Introduction
First Nations people experience significant socio-economic and health disparities compared with non-Indigenous populations in Canada (
Reading and Wein 2009). They continue to face serious health challenges, including shorter life expectancy, high rates of mortality, and chronic diseases such as obesity, diabetes, and cardiovascular problems (
Reading and Wein 2009;
FNIGC 2012;
Tjepkema et al. 2012;
Young 2012;
Bruce et al. 2014). Cardiovascular diseases (CVDs) are conditions that describe diseases of the heart and/or blood vessels, including ischemic heart diseases (IHD), cerebrovascular disease (stroke), heart failure, congenital heart disease, inflammatory, rheumatic, and hypertensive heart diseases (
WHO/WHF/WSO 2011). Overweight and obesity, a sedentary lifestyle, smoking, and eating a diet high in saturated fat, sodium, and sugar are well-known risk factors for CVD (Cannon 2007).
Among First Nations, CVD remains the second leading cause of death (
FNHA 2012;
Health Canada 2014;
Reading 2015). Historically, the mortality from CVD in First Nations was lower than that among the general Canadian population (
Young 2012). Studies conducted in recent decades, however, have demonstrated that heart diseases in First Nations have increased compared with the general Canadian population (
RHS 1999). The First Nations Regional Health Survey (RHS) (2002/03) found that the overall prevalence of self-reported heart disease was slightly higher in First Nations than in non-Indigenous people in Canada (7.6% vs. 5.6%) whereas, among older individuals (50–59 years), the rate of heart disease was more than two times higher than in the general Canadian population (11.5% vs. 5.5%) (
FNC 2005).
In British Columbia (BC), First Nations living on reserves had rates for ischemic heart diseases 25% higher than the rates among other residents in the province (
BC Provincial Health Officer 2009). In 2008/10, the RHS reported that 5.7% of First Nations in Canada (
FNIGC 2012) and 6.4% of First Nations in BC were diagnosed with heart disease (
First Nations Health Authority 2012). The data on the prevalence of acute myocardial infarction (MI) in First Nations, however, are limited (
Reading 2015). Nevertheless, the mortality rates due to MI were estimated to be 25% higher in First Nations men and 55% higher in First Nations women compared with non-Indigenous populations in Canada (
Tjepkema et al. 2012).
Harvesting, consuming, and sharing traditional foods (i.e., all culturally acceptable foods within a particular local, natural environment) contribute to physical fitness, provide an abundance of essential nutrients, and are vital for the cultural identity, spiritual and mental health, and social well-being of First Nations (
Egeland et al. 2001;
Kuhnlein and Receveur 2007;
Sheehy et al. 2015). In BC, traditional diets of First Nations include a rich diversity of marine foods such as fish, shellfish, seaweeds, and marine mammals (
Mos et al. 2004;
Chan et al. 2011), which supply significant sources of protein, micronutrients, and essential polyunsaturated omega-3 fatty acids (
n-3 FAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (
Sheehy et al. 2015).
Health effects of fish and dietary
n-3 FAs have been intensively investigated since the first study in the 1970s, demonstrating an inverse relationship between
n-3 FAs and IHD among Greenland Inuit (
Bang et al. 1971). Following this observation, several clinical and epidemiological studies continue to demonstrate protective effects of fish and dietary
n-3 FAs intakes against CVD, such as stroke, MI (
Leung Yinko et al. 2014;
Elagizi et al. 2018), reduced cardiac death, and total mortality (
Breslow 2006;
Leung Yinko et al. 2014;
Shahidi and Ambigaipalan 2018). These cardioprotective effects of EPA and DHA are attributed to the beneficial modulation of several risk factors for CVD (
Innes and Calder 2020). Many prospective studies have demonstrated that EPA and DHA lower plasma triglycerides, improve high-density lipoprotein (HDL) cholesterol, lower blood pressure, inhibit platelet aggregation, and improve endothelial function and vascular reactivity (
Yashodhara et al. 2009;
McLennan 2014;
Shahidi and Ambigaipalan 2018). EPA and DHA have also been reported to reduce inflammatory markers such as acute-phase protein, C-reactive protein, pro-inflammatory cytokines, tumour necrosis factor (TNF)-α, and interleukin (IL)-6 (
McLennan 2014;
Innes and Calder 2020).
