatures rise above the preferred thermal niche for Atlantic halibut, as decreases have been predicted for other fish and invertebrate species (
Stanley et al. 2018). This analysis focuses on juvenile Atlantic halibut due to ontogenetic coverage by synoptic surveys, but changes in thermal regime likely also affect other life history stages. For example, decreases in probability of occurrence could be caused by water column temperatures exceeding thresholds that result in increased deformities (6 °C) and mortality (12 °C) in Atlantic halibut larvae (
Lein et al. 1997), thus reducing survival and consequently juvenile recruitment.
Predicted changes in the probability of occurrence indicate abundance and biomass increases in future climate scenarios, even under an emission reduction scenario (RCP 4.5). The average probability of occurrence in the near future under RCP 8.5 was higher than in the far future under RCP 4.5. Therefore, a greater increase in Atlantic halibut occurrence, and, consequently, abundance in a shorter period in a high-emissions scenario (RCP 8.5), is anticipated. Following the model predictions, an increase in Atlantic halibut abundance in Canadian waters (with the exception of NAFO Division 4X in RCP 4.5 in the near future) and an unchanged abundance in U.S. waters from 2021 to 2085, regardless of emission mitigation efforts, are expected. The resultant spatial patterns accord well with other models of Atlantic halibut occurrence (
Boudreau et al. 2017;
French et al. 2018;
Shackell et al. 2022). However, these predictions are habitat-specific and do not consider broader ecological responses to climate change. Prey availability and predator abundance play important roles in survival and recruitment; thus, the spatial distribution of Atlantic halibut based on a broader ecological scope is warranted.
Increasing bottom temperatures have correspondingly increased the amount of suitable thermal habitat and GDD for Atlantic halibut between its near collapse and its current population state. This increase coincides with a period of increased recruitment and abundance, suggesting that increases in Atlantic halibut landings since the 2000s are very likely linked to environmental change in the study domain. Our model of the relationship between bottom temperature and the occurrence of Atlantic halibut provides important insight into the potential influence of climate change on halibut stocks and demonstrates that even under an emission reduction scenario (RCP 4.5), the trend of increasing habitat, abundance, and changing distribution for Atlantic halibut will likely continue. Our results contribute to growing calls for climate change to be incorporated into adaptive management decisions for fisheries in the Northwest Atlantic.