α1-adrenergic stimulation increases ventricular action potential duration in the intact mouse heart
Abstract
The role of α1-adrenergic receptors (α-ARs) in the regulation of myocardial function is less well-understood than that of β-ARs. Previous reports in the mouse heart have described that α1-adrenergic stimulation shortens action potential duration in isolated cells or tissues, in contrast to prolongation of the action potential reported in most other mammalian hearts. It has since become appreciated, however, that the mouse heart exhibits marked variation in inotropic response to α1-adrenergic stimulation between ventricles and even individual cardiomyocytes. We investigated the effects of α1-adrenergic stimulation on action potential duration at 80% of repolarization in the right and left ventricles of Langendorff-perfused mouse hearts using optical mapping. In hearts under β-adrenergic blockade (propranolol), phenylephrine or noradrenaline perfusion both increased action potential duration in both ventricles. The increased action potential duration was partially reversed by subsequent perfusion with the α-adrenergic antagonist phentolamine (1 μmol L−1). These data show that α1-receptor stimulation may lead to a prolonging of action potential in the mouse heart and thereby refine our understanding of how action potential duration adjusts during sympathetic stimulation.
Introduction
Adrenergic stimulation modulates myocardial function by acting upon α- and β-adrenergic receptors (ARs). The latter, especially β1-ARs, are found in the greatest abundance (Baker 2014; Myagmar et al. 2017), and their functions, including mediating a powerful positive inotropic effect in the heart, are well-established (Lymperopoulos et al. 2013; Baker 2014). Conversely, α-ARs, namely the α1-ARs expressed in cardiomyocytes, remain much more enigmatic and controversial (Mohl et al. 2011; Endoh 2016), as the literature reveals considerable variation in cardiac effects between different species, tissues, and experimental preparations (Wagner and Brodde 1978; Tohse et al. 1992; Zhang et al. 1998; Ross et al. 2003; Endoh 2016; Joyce and Wang 2020). Nevertheless, it is clear that as β-adrenergic function declines during heart failure, due to the desensitization of β1-AR, the relative importance of cardiac α1-ARs increases (Skomedal et al. 1997; Sjaastad et al. 2003; Jensen et al. 2011) due to the desensitization of β1-ARs (Bristow et al. 1982). Accordingly, the α1-adrenergic signalling pathway is emerging as a potential therapeutic target (Jensen et al. 2011; Perez and Doze 2011; Baker 2014; O’Connell et al. 2014; Endoh 2016).
The mouse has become a cornerstone to study α-adrenergic regulation in the heart, particularly given the genetic amenability that enabled the generation of various α1-AR sub-type specific transgenic and knockout lines (Koch et al. 2000; Ross et al. 2003; Turnbull et al. 2003; Mohl et al. 2011; Jensen et al. 2014). However, whilst in most mammals (Brückner et al. 1978; Wagner and Brodde 1978; Brückner and Scholz 1984; Endoh et al. 1991; Endoh 2016), including humans (Landzberg et al. 1991; Janssen et al. 2018), α1-adrenergic stimulation of ventricular myocardium results in a positive inotropic effect, the effects in mice are particularly complex, and both positive and negative inotropic have been reported in cardiomyocytes, isolated muscle, and whole heart preparations (Ross et al. 2003; Petrashevskaya et al. 2004; Wang et al. 2006; Mohl et al. 2011). In accordance with the positive inotropic effect, most mammals exhibit a lengthening of action potential duration (APD) with α1-adrenergic stimulation (Brückner and Scholz 1984; Tohse et al. 1987, 1990; Apkon and Nerbonne 1988; Endoh et al. 1991; Fedida and Bouchard 1992). By contrast, previous studies in mice utilizing direct measurements of transmembrane potentials in isolated right ventricular papillary muscle or isolated cardiomyocytes have reported shortened APD in response to α1-adrenergic stimulation (Nishimaru et al. 2001; Petrashevskaya et al. 2004).
To get insight into the regulation of APD during sympathetic stimulation, we investigated the effects of α1-adrenergic stimulation on APD using optical mapping in whole Langendorff-perfused mouse hearts. In our first series of experiments, Protocol 1, we studied the effect of selective α-adrenergic stimulation with the pharmacological agonist phenylephrine. Because the heart primarily relies on stimulation via the neurotransmitter noradrenaline in vivo, in Protocol 2 we investigated if this nonselective agonist of adrenergic receptors is also able to induce electrophysiological effects mediated by α1-ARs when β-ARs are inhibited by propranolol, which mimics the β-AR desensitization during heart failure.