Although dietary
n-3 FAs confer a wide range of cardiovascular benefits, they can be attenuated by the adverse effects of the presence of methylmercury (MeHg) in fish (
Lipfert and Sullivan 2006;
Laird et al. 2013). Indeed, consuming traditionally harvested fish and marine mammals constitutes the major pathway of MeHg exposure among Indigenous people (
Laird et al. 2013). The adverse effects of MeHg on the nervous system, growth and development of fetuses and children, and immune function are well-established (
Bjørklund et al. 2017;
Ha et al. 2017). Recent evidence also indicates that chronic exposure to MeHg may increase the risk of CVD (
Hu et al. 2018), including MI (
Genchi et al. 2017). The mechanism by which MeHg produces toxic effects on the cardiovascular system is not fully elucidated, but it is believed to increase oxidative stress, increase the production of free radicals, affect heart rate variability, and promote inflammation, hypertension, and plaque development (
Genchi et al. 2017). The intake of EPA + DHA and MeHg from seafood depends on the concentrations of EPA + DHA and MeHg, which significantly vary between seafood species and across different regions (
Laird et al. 2013;
Hu et al. 2017;
Chan et al. 2020).
Current seafood consumption contributes to reaching the daily recommendations of EPA and DHA (
Marushka et al. 2018a,
2018b). However, traditional food consumption among First Nations has been declining over the past decades and has been gradually replaced with nutrient-poor market foods (
Kuhnlein and Receveur 1996;
Johnson-Down and Egeland 2013;
Sheehy et al. 2013). Several socio-economic and environmental factors, such as poverty, food insecurity, and concerns about the risk of environmental exposure, are contributing to the diet transition (
Chan et al. 2011;
Kuhnlein et al. 2013). Furthermore, climate change exacerbates the nutrition transition through its impacts on traditional food systems (
Ford et al. 2010). Climate change was documented to decrease the abundance and availability of wildlife species by affecting the magnitude and cycles of animals’ growth, reproduction, distributions, and migrations in oceans, rivers, and on land (
Hori 2010;
Lemmen et al. 2016).
Weatherdon et al. (2016) found that climate change may reduce the abundance and alter the distribution of the majority of marine species harvested by coastal First Nations in BC (
Weatherdon et al. 2016). We have previously projected the impact of climate change on seafood consumption and the diet quality of coastal First Nations in BC and estimated a decrease in the intake of essential nutrients from seafood by 21% under lower (Representative Concentration Pathway [RCP] 2.6) and 31% under higher (RCP 8.5) climate change scenarios by 2050 relative to 2000 (
Marushka et al. 2018b). This follow-up study further investigated the potential implications for human health. The objective of this study was to model the effects of projected declines in seafood consumption on the relative risk (RR) of MI in coastal First Nations in BC under lower and upper climate change scenarios (i.e., 21% and 31%). Specifically, we modelled the combined effects of reduced EPA + DHA and MeHg intake from seafood on the RR of MI.
Results
This study included 369 participants (140 men and 229 women) with an average age of 47.6 (±14.3), ranging from 21 to 90 years old. Descriptive characteristics and hair Hg concentrations of the study population are presented in Tables S1
a and S1
b. The hair Hg concentration was relatively higher in men and older individuals than in women and younger respondents. However, the hair Hg means were considerably below the established Health Canada Hg guideline across all genders and age groups (
Legrand et al. 2010).
