Introduction
Diabetes mellitus type 2 (T2DM) occurs in epidemic proportions globally and was reported by the International Diabetes Federation to involve a worldwide diabetic population of 366 million in 2011 with projections of reaching 552 million by 2030 (
Whiting et al. 2011). While diagnostic tools are available for early risk assessment of T2DM (fasting glucose measurement, glucose tolerance tests, insulin sensitivity indexes, and anthropometric measures), new biomarkers are needed to predict early development of T2DM, which may help to ease the global healthcare burden of this disease. Previously, we identified several biomarkers (TRX, TXNIP, NLRP3, 25(OH)D) shown by receiver operating characteristic (ROC) analysis to have predictive value for prediabetes and T2DM (
Xie et al. 2021). Now we investigate the predictive value of copeptin, a prognostic biomarker in neurological and cardiovascular diseases (
Baranowska and Kochanowski 2019), for potential use in early diabetes risk assessment.
In chronic psychosocial stress, arginine vasopressin (AVP) is not only regulated by plasma osmotic pressure, but it is also associated with endogenous stress stimulation, which activates the hypothalamic–pituitary–adrenal (HPA) axis, resulting in insulin resistance, obesity, diabetes, and metabolic syndrome (
Baranowska and Kochanowski 2019;
Enhorning et al. 2010). However, AVP is largely bound to platelets in the circulation, is frequently cleared from plasma, and is relatively unstable both in vivo and ex vivo (
Enhorning et al. 2010). In contrast, C-terminal pro-AVP fragment (copeptin) directly reflects AVP concentration (
Dobsa and Edozien 2013) and is considered to be a marker of insulin resistance, metabolic disorders, and diabetes (
Saleem et al. 2009).
Multiple studies have shown that copeptin affects glucose metabolism in several ways by: (
i) stimulating glycogen decomposition in the liver (
Grazzini et al. 1999;
Montero et al. 2006), (
ii) activating V1a receptor of hepatocytes to promote gluconeogenesis (
Hems and Whitton 1973), (
iii) activating the V1b receptor on pancreatic α-cells to increase glucagon secretion (
Kim et al. 2021), (
vi) activating the phosphatidylinositol receptor pathway to enhance glucose-dependent pancreatic β-cell secretion and insulin release (
Lu et al. 1993), and (
v) activating the V1b receptor on the adrenal medulla chromaffin cells to increase the adrenaline level and stimulate glycogen decomposition in the liver, leading to hyperglycemia (
Montero et al. 2006;
Saleem et al. 2009).
When under continuous chronic psychosocial stress such as depression, anxiety, stressful life events, poverty, or lack of social support (
Feller et al. 2019), increased circulating copeptin concentration may lead to HPA axis dysfunction, resulting in increased cortisol levels, reduced energy consumption, increased appetite and food intake, increased insulin levels, increased peripheral vascular resistance, and subsequent metabolic disorders such as abdominal obesity, insulin resistance, dyslipidemia, and hypertension (
Melander 2016;
Roussel et al. 2016;
Saleem et al. 2009;
Wannamethee et al. 2015). However, the relationship between changes in copeptin levels and blood pressure during the early stage of T2DM and the relationship between copeptin and related metabolic indicators are still unclear. This study aimed to evaluate the clinical significance of changes in copeptin levels in prediabetes and T2DM and relationships between copeptin and various clinical indicators.
Materials and methods
Study sample
Individuals undergoing physical health examinations in the Physical Examination Center of the Second Affiliated Hospital of Ningxia Medical University between September 2020 and November 2020 were recruited. The diagnostic criteria for prediabetes and T2DM met the 2021 American ADA diagnostic standard (
American Diabetes 2021), and the diagnostic criteria for hypertension met the 2020 ACC/AHA hypertension guidelines (
Flack and Adekola 2020). Prediabetes and T2DM were newly diagnosed in the included subjects, and all included subjects did not receive previous lifestyle interventions or antidiabetic medications. Exclusion criteria were: individuals younger than age 20 years or older than 60 years (we used 60 instead of 65 to avoid the possible effect of older age on the copeptin levels) (
Smaradottir et al. 2017); those with previously diagnosed prediabetes, T2DM, or Type 1 diabetes mellitus (T1DM); patients with secondary hypertension, chronic kidney or liver disease or cancer, acute or chronic infection, acute or chronic autoimmune diseases, history of cardiovascular or cerebrovascular diseases, thyroid dysfunction, or any blood disease; individuals who abuse alcohol or drugs or who smoke and women who are receiving hormone replacement; and patients with an incomplete medical record.