Methods
Experimental animals and preparation
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and the European Commission Directive 2010/63/EU and was approved by the institutional review board (Amsterdam University Medical Centers).
Six-week-old male FVB/N wild-type mice were anesthetized in CO2 and killed by cervical dislocation. The heart was rapidly excised and the aorta was cannulated and Langendorff- perfused with Tyrode’s solution (mmol L−1: NaCl (128), KCl (4.7), CaCl2 (1.45), MgCl2 (0.6), NaHCO3 (27), NaH2PO4 (0.4), glucose (11), and maintained at pH 7.4 by bubbling with carbogen (95% O2, 5% CO2)) within 10 min. The preparation was placed in a water-jacketed organ bath, containing Tyrode’s solution, with the ventral surface facing upwards. Both the perfusate reservoir and organ bath were maintained at 37 °C. For the duration of the experiment, the heart was perfused at a rate of 3–4 mL min−1.
Immediately after starting perfusion, blebbistatin (±) (Bio-Techne Ltd, United Kingdom) was added to Tyrode’s reservoir to achieve a final concentration of 16 μmol L−1, which was maintained throughout the experiment. Five min later, 100–125 μL of 0.1 mmol L−1 di-4-ANEPPS (Molecular Probes, Eugene, Oregon, USA) (dissolved in ethanol) was diluted into 300 μL of Tyrode’s solution and delivered as bolus into the perfusate line. Optical action potentials were measured with an excitation light provided by a 5-W power LED (filtered 510 ± 20 nm). Fluorescence (filtered >610 nm) was transmitted through a lens system on a CMOS sensor (100 × 100 elements; MICAM Ultima), and the data were acquired with MICAM Ultima software on a personal computer. Pseudo-electrograms were recorded by placing 3 electrodes at ±0.5 cm distance from the heart in the Einthoven configuration as published previously (Boukens et al. 2013). Electrode R and L were placed alongside the right and left atrium, respectively, whereas electrode F was placed alongside the apex. Electrode R was used as negative input for both lead I and lead II. Recordings were made using Labchart amplifier (ADInstruments, Model 15T, sample frequency 1 kHz) and analyzed in LabChart Pro v8.1.13. A silver electrode was used for stimulation at the apex via a custom build current controlled stimulator (Antronics).
Experimental protocol
Ten to fifteen minutes after the di-4-ANEPPS treatment, the experimental protocol started. For each trial, optical action potentials were measured during apical simulation at 200 ms intervals before the duration was decreased in 20 ms steps to 120 ms, or until the heart failed to capture. At each frequency, stimulation was maintained for approximately 40–60 s to allow sufficient stabilisation before action potentials were measured.
Two protocols were employed (Fig. 1); each included serial additions of pharmacological agents to the Tyrode’s solution perfusate. Each initially used propranolol to block stimulation of β-adrenergic receptors (Brückner and Scholz 1984; Nishimaru et al. 2001; Montgomery et al. 2002; Ross et al. 2003) and ended with phentolamine, an α-adrenergic antagonist. In the first protocol (N = 4), the trial was conducted under control conditions with propranolol (1 μmol L−1), phenylephrine (1 μmol L−1), and phentolamine (1 μmol L−1). In the second protocol (N = 5), phenylephrine was substituted with noradrenaline (500 nmol L−1). Each agent was perfused for 10 min prior to the pacing trial to achieve steady state (Ross et al. 2003; Wang et al. 2006; Baker 2014). We additionally conducted a series of trials (N = 3) without the addition of phenylephrine/noradrenaline and phentolamine to account for time-dependent changes in APD after the addition of propranolol. Noradrenaline bitartrate, phenylephrine hydrochloride, propranolol hydrochloride, and phentolamine hydrochloride were purchased from Sigma-Aldrich (St Louis, MO, USA).
Fig. 1.