Tables 1A and
1B summarize the mean daily intake of the most frequently consumed seafood species and the corresponding EPA + DHA and MeHg exposure by gender and age groups. The average daily intake of seafood was 78 g/day in men and 50 g/day in women. Older participants reported significantly higher seafood consumption compared with younger individuals, with a mean intake of 34.8, 45.7, and 78.2 g/day among the 19–34, 35–49, and ≥50 years age groups, respectively. The mean EPA + DHA intake from seafood was higher in men compared with women (0.81 and 0.50 g/day, respectively). Likewise, older individuals (≥50 years) consumed more EPA + DHA (0.81 g/day) than the middle age group (35–50 years) (0.49 g/day) and younger individuals (19–34 years) (0.29 g/day). The average daily intake of MeHg from seafood was 5.10 µg/day in men and 3.14 µg/day in women. Among older participants (≥50 years), the average daily MeHg intake was 5.02 µg/day, which is almost two times higher than in younger age groups (2.60 µg/day in 19–34 years and 2.64 µg/day in 35–49 years). The top 10 most consumed species contributed about 65% to the total seafood consumption, 71% to the EPA + DHA intake, and 67% to the MeHg intake. Salmon species (i.e., sockeye, chinook, coho, and salmon eggs) were the most consumed fish types. Salmon was also the main source of EPA + DHA and the second-highest contributor to the MeHg intake. Halibut, the second most consumed fish, was the major source of MeHg and contributed 31% and 47% of total MeHg intake in men and women, respectively. However, halibut provided a low intake of EPA + DHA (Table S2). Herring roe was the fourth most consumed species and provided a good source of EPA + DHA. Shellfish, such as prawn, clam, and crab, contributed little to either EPA + DHA or MeHg. Overall, the daily intake of MeHg in First Nations was below the established Tolerable Daily Intake (TDI) of 0.47 µg/kg bw/day (
Legrand et al. 2010). Men and older respondents had relatively higher estimated MeHg exposure than women and younger individuals due to higher seafood consumption.
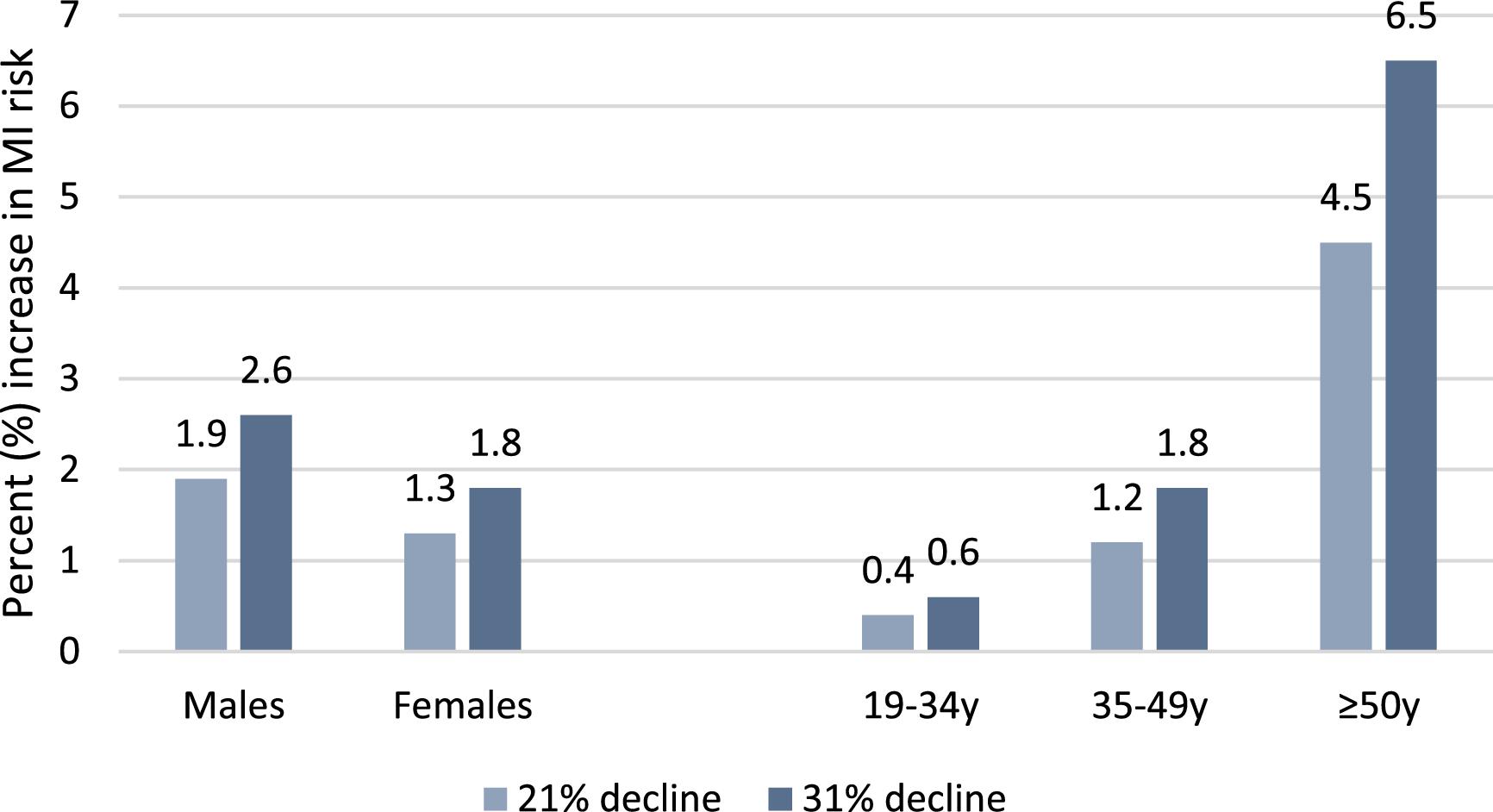
The modelled effects of the projected decline in seafood consumption on MI risk among coastal First Nations are presented in
Fig. 2 and Table S3. Due to the small effect size estimates, we present the percentage change in RR and translate it into the percentage increase in MI risk. We reported the combined effects of reduced EPA + DHA and MeHg intake under lower and upper climate change scenarios (i.e., 