Introduction
Oxidative stress refers to the various harmful processes resulting from an imbalance caused by the excessive formation of reactive oxygen species (ROS) and the limited antioxidant defenses of cells (
Turrens 2003). Doxorubicin (DOX) and (or) γ-radiation treatments are known to induce oxidative stress through the generation of ROS, which causes an imbalance in antioxidant activity and ultimately results in cell death (
Elsadek et al. 2017;
Fathy et al. 2017a). ROS can react with biological molecules and destroy the structure of cells (
Baatout et al. 2004). They are often responsible for protein denaturation, lipid peroxidation, and impaired enzyme activity (
Karbowink and Reiter 2000;
Fathy et al. 2017a). DOX, also called adriamycin, is a potent antibiotic that is widely used for the treatment of different solid and hematopoietic tumors. However, in addition to its anti-tumoricidal activity, it has several well-known side effects that include chronic and irreversible toxicity (
Asmis et al. 2005;
Patil et al. 2008).
The chronic administration of plant extracts might augment the major cellular endogenous antioxidants, and is identified as a promising approach to combat oxidative stress (
Bashandy et al. 2014;
Fathy et al. 2017b). The major benefit of the Mediterranean diet is its high level of antioxidants derived from fruits and vegetables, including olive oil, figs, and date palm fruits, which contribute antioxidant vitamins, minerals, flavonoids, and polyphenol content (
Solomon et al. 2006). In addition, mixed plant extracts showed a higher diversity of polyphenols resulting in greater stability and bioaccessibility of antioxidants compared with a single fruit extract (
Bashandy et al. 2014;
Kamiloglu et al. 2014;
Fathy et al. 2018).
Our study aimed to investigate the protective synergistic effects of olive oil with fig and date palm fruit extracts on the toxicity hazards of DOX- and (or) γ-radiation-induced oxidative stress in Wistar albino rats.
Materials and methods
Ethics statement
All animals in our study were handled in accordance with the ethical guidelines for investigations using laboratory animals and complied with the guide for the care and use of laboratory animals (
Institute of Laboratory Animal Resources 1996). The study was also approved by an independent ethics committee of the National Research Center, Egypt.
Experimental animals and study design
We used 120 male Wistar albino rats (150–170 g body mass). The rats were obtained from the Egyptian Holding Company for Biological Products and Vaccines (VACSERA, Giza, Egypt) and allowed to acclimatize in the experimental laboratory for two weeks. Rats were then divided into eight groups (n = 15 rats) according to the treatment and the requirements of the experiment. The rats were maintained under standard laboratory conditions at the animal center, Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt. They were kept in a temperature-controlled environment (20–25 °C) and 50%–60% relative humidity with an alternating 12 h light:dark cycle. Five rats were placed into each cage and provided with standard diet pellets and drinking (tap) water ad libitum throughout the experimental period.
Group I (Control): Rats of this group were neither treated nor irradiated and were provided with standard diet pellets and drinking (tap) water ad libitum during the experiment (six weeks).
Group II (OFD): Rats of this group were administered extra virgin olive oil (7 g/kg) and freshly prepared fig (1 g/kg) and date palm fruit (1 g/kg) extracts daily via oral gavage for six weeks.
Group III (DOX): Rats of this group received DOX in doses of 2.5 mg/kg body weight via intravenous (IV) injection weekly for four consecutive weeks (cumulative doses of 10 mg/kg body weight).
Group IV (R): Rats of this group were exposed to whole-body γ-radiation with fractioned doses of 2 Gy every week for four consecutive weeks (up to 8 Gy total doses).
Group V (DOX-R): Rats of this group were irradiated following 20 h of DOX injection on the same schedule as Groups III and IV.
Group VI (OFD-DOX): Rats of this group were treated with OFD (for two weeks prior to DOX dosing and during the four weeks of treatment during the experiment) and injected with DOX on the same schedule as Group III.
Group VII (OFD-R): Rats of this group were treated with OFD (for two weeks prior to irradiation and during the four weeks of treatment during the experiment) and irradiated on the same schedule as Group IV.