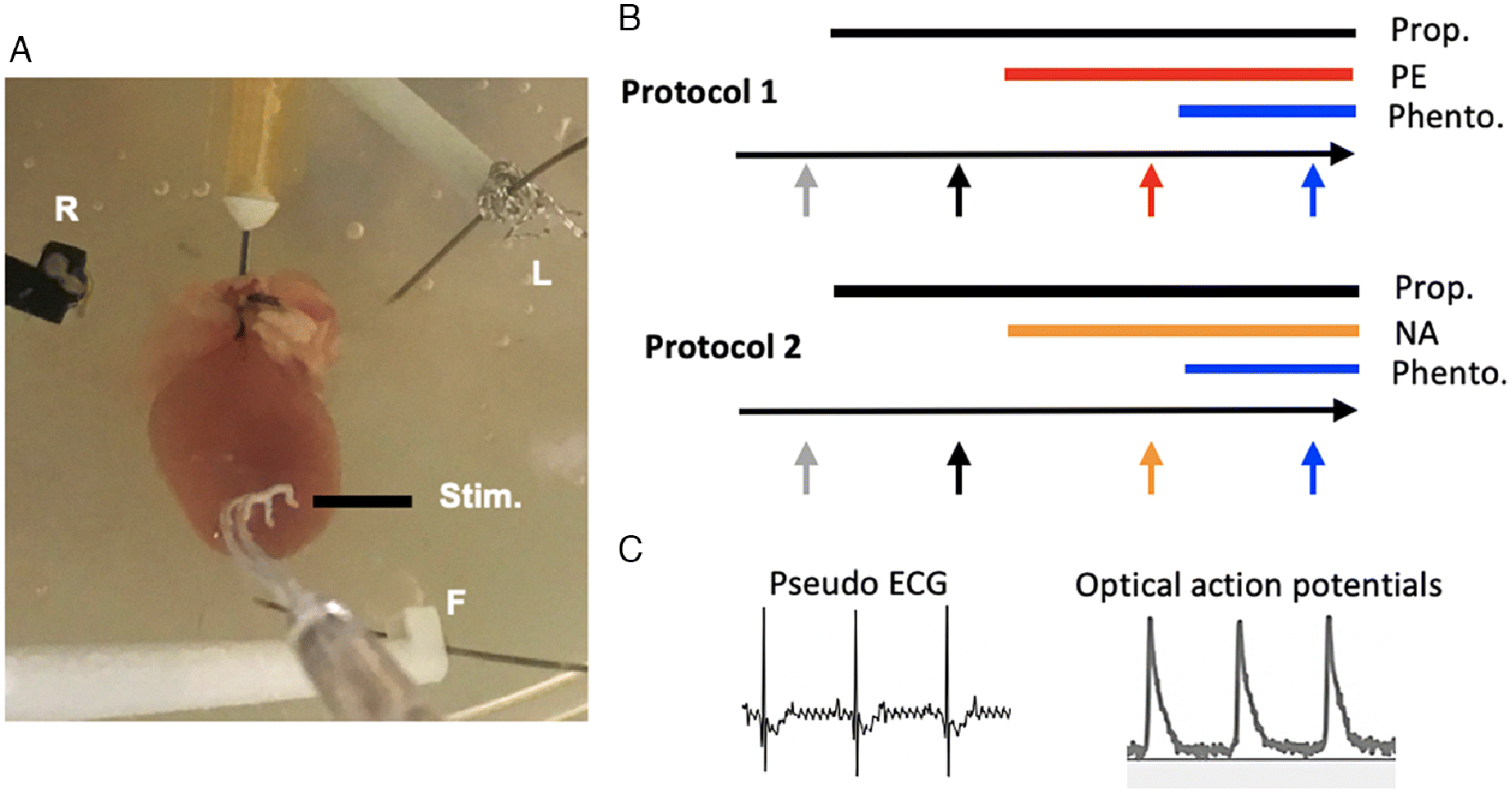
Data analysis
Optical action potentials were analysed with custom software in MATLAB (version 2017a, MathWorks, Inc. Nattick, Massachusetts, USA). In brief, action potentials were normalized based on the peak of the upstroke after spatial binning of 7 × 7. Ten action potentials were averaged after which APD80 was determined based on 80% of repolarization. We took the average APD80 of a region of 1 mm2 at the base of the left and right ventricles at each frequency and with each drug treatment. Total ventricular activation time and repolarization time were measured during pacing at 180 ms intervals using computer generated activation maps.
Statistical analysis was performed in GraphPad Prism version 8.4.0 (GraphPad Software, San Diego, CA, USA). Mixed-effects models (with repeated measures for stimulation frequency and drug treatment) followed by Dunnett’s post-hoc tests were used to determine significant (p < 0.05) effects of propranolol, noradrenaline/phenylephrine, and phentolamine on APD80 at different stimulation frequencies. A repeated measurement one-way analysis of variance was performed to analyse the effect of propranolol, noradrenaline, and phentolamine on end QRS-complex time, end T-wave time, repolarisation time, and activation time. Data are presented as means ± standard error of the mean (SEM). In Supplementary Material 1, we present figures depicting data points of individual hearts.