Subjects were divided into four groups based on fasting plasma glucose (FPG) levels: normal control group (NGT), FPG < 5.6 mmol/L; prediabetes A (PDA), 5.6 mmol/L ≤ FPG < 6.1 mmol/L; prediabetes B (PDB), 6.1 mmol/L ≤ FPG <7.0 mmol/L; and T2DM group, FPG ≥ 7.0 mmol/L. Each group was further divided into two subgroups according to whether they had hypertension (-h) or not (-n). All study groups were gender and age matched, and the same number of individuals was enrolled in each subgroup.
Ethical considerations
This study was carried out in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved by the ethics committee of Second Affiliated Hospital of Ningxia Medical University (No. 2021050). Signed informed consent was provided by all subjects before they participated in the study.
Study design and methods
This retrospective observational study analyzed the data of subjects with different FPG levels. Data of all included subjects were collected using a standardized questionnaire, including family history of T2DM, history of complications, medical history, measurement of systolic blood pressure (SBP), diastolic blood pressure (DBP), height, weight, waist circumference (WC), hip circumference, calculated body mass index (BMI (kg/m2)) and waist-to-hip ratio (WHR (cm/cm)).
Peripheral venous blood samples were collected after an 8- to 12-h fast. Clinical laboratory testing included: FPG, total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr), and uric acid (UA). These parameters were detected using the Beckman Coulter, AU5821 automatic biochemical analyzer (Beckman Coulter, Brea, CA, USA). Serum samples of 2 mL from all subjects were stored at −80 °C. After all blood samples were collected, fasting insulin (FINS) and glycosylated hemoglobin (HbA1c) were detected by ELISA using a fluorescence microplate reader (Promega-GloMax, USA), and the concentrations of copeptin was measured using the ELISA kit from China Jianglai Biological Co., Ltd. (Cat. No.: JL46021; Shanghai, China). The intra-precision and inter-precision of this ELISA kit is <10% and <15%, respectively, as tested with human asprosin. β-cell function was evaluated according to the homeostatic model. Insulin resistance index (HOMA-IR) = FPG × FINS/22.5, and insulin secretion index (HOMA-β) = 20 × FINS/(FPG-3.5).