21% and 31%). Reduced seafood intake was estimated to increase the risk of MI by 1.9% and 2.6% in men and 1.3% and 1.8% in women under lower and upper climate change scenarios, respectively, by 2050 relative to 2009. When considering age groups, the most prominent adverse effects were observed in participants 50 years of age and older, with the risk of MI projected to increase by 4.5% under lower and 6.5% under upper scenarios of climate change (
Fig. 2).
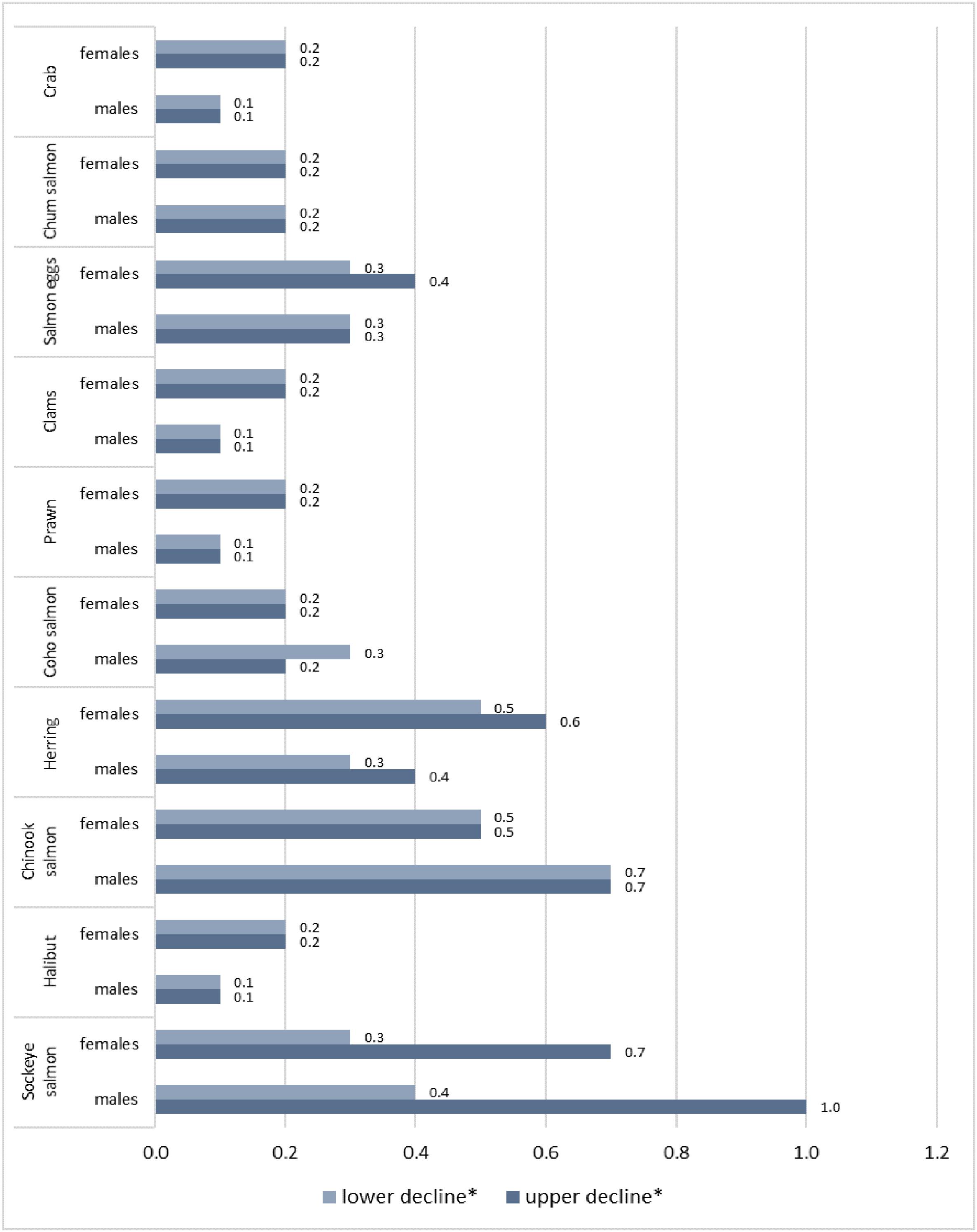
Figure 3 presents the increased MI risk due to the projected decline of the top 10 most consumed seafood species, individually, considering species’ specific data on the decline (Table S4). The most pronounced adverse effects on MI were observed for salmon species and herring, with the increased risk of MI ranging from 0.2% to 0.7% under lower and from 0.2% to 1.0% under upper climate change scenarios (
Fig. 3 and Table S5). These species were projected to experience the greatest relative impact of climate change (Table S4). In addition, they contributed relatively high sources of EPA + DHA. The individual effects of reduced halibut and shellfish species were not significant due to their relatively low daily consumption as well as low concentrations of EPA + DHA.