Results
To investigate the role of α1-ARs on ventricular repolarization, we administered phenylephrine (Protocol 1) and noradrenaline (Protocol 2) to Langendorff-perfused mouse hearts (Fig. 1). We first administered propranolol to exclude possible binding of phenylephrine and noradrenaline to β-adrenergic receptors. We also tested the effect of propranolol alone on action potential duration in a separate set of hearts (N = 3) to account for time-dependent changes during the course of the experiment. Figure 2 shows optical action potentials recorded 0, 15, and 45 min after administration of propranolol, during which APD did not change.
Fig. 2.
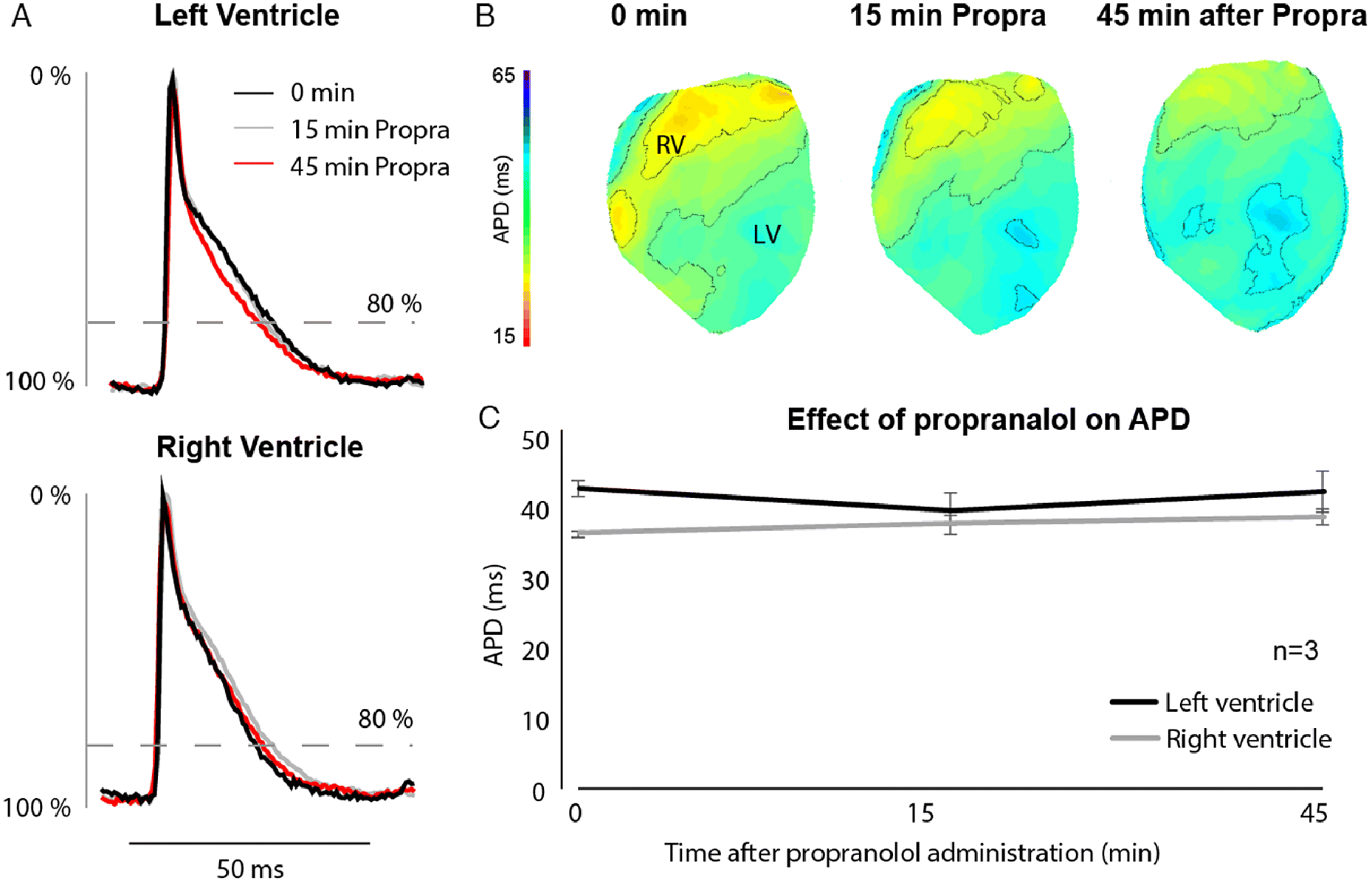
In Protocols 1 and 2 propranolol administration increased intrinsic RR interval (sinus rhythm) by ∼40 ms and ∼20 ms, respectively. By the end of both protocols, intrinsic RR interval had increased from 201 ± 21 ms to 300 ± 26 ms in protocol 1 and from 184 ± 22 ms to 247 ± 25 ms in protocol 2 (Table 1). To exclude the influence of heart rate on QT interval, we therefore measured the effect of phenylephrine and noradrenaline on repolarization during stimulation at the ventricular apex at 180 ms interval. Figures S1 and S2 show that neither QRS duration nor QT time changed during administration of either drug.