Group VIII (OFD-DOX-R): Rats of this group were treated with OFD (for two weeks prior to DOX and R treatment and during the four weeks of treatment during the experiment) and irradiated following 20 h of DOX injection on the same schedule as Groups III and IV.
DOX
Adricin® (doxorubicin hydrochloride) vials were obtained from EIMC United Pharmaceuticals, Cairo, Egypt. Rats in our study were dosed with 2.5 mg/kg body weight of DOX via IV injection weekly for four consecutive weeks, for a cumulative dose of 10 mg/kg body weight.
Irradiation (R)
We used the Canadian Gamma cell-40 (137Cs) facility housed at the National Center for Radiation Research and Technology (NCRRT), Cairo, Egypt for irradiation treatment. Rats in the R-treated groups were exposed to whole-body γ-irradiation with fractioned doses (2 Gy every week for four weeks, up to 8 Gy cumulative doses). The dose rate at the time of the experiment was 0.45 Gy/min and the time of exposure was 4.44 min exactly.
Extra virgin olive oil (Olea europaea L., Family Oleaceae)
Monumental brand extra virgin olive oil was procured from the Grup Pons company (Lleida, Spain). The purchased extra virgin olive oil density was 920 g/L and the selected olive oil dose was 7.6 mL/kg (7 g/kg) body weight (
Bashandy et al. 2014). The extra virgin olive oil was provided to the rats in the treatment group via oral gavage.
Fig fruit extract (Ficus carica L., Family Moraceae)
Dried ripe fig fruits were procured from Kafoods Ltd. (Istanbul, Turkey). The fig fruits were cut into small pieces, dried, and coarsely ground using an electrical device. The powdered material was soaked in five times its volume of 80% ethanol for 72 h with occasional shaking. The soaked material was filtered through fine filter paper, then subjected to evaporation under reduced pressure on a rotary evaporator until it dried (
Gilani et al. 2008). Each rat in the treatment group received crude fig fruit extract at a concentration of 1 g/kg body weight (equivalent to about three figs) during the experimental period (six weeks) via oral gavage. The dose of the fig fruit extract was based on the recommended antioxidant dose of dry fig fruits for humans (
Vinson et al. 2005) and converted to a suitable dose for albino rats (
Reagan-Shaw et al. 2008).
Date palm fruit extract (Phoenix dactylifera L., Family Arecaceae)
The plant material was rendered free from soil and the date palm fruits were manually separated from the pits, and the flesh of the fruits was cut into small pieces, dried in an oven at 40 °C, and coarsely ground using an electrical device. The ground date palm fruits were added to ethanol (50%) (1:3 weight to volume) for 48 h in a refrigerator (4 °C) with continuous stirring (
Al-Qarawi et al. 2005). The whole solution was ground, then centrifuged at 4 °C for 20 min at 1788
g. The supernatant was collected and stored at −20 °C until used (
Vayalil 2002). This suspension was given to rats via oral gavage, and each rat in the treatment group received crude date palm extract at a concentration of 1 g/kg body weight (equivalent to the flesh of seven dates) during the experimental period (six weeks). The dose of the crude date palm fruit extract was based on the recommended antioxidant dose of date palm fruits for humans (
Vinson et al. 2005) and converted to a suitable dose for albino rats (
Reagan-Shaw et al. 2008).