Statistical analysis
Data are expressed as mean ± standard deviation or count (n) and percentage. Comparisons between different groups were performed using one-way ANOVA, and categorical variables were analyzed using the χ2 test. Correlations between different parameters were analyzed using the Pearson correlation test. Sensitivity and specificity of copeptin and other indicators in the diagnosis of prediabetes and T2DM were analyzed using the ROC curve analysis. A two-sided P value (P < 0.05) was established as statistical significance. All statistical analysis was performed using SPSS version 25.0 (IBM Corp, Armonk, NY, USA)
Discussion
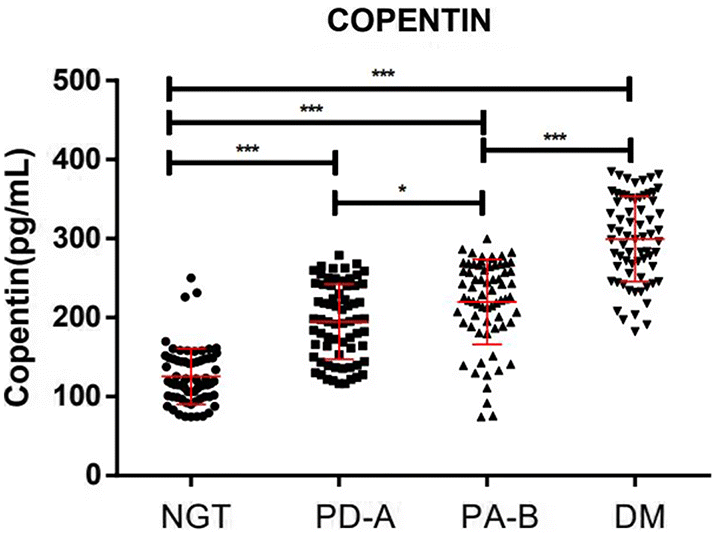
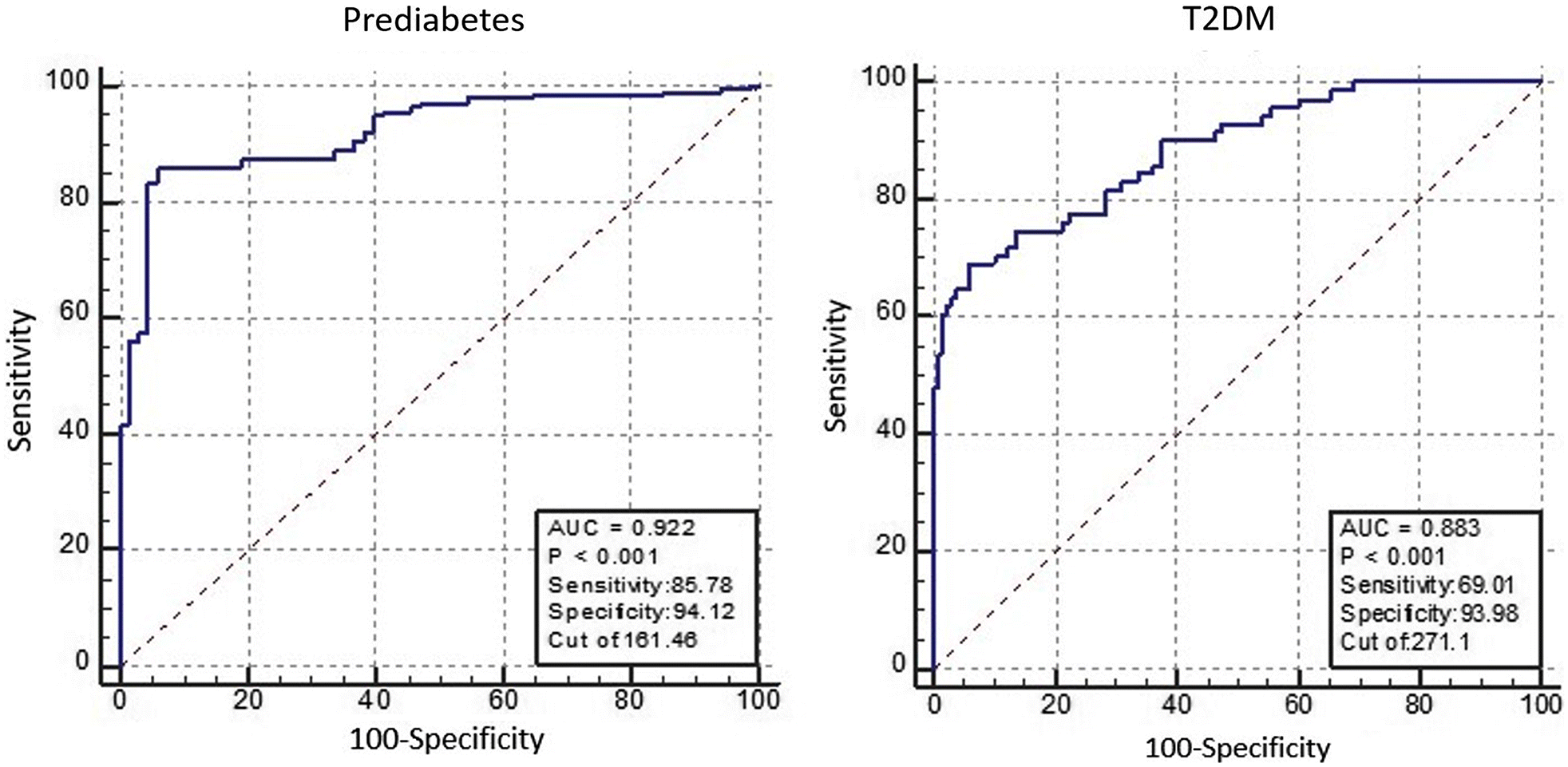
In the present study, we examined copeptin levels and other related indicators in subjects with different FPG levels who had undergone health checkups. The plasma levels of copeptin were significantly higher in individuals diagnosed with prediabetes or T2DM. Copeptin, HbA1c, FINS, and HOMA-IR were significantly increased in patients with prediabetes and T2DM, corresponding with the increase in FPG. Moreover, although copeptin correlated positively with HbA1c, FPG, FINS, and HOMA-IR, it did not correlate with HOMA-β. Also, regression analysis with copeptin as the dependent variable showed that copeptin independently associated with HbA1c and HOMA-IR, but again no association with HOMA-β. These results also agreed with results of quartile analysis, where subjects with T2DM accounted for 100% of all subjects in the top quarter of copeptin levels. Moreover, in ROC curve analysis, the AUC of copeptin for prediabetes and for T2DM were significantly elevated, suggesting that copeptin may play a deleterious role in early T2DM development.