Table 1.
| RR | PR | QRS | QT | |||||
|---|---|---|---|---|---|---|---|---|
| Average | SEM | Average | SEM | Average | SEM | Average | SEM | |
| Protocol 1 | ||||||||
| Before | 200.7 | 21.4 | 42.2 | 7.7 | 13.3 | 2.6 | 63.0 | 3.7 |
| Propranol | 243.4 | 12.0 | 61.3 | 10.7 | 13.9 | 2.4 | 65.1 | 6.8 |
| Phenylephrine | 271.2 | 20.6 | 71.5 | 5.0 | 16.4 | 3.9 | 78.3 | 8.9 |
| Phentolamine | 299.5 | 25.5 | 91.0 | 13.1 | 22.8 | 6.2 | 83.1 | 4.4 |
| Protocol 2 | ||||||||
| Before | 184.2 | 21.7 | 45.3 | 2.6 | 18.3 | 1.8 | 89.1 | 17.6 |
| Propranol | 202.0 | 26.2 | 47.9 | 4.4 | 19.6 | 4.3 | 74.1 | 6.9 |
| Noradrenaline | 234.7 | 20.5 | 63.9 | 10.3 | 19.1 | 3.3 | 88.0 | 3.6 |
| Phentolamine | 247.3 | 24.8 | 62.8 | 8.7 | 20.7 | 2.0 | 90.3 | 7.5 |
During ventricular pacing we also determined local moments of activation and repolarization (RT80). Panel A and B of Fig. 3 show two typical examples of the activation (upper) and repolarization (lower) sequence at the ventral side of the heart during stimulation on the apex. In both protocols, last moment of activation delayed significantly after administration of propranolol but remained unchanged after adding the other drugs. On the other hand, last moment of