Biochemical study
Blood samples were collected at the end of the experiment from the retro-orbital venous plexus puncture of each animal (under anesthesia) using blood capillary tubes. One part (0.50 mL) of the blood sample was collected in ethylenediaminetetraacetic acid (EDTA) tubes for hematological study and the remaining sampled blood was left to clot at room temperature for 15 min. Sera were separated by centrifugation at 1006g at 20 °C for 15 min and the clear serum was extracted and kept frozen at −80 °C for use in the biochemical analyses. After blood sampling, the animals were sacrificed and the livers were isolated, quickly dissected out, and washed with isotonic ice-cold saline. A portion of each animal’s liver tissue was taken from all test groups. Each tissue sample was homogenized in ice-cold Tris–HCl lysis buffer (pH 7.4) containing 1% protease inhibitor cocktail (Cell Signaling Technology, Inc., Danvers, Massachusetts, USA) using Potter-Elvehjem rotor–stator homogenizer fitted with a Teflon pestle (Omni International, Kennesaw, Georgia, USA). The homogenates were centrifuged under cooling at 1006g for 20 min. All tissue samples were kept cold on a crushed ice at all times the preparation, and then supernatants were subsequently aliquot and stored at −80 °C until used for determination of hepatic thiobarbituric acid reactive substances (TBARS), reduced glutathione (GSH), and nitric oxide (NO) concentration.
The total number of erythrocytes (RBCs), total number of leukocytes (WBCs), differential leukocyte count, platelet count, hematocrit (Hct) percentage, and hemoglobin (Hb) concentration in the blood were estimated using a complete blood count (CBC) analyzer (Sinothinker sk9000, Shenzen, China).