Discussion
Although CVD has been decreasing in Canada (
PHAC 2018), First Nations continue to experience a growing burden of CVD morbidity and mortality (
Reading 2015). In this study, we modelled the effects of the climate-related decline in seafood consumption and consequent dietary EPA + DHA intake on the increased risk of MI among coastal First Nations in BC. Our results showed that reduced EPA + DHA intake may increase the risk of MI by 1.9% and 2.6% in men and by 1.3% and 1.8% in women under lower and upper climate change scenarios, respectively, by 2050 relative to 2009. The most significant impact was observed among older individuals 50 years of age and over, with the risk of MI increasing by 4.5% and 6.5% under the two climate change scenarios, respectively. When considering individual seafood driving this health risk, the most prominent adverse effects on MI risk were the estimated decline in salmon species, such as sockeye (0.4% to 1.0%) and chinook (0.5% to 0.7%) since they are the major source of EPA + DHA intake. In addition, salmon was the most consumed species, which reflects its status as a cultural keystone and dominant food source for coastal First Nations. On the other hand, salmon species are among those that are most sensitive to climate change (
Weatherdon et al. 2016). For instance, the abundance of chinook salmon was projected to decline by 47.8% and 46.8% under lower and upper climate change scenarios, respectively (
Weatherdon et al. 2016). Traditional seafood continues to play an essential role for coastal First Nations, providing significant sources of
n-3 FAs. In the present study, seafood supplied 810 mg/day and 500 mg/day of EPA + DHA in men and women, respectively, and 38% (44% of men and 34% of women) of individuals met the recommended daily intake of 500 mg/day, which is considered sufficient to obtain protective effects for primary prevention of CVD (
Academy 2014). Overall, First Nations people obtain more
n-3 FAs from fish consumption compared with the general Canadian population. A study in a French-Canadian population found that, on average, daily intake of EPA + DHA was 250 mg among males and 270 mg among females (
Garneau et al. 2012). Fish and seafood consumption was estimated to be, on average, 100 g/day (ranging from 98 g/day to 108 g/day) among the general Canadian population, while contributing up to 27% of EPA and 75% of DHA from all food sources (
Hu and Chan 2020). In contrast,
n-3 FAs intake in coastal First Nations in BC was lower than in the Canadian Inuit people, whose traditional diets were mainly composed of fish and marine mammals rich in
n-3 FAs. According to
Hu et al. (2017), traditional diets provided about 2.3 g and 1.5 g of EPA + DHA daily for men and women, respectively (
Hu et al. 2017). Likewise,
Dewailly et al. (2001) reported that the Inuit of Nunavik consumed on average 2.1 g/day of EPA + DHA (
Dewailly et al. 2001). Indeed, low rates of IHD in the Inuit population were attributed to the high consumption of
n-3 FAs (
Bang et al. 1971;
Dewailly et al. 2001). At the same time, Inuit people are exposed to higher levels of MeHg through the consumption of marine foods, which diminishes the cardioprotective effect of EPA + DHA (
Lipfert and Sullivan 2006).
Hu et al. (2017) estimated that seafood accounted for about 38 µg/day intake of MeHg for Inuit men and 25 µg/day for Inuit women. In contrast, First Nations in British Columbia had a MeHg intake of about 5.10 µg/day in men and 3.14 µg/day in women, which is 7.5–8 times lower than among the Inuit population. Compared with inland First Nations living in Manitoba and Ontario, coastal First Nations in British Columbia consumed higher amounts of
n-3 FAs (
Marushka et al. 2017a,
2017b), which reflects differences in geographical diversity and availability of species. In the current study, younger individuals consumed less seafood and consequently had a lower intake of EPA + DHA than older participants. These findings are consistent with previous studies among Indigenous people (
Dewailly et al. 2001;
Hu et al. 2017;
Marushka et al. 2018a) that there might be a nutrition transition from a traditional diet towards store-bought food, resulting in a decline in fish consumption.
Sensitivity analyses showed that EPA + DHA intake and their association with MI are the dominant contributors to MI risk under current and projected seafood consumption scenarios among coastal BC First Nations, given that their exposure to MeHg from seafood is very low (Tables S6 and S7). In addition to omega-3 FAs, seafood is rich in other essential nutrients, such as selenium, that may provide many cardiovascular health benefits. Recent systematic reviews and meta-analyses suggested that selenium intake was associated with decreased risks of CVD incidence and mortality (
Jenkins et al. 2020;
Kuria et al. 2021). A study among the Inuit population found that high selenium intake from country food may lower the odds ratios for MI associated with mercury exposure (
Hu et al. 2017a). However, results on the protective effects of selenium against CVD are still inconclusive.