repolarization occurred later after administration of phenylephrine and noradrenaline. Additional administration of phentolamine, during perfusion with phenylephrine or noradrenaline, did not affect repolarization (see Fig. S3 for data points of the individual hearts).
Fig. 3.
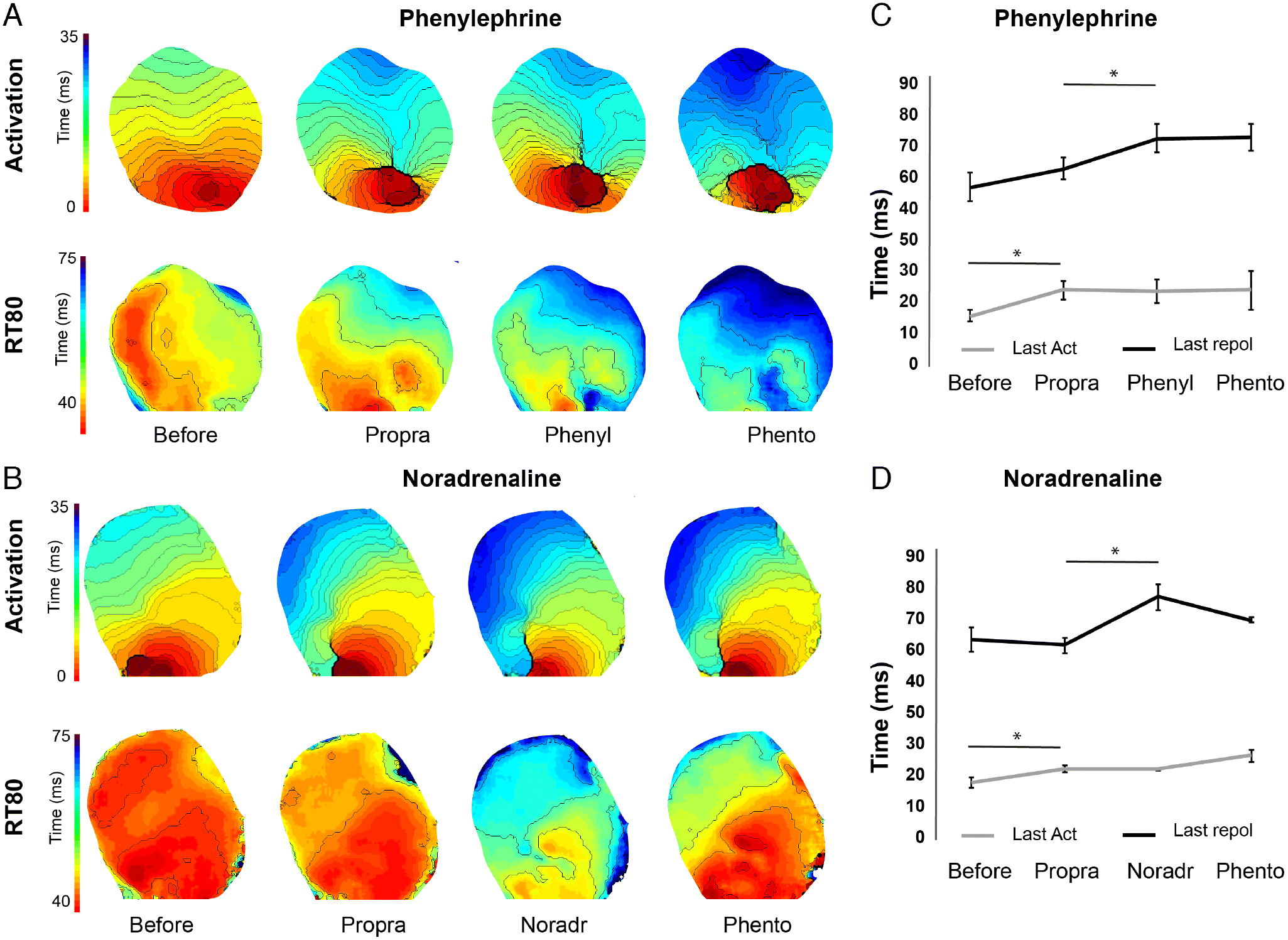
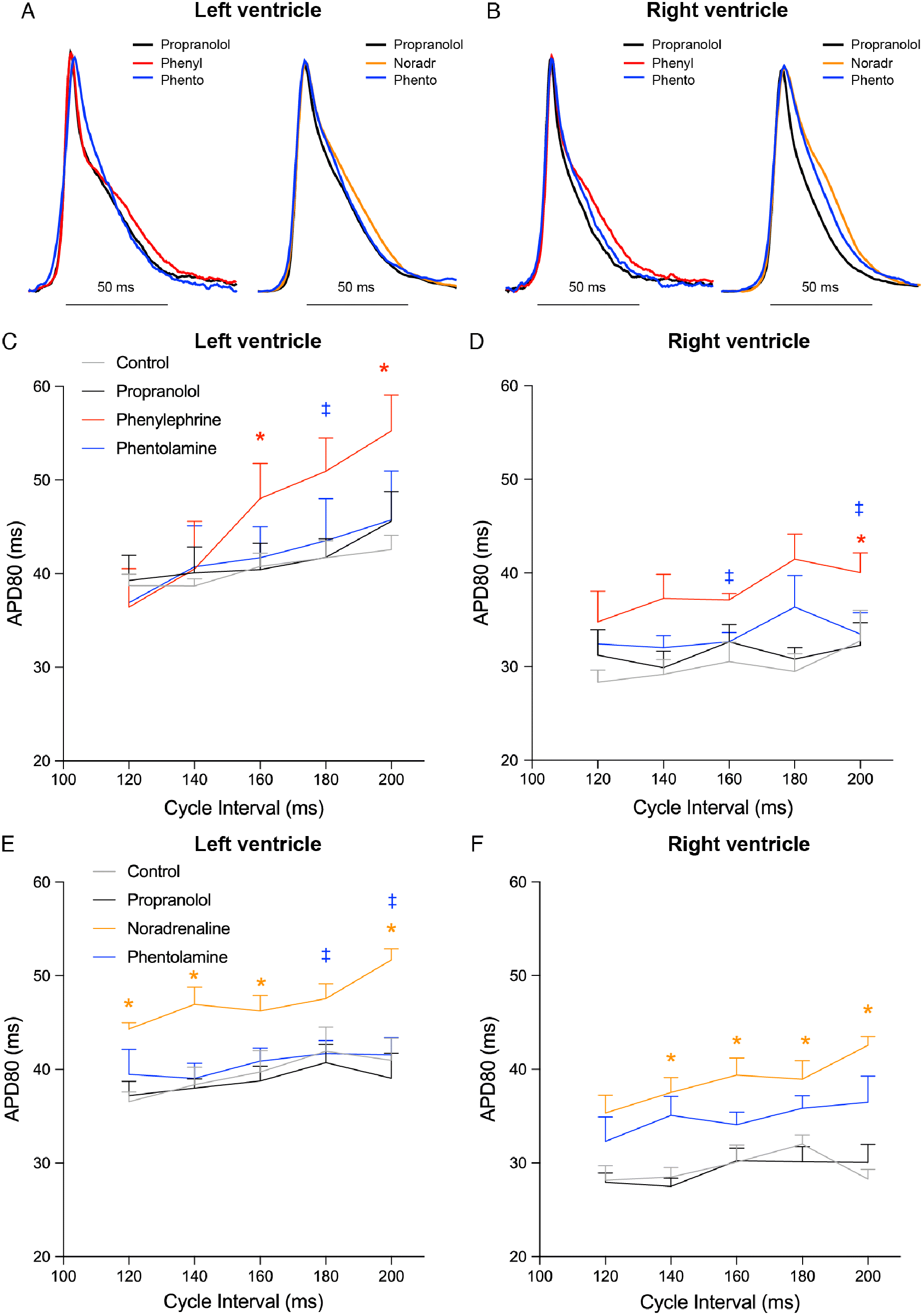
Figure 4 shows APD maps during Protocol 1 (Fig. 4A) and Protocol 2 (Fig. 4B). The initial treatment with the general β-adrenergic receptor blocker propranolol did not affect APD in either ventricle (Fig. 4). Administration of either phenylephrine or noradrenaline prolonged APD, which then became shorter after administration of phentolamine (Figs. 5A and 5B). Phenylephrine significantly (p < 0.05) increased APD80 in the right ventricle at 200 ms stimulus intervals and in the left ventricle at 200 and 160 ms intervals (p = 0.052 at 180 ms). Following phentolamine, APD80 was reduced at 200 and 160 ms in the right ventricle and 180 ms in the left ventricle (p < 0.05) (Figs. 5C and 5D). Noradrenaline significantly increased APD80 (p < 0.05) at all frequencies in the right ventricle, with the exception at 120 ms, which was borderline significant (p = 