Statistical analysis
Statistical analysis of the results was performed using statistical package for social sciences (SPSS) PC computer program (version 19, IBM Analytics, New York, New York, USA). All values were expressed as mean ± SE and the results were analyzed using one-way analysis of variance (ANOVA) test followed by least significant difference test (LSD) for multiple comparisons. Differences were considered statistically significant at p < 0.05.
Results
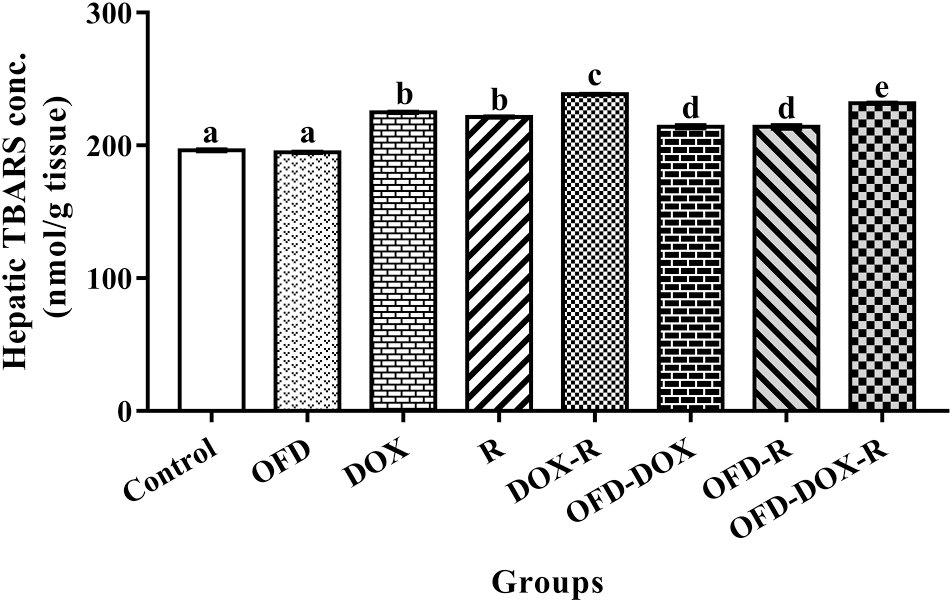
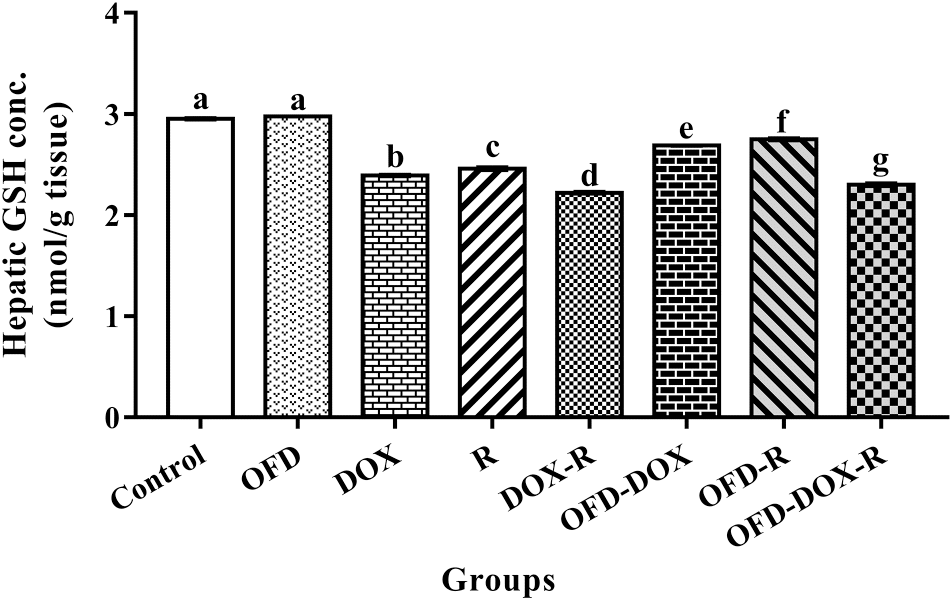
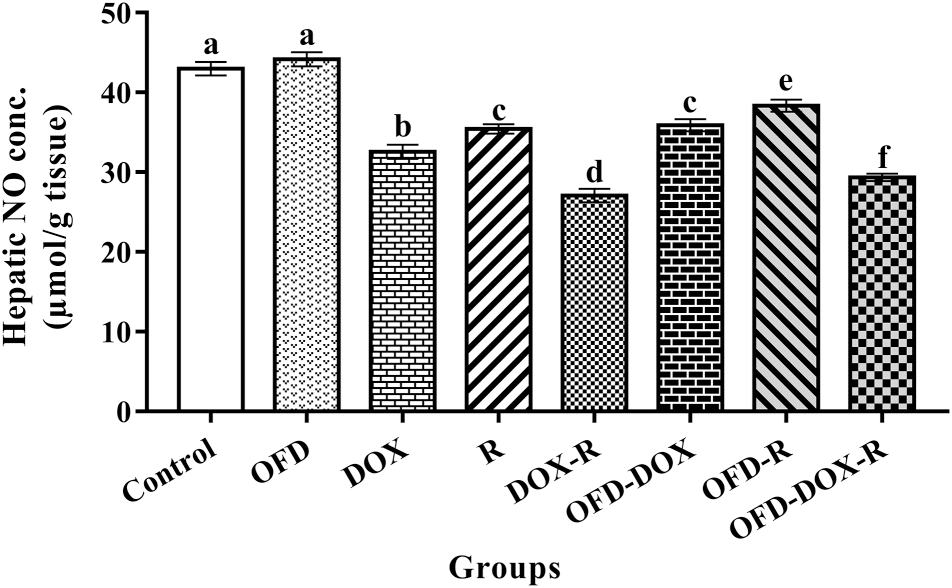
The DOX-treated and (or) R-treated rats showed a significant increase (
p < 0.05) in hepatic TBARS and a significant decrease (
p < 0.05) in hepatic GSH and NO concentrations compared with the corresponding values in the control group (
Figs. 1,
2, and
3).
In addition, the DOX-treated and (or) R-treated groups showed a significant increase (
p < 0.05) in the serum levels of TG, TC, LDL-C, TG/HDL-C, TC/HDL-C, and LDL-C/HDL-C risk ratios, and a significant decrease (
p < 0.05) in serum HDL-C compared with the corresponding values in the control group (
Table 1).
Moreover, the DOX-treated and (or) R-treated groups showed a significant decrease (
p < 0.05) in RBC count, Hb concentration, Hct percentage, platelet count, WBC count, and lymphocyte percentage, and a significant increase (
p < 0.05) in neutrophil and monocyte percentage compared with the corresponding values in the control group (
Table 2).