Previous studies have reported that plasma copeptin levels increase significantly in individuals with prediabetes, diabetes and certain vascular complication (
Li et al. 2020;
Noor et al. 2020;
Piani et al. 2021;
Sujana et al. 2020;
Yin et al. 2020). In patients with prediabetes, diabetes without nephropathy, and diabetic nephropathy, copeptin levels were increased (mean 215.10 pg/mL) and were significantly increased in patients with prediabetes (mean 252.85 pg/mL); copeptin levels also correlated positively with HbA1c (
Noor et al. 2020). The presence of newly diagnosed prediabetes and T2DM correlated positively with copeptin, and increased by 1.29 times for every one SD increase of copeptin (
Sujana et al. 2020). The present study showed that about 75% of patients with prediabetes had copeptin levels between 151.96 and 229.63 pg/mL, and all patients with T2DM had copeptin levels >307.29 pg/mL. Comparing with copeptin levels in healthy individuals from other literature (6.84–45.24 pg/mL) (
Dobsa and Edozien 2013), copeptin measured in the present study were about 10-fold or higher. This was probably due to the use of ELISA method rather than the gold standard (BRAHMS Kryptor system, Thermo Fisher Scientific BRAHMS GmbH) to assess copeptin levels. Nevertheless, although our results showed that the copeptin and HbA1c levels increased significantly with increased FPG levels, regression analysis with copeptin as the dependent variable revealed that the copeptin level associated independently with HbA1c, but not with FPG. Therefore, we suggest that the increase in the plasma copeptin level is closely associated with the gradual increase of glycated hemoglobin following increasing FPG levels during early diabetes development. Changes in copeptin levels may appear in the earlier stage of T2DM; that is, a chronic stress response may occur when FPG is ≥ 5.6 mmol/L, which may stimulate the hypothalamus to secrete a large amount of copeptin into the blood circulation.
Copeptin (reflecting AVP) is a hypothalamic-regulating neuroendocrine factor and has an important influence on the body’s glucose metabolism, as AVP has been shown to stimulate insulin-induced glucagon secretion during hypoglycemia (
Kim et al. 2021). In the normal adolescent population, insulin resistance correlates positively with copeptin (
Thomsen et al. 2019). In obese and insulin resistant children, serum copeptin levels are significantly increased, and regression analysis shows that when copeptin is used as the dependent variable, it correlates independently with BMI, WC, WHR, TG, FPG, ALT, SBP, DBP and HOMA-IR (
Yin et al. 2020). The copeptin level in patients with metabolic syndrome is significantly higher than that in patients without metabolic syndrome, and the incidence of metabolic syndrome in the fourth quartile of the plasma copeptin is as high as 70%–100% (
Penit et al. 1983). Other scholars suggest that the copeptin level of patients with newly diagnosed T2DM is significantly higher than that of normal people, but is not different from that of patients with prediabetes (impaired glucose tolerance and (or) impaired fasting plasma glucose), and the increased serum copeptin level in subjects with newly diagnosed T2DM correlates positively with stress (
Madhu et al. 2020).