First Nations continue to experience a disproportional burden of food insecurity (i.e., the inability to afford nutritionally adequate and safe foods), with prevalence rates greatly exceeding those of the non-Indigenous population in Canada (
Chan et al. 2011;
Tarasuk 2012). Indeed, 41% of on-reserve First Nations in British Columbia reported living in food-insecure households, compared with 8.4% of the general population in British Columbia (
Statistics Canada 2010;
Chan et al. 2011). Food insecurity is directly related to unhealthy diets reflecting a low intake of essential nutrients and an increased intake of energy from sugar and fatty foods, which in turn increases the risk of CVD. Several studies have demonstrated that trans-fats increase the “bad” LDL-cholesterol and decrease the “good” HDL-cholesterol levels. Also, trans-fats promote inflammation, increase abdominal fat, and decrease the health of the endothelium, which has metabolic consequences including obesity, diabetes, and CVD (
de Souza et al. 2015). Several factors, such as limited income, high cost, and insufficient availability and access to affordable, healthy, and nutritious market food, contribute to food insecurity (
Willows 2005).
Traditional food systems undergo significant pressure from various environmental factors, such as climate change, which negatively impacts the availability, diversity, and access to traditional foods, including seafood. Climate change may also affect the bioaccumulation rates of environmental contaminants (such as MeHg) in marine food webs (
Alava et al. 2017,
2018). Indigenous people, especially remote and coastal communities, are particularly vulnerable to climate change impacts as they live off the land and the ocean and have limited resources and abilities to adapt to changing conditions. Most of the previous research on the impact of climate change on traditional food systems was performed among Inuit and Northern First Nations in the Canadian Arctic (
Ford et al. 2010;
Nancarrow and Chan 2010;
Rosol et al. 2016). The main documented effects of climate-related changes by the Inuit and Northern First Nations included: warming, increased precipitation, more unpredictable weather patterns, stronger winds, increased coastal erosion, and alteration in sea-ice dynamics (
Ford et al. 2010;
Rosol et al. 2016). These factors have been reported to affect the availability, distribution, and health of wildlife species, with consequent serious implications for food security in Indigenous communities (
Ford et al. 2010;
Rosol et al. 2016). Among First Nations in British Columbia, the majority (75%) of participants observed that climate change decreased the availability of traditional foods for harvest through declining abundances, altered growth and migration patterns, and observed diseases in animals (
Chan et al. 2011). In addition to climate change, First Nations noted other constraints on traditional food consumption, including government restrictions and industrial activities such as forestry, hydroelectricity, mining, farming, and oil/gas industries. These barriers combined were perceived by 68% of BC First Nations to decrease access to salmon, shellfish, and other fish species (
Chan et al. 2011).
This is the first study that models the potential impacts of the climate-related decline in seafood consumption and consequent reduced EPA + DHA intake on the risk of MI among coastal First Nations. The main strengths of this study are a representative sample of coastal First Nations in BC, the use of a comprehensive traditional food frequency questionnaire over the prior year to estimate seafood consumption patterns, as well as empirical analysis of mercury concentrations in seafood species and the hair of the study participants.
There are several uncertainties in this study. First, the strength of associations between
n-3 FAs, MeHg, and MI among First Nations in BC may not be the same as observed by
Wennberg and colleagues (2012). Second, the baseline serum EPA + DHA concentrations were not measured in the study participants but were adopted from the Cree Nation of Eeyou Istchee. This may introduce bias in the RR estimates. RR estimates with converted serum EPA + DHA values are smaller compared with the corresponding estimates with plasma phospholipid values (Table S3). However, the conclusion will not change since the modelled increase in the MI risk due to seafood decline scenarios mainly depends on the proportional change in EPA + DHA, rather than the absolute value. Cardiovascular health outcomes can be further exacerbated due to significant dietary changes and the consequent increased intake of processed food. Therefore, the estimated increase in MI risk might be underestimated. The levels of MeHg in seafood species may change over time (increase or decrease) due to different environmental factors, including climate change. Our model assumes that the MeHg concentrations in seafood will remain constant over time. Finally, there are some uncertainties about the DBEM model in the projected seafood declines (see
Weatherdone et al. 2016).