The pretreatment of the DOX and (or) R treatment groups with OFD for two weeks as well as OFD treatment during the four weeks of the experiment significantly reduced (
p < 0.05) the hepatic TBARS and significantly increased (
p < 0.05) the hepatic GSH and NO compared with the DOX and (or) R treatment groups (
Figs. 1,
2, and
3).
In addition, pretreatment of the DOX and (or) R treatment groups with OFD for two weeks as well as OFD treatment during the four weeks of the experiment significantly reduced (
p < 0.05) the serum TG, TC, LDL-C, TG/HDL-C, TC/HDL-C, and LDL-C/HDL-C risk ratios and significantly increased (
p < 0.05) serum HDL-C compared with the DOX and (or) R treatment groups (
Table 1).
Moreover, pretreatment of the DOX and (or) R treatment groups with OFD for two weeks as well as OFD treatment during the four weeks of the experiment significantly increased (
p < 0.05) RBC count, Hb concentration, Hct percentage, platelet count, WBC count, and lymphocyte percentage and significantly decreased (
p < 0.05) neutrophil and monocyte percentages compared with the DOX and (or) R treatment groups (
Table 2).
Discussion
Oxidative stress and generation of ROS may contribute to DOX and (or) R cytotoxicity during chemotherapy and radiotherapy (
Elsadek et al. 2017;
Fathy et al. 2017b). Among the major forms of cellular damage induced by DOX and (or) R exposure are DNA damage and lipid peroxidation. The increased levels of hepatic TBARS in the DOX and (or) R treatment groups and the decreased levels of GSH and NO compared with the control group indicate high levels of oxidative stress.
NO is a small, diffusible, highly reactive molecule that can generate oxidative stress (
Millar 2004;
Fathy et al. 2018). The significant decrease in NO levels recorded in the liver tissue after DOX and (or) R treatment might be the result of its interaction with superoxide to form peroxynitrite (
Pryor and Squadrito 1995).
GSH deficiency contributes to oxidative stress and, therefore, may play a key role in aging and many diseases (
Wu et al. 2004). In addition, GSH depletion after DOX and (or) R exposure may result from its diffusion through impaired cellular membranes and (or) inhibition of GSH synthetase and glutathione reductase enzymes (
Zahran et al. 2006). Moreover,
Srinivasan et al. (2006) demonstrated that decreased levels of GSH from oxidative stress might be due to its utilization by ROS.
The products of lipid peroxidation (TBARS) are used as an indicator of tissue damage (
Zhou et al. 2006;
Fathy et al. 2017a). The observed increase in TBARS levels may be attributed to increased ROS in the aqueous media of the cells and the interaction of the hydroxyl radical with the polyunsaturated fatty acids of membranes in the phospholipid portion of the cellular membranes initiating lipid peroxidation and the resulting damage of the cell membranes (
Azab et al. 2001).
The depletion of NO and GSH and the increase in TBARS in DOX-treated and (or) R-treated groups match the results of studies by
Bhatia and Jain (2004) and
Abd Elbaky et al. (2010) who reported a significant depletion in the antioxidant system accompanied by the increase in lipid peroxides in rats treated with DOX or R.
Our results also showed an increase in the lipid profile and lipid risk ratios in the serum of rats treated with DOX and (or) R. Hypercholesterolemia conditions might result from the stimulation of cholesterol synthesis in the liver due to its release from tissues, the destruction of cell membranes and an increase in the rate of cholesterol biosynthesis in the liver and other tissues (
Fathy 2014;
Al-Saedi et al. 2015), the mobilization of fats from the adipose tissues into the bloodstream and mitochondrial dysfunction (
Said and Azab 2006), interesterification, or random reaction changes in fatty acid positional distribution and the solid fat content of fats, which may consequently affect fat absorption and metabolism (
Wang et al. 2016). Moreover, (
Bok et al. 1999) attributed hypercholesterolemia to the increased activation of 3-Hydroxyl-3-methyl glutaryl coenzyme A (HMG-CoA) reductase enzyme, the key regulatory enzyme in the reduction of the overall process of cholesterol synthesis.