Several studies have confirmed that achieving aerobic fitness (
Traustadottir et al. 2005) and increasing water intake to promote metabolism (
Brunkwall et al. 2020) decreases plasma copeptin levels and reduces the HPA axis stress response. This suggests that copeptin may be a potential indicator for early medical intervention to prevent progression of T2DM and its complications. Copeptin activates the HPA axis, resulting in various endocrine disorders, including decreased levels of thyroid hormone, growth hormone, and gonadal hormones, and increased levels of cortisol (
Volpi et al. 2004). Cortisol is an important endocrine hormone that increases the blood glucose level by reducing insulin-dependent glucose uptake, promoting glucagon secretion, and increasing glycogenolysis. At the same time, the elevated cortisol level can also induce eating behavior and increase food intake, and thereby increase the risk of obesity (
Cavagnini et al. 2000). During the prediabetes stage, the elevated copeptin concentration can predict diabetes onset and abdominal obesity, suggesting that the development of T2DM may be associated with the increase of copeptin secretion caused by fat accumulation in the abdominal wall (
Enhorning et al. 2013). However, in the present study, copeptin in patients with prediabetes and diabetes did not correlate with the main obesity-related indexes, such as BMI, WC, and WHR, nor with the blood lipid indicators, such as TC, HDL, LDL, and TG, which agrees with the results of studies investigating patients with symptoms related to the metabolic synthesis (
Penit et al. 1983). Although earlier animal experiments found that stress is associated with plasma cortisol levels (
Kaplan et al. 1996), studies of patients with obesity and cardiometabolic diseases have not found that increased plasma copeptin levels are associated with cortisol levels (
van der Valk et al. 2020). In the present study, BMI, WC, WHR, TG, and LDL increased significantly with the gradual increase in FPG, but increases in these indicators did not correlate with plasma copeptin levels.
Patients with prediabetes and T2DM often have comorbid obesity and nonalcoholic fatty liver disease (NAFLD). A study of patients with NAFLD showed that obese patients with NAFLD had significantly higher copeptin levels than obese patients without NAFLD and non-obese patients, and the increased plasma copeptin level correlated independently with NAFLD severity (
Barchetta et al. 2019). In the present study, although we found a significant increase in ALT and AST corresponding with the gradual increases in FPG, abnormal liver function did not correlate with changes in the plasma copeptin level. Consequently, we speculated that obesity and NAFLD in the early stage of T2DM may not be associated with increased plasma copeptin levels; the main cause of obesity may not be AVP-induced increases in cortisol levels; it is more likely that unhealthy lifestyles, including sitting for long periods, lack of exercise, and excessive energy intake, contribute more directly to the development of obesity, insulin resistance, and T2DM.
In the present study, under the same blood glucose levels, no significant differences were found in copeptin levels between the groups with and without hypertension. This result is different from the results of previous studies reporting that increased copeptin levels were closely associated with hypertension (
Saleem et al. 2009). This explains in part the critical role of AVP in regulating fluid and electrolyte balance, and its release is stimulated by changes in plasma tension, hypovolemia, or hypotension (
Penit et al. 1983). Moreover, AVP is associated with activation of the renin–angiotensin–aldosterone system and renal hemodynamic function (
Piani et al. 2021). However, when copeptin is used as the dependent variable for multiple regression analysis, it did not correlate with blood pressure, and changes in copeptin correlated mainly with HbA1c and HOMA-IR independently, suggesting that increased plasma copeptin levels have little effect on blood pressure during the development of T2DM, but appears to associate significantly with insulin resistance and glucose metabolism. Indeed, as indicated in other literature, copeptin has larger molecular weight and its clearance from the circulation is slower than AVP (
Fenske et al. 2018;
Roussel et al. 2014). The copeptin dynamics and how well this reflects changes in AVP in all patient populations remain to be elucidated.
Diabetes or glucose homeostasis is usually assessed or identified using the surrogate biomarkers FPG and HbA1c in clinical practice (
Agnello et al. 2019;
Bellia et al. 2019). However, as suggested by the Diabetes Control and Complications Trial (
Rohlfing et al. 2002), using a single measurement for FPG could be misleading. In addition, only looking at HbA1c is not enough under conditions that survival of erythrocytes is reduced (
Bellia et al. 2019;
Giglio et al. 2020). As subjects with impaired FPG are usually associated with other metabolic syndrome, other surrogate biomarkers could be considered for a more comprehensive interpretation of the underlying pathological mechanisms.
The present study intended to ascertain how well copeptin distinguish between prediabetes and T2DM by identifying the optimal cut-off points. For prediabetes (FPG level ≥5.6 and < 7.0 mmol/L), the optimal cut-off point was 161.46 ng/mL. This suggests that in early development of T2DM, the level of copeptin should be above 162 ng/mL. For T2DM (FPG level ≥7.0 mmol/L), the optimal cut-off point was 271.10 ng/mL. This suggests that when the copeptin level is >271 ng/mL, it is very likely that T2DM has developed. In the present study, high AUC values in ROC analysis revealed that copeptin may be a potential predictor for prediabetes and T2DM (prediabetes 0.922; T2DM 0.883).