Molchanova and Ahlers (1989) attributed the increase in serum triglyceride levels to the inhibition of lipoprotein lipase activity as well as an increase in cell damage and an efflux of triglycerides from the adipose tissues. Free radicals impair liver functions and cause hormonal imbalance, which induces hyperlipidemia through its multiple effects on lipid metabolism, including increased synthesis of cholesterol, triglyceride, and LDL-C (
Bowden et al. 1989;
Fathy et al. 2017a).
The hematopoietic system is highly sensitive to DOX and ionizing radiation. The reduction in the hematological parameters from DOX and (or) ionizing radiation treatments might be due to damage in the hematopoietic system (
Fathy 2014) or the increased permeability of cell membrane, which in turn caused osmotic swelling and erythrocyte hemolysis (
Asmis et al. 2005). It might also be due to the increased destruction of mature cells or increased plasma volume (
Patil et al. 2008), or a decreased hemoglobin affinity for oxygen that induced hypoxia via diminished O
2 transport from the lungs to the blood and decreased O
2 release from oxyhemoglobin to the tissues (
Jagetia et al. 2006).
OFD is rich in polyphenolic substances, which have received widespread attention because of their potential for preventing some common chronic diseases. Polyphenols are reported to have anti-inflammatory, antioxidant, antidiabetic, and hepatoprotective effects (
Bashandy et al. 2014;
El Arem et al. 2014).
OFD treatment for two weeks prior to DOX and (or) R treatment and during the four weeks of treatment during the experiment protected against oxidative stress. This was demonstrated by significantly ameliorated oxidative stress markers (TBARS, GSH, and NO) in the liver tissue compared with the DOX-treated and (or) R-treated rats. This might be a result of the protective action of OFD active ingredients modifying membrane organization and their ability to scavenge oxidation-initiating agents (
Bashandy et al. 2014;
Fathy et al. 2018). The antioxidant effect of OFD is mainly due to phenolic compounds, which are able to donate a hydrogen atom to the free radicals, thus stopping the chain reaction propagation during the lipid peroxidation process (
Sanchez-Mareno et al. 1998). The two-week pretreatment of DOX-treated and (or) R-treated rats with OFD was also found to increase GSH and NO production. The antioxidant properties of GSH and NO stem from their reaction with oxygen, carbon, and nitrogen-centered radicals, and they play a scavenger role against free radical attack (
Grisham et al. 1999). In addition, NO modulates the inflammatory response by inhibiting the formation of proinflammatory lipids (
Rubbo et al. 1994).
The results of our study are in agreement with the findings of
Gorinstein et al. (2002) who reported that polyphenols decreased plasma LDL-C levels and prevented their oxidation in vivo. The mechanism of this hypocholesterolemic action may be the inhibition of dietary cholesterol absorption in the intestine, inhibition of cholesterol production by the liver (
Krzeminski et al. 2003), or stimulation of the biliary secretion and cholesterol excretion in the feces (
Prasad and Kalra 1993;
Katan et al. 1995). The intake of unsaturated fat decreases plasma cholesterol, whereas the intake of saturated fat increases it (
Grundy and Denke 1990;
Visioli et al. 2005;
Alfieri et al. 2017). Moreover,
Fathy (2014) found that the administration of fig extract and (or) olive oil to R-treated rats significantly ameliorated the lipid profile parameters and lipid risk ratios.
The administration of OFD to DOX-treated and (or) R-treated rats improves the hematological parameters (RBC, Hb, Hct, platelets, total, and differential WBCs), which might be attributed to increased antioxidant enzymes leading to diminished oxidative stress in bone marrow and the spleen. The results of our study are in agreement with the results of studies by
Viola and Viola (2009) and
Fathy et al. (2018) who attributed the improved hematological parameters to oleuropein, a component of olive oil, which also exerts a favorable action on the platelets.