0.057). Likewise, noradrenaline increased APD80 at all frequencies (p < 0.05) in the left ventricle with the marginal exception at 180 ms stimulus interval (p = 0.059). APD80 was significantly reduced towards control levels by α-AR block with phentolamine at 200 and 180 ms stimulus intervals in the left ventricle (Figs. 5E, 5F, and S4).
Fig. 4.
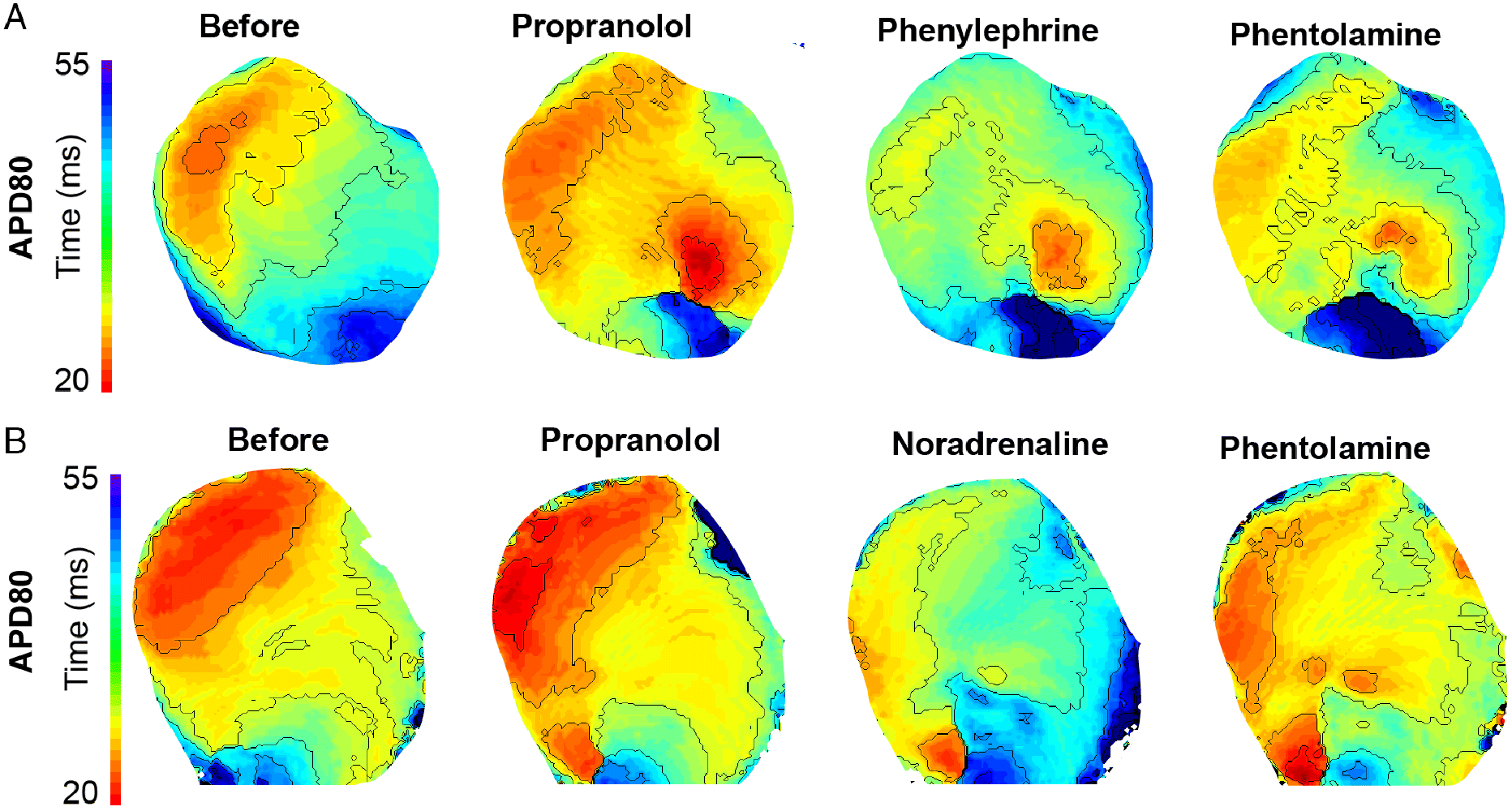
Fig. 5.
Discussion
Our data show that stimulation of α1-ARs prolongs APD in both the left and right ventricle of mice at various stimulation frequencies. Moreover, we show that noradrenaline is able to prolong APD in mice when β-ARs are blocked.
The important role of α1-ARs in the regulation of myocardial function is less well appreciated than that of β-ARs (Brückner et al. 1985; Baker 2014; Endoh 2016). It has previously been shown that α1-AR stimulation shortens the APD in mice (Nishimaru et al. 2001; Petrashevskaya et al. 2004). Nishimaru et al. (2001) demonstrated that APD reduces rapidly at the onset of phenylephrine treatment and remained shortened for at least 15 min, whereas our measurements within the same timeframe (10 min after the initiation of α1-AR stimulation) indicated an increased APD. The disparate aspects of the results between previous work and our own remain to be clarified, but may be due to the complex cellular heterogeneity in the heart (Chu et al. 2013; Myagmar et al. 2017). Indeed, having established that the positive inotropic effect in response to α1-AR stimulation in a subset of cells was not due to altered sarcoplasmic reticulum Ca2+ load, Chu et al. (2013) speculated that increased APD may be implicated in at least some cardiomyocytes. As we solely measured epicardial action potentials, it is possible that the difference stems from transmural differences. APD is shorter in epicardial than endocardial cardiomyocytes in mice (Bondarenko and Rasmusson 2010), potentially providing more scope for it to increase during α1-AR stimulation. It is also possible that studies on isolated cells or tissues interrupt the integrity of the preparation and lead to differences in α1-AR signalling (Ross et al. 2003). Indeed, it was previously reported that α1-AR subtype expression changed considerably during isolation of hepatocytes (González-Espinosa et al. 1999).
The previous studies operated with a very low stimulation frequency (1 Hz; 60 beats min−1), whereas our study was performed close to the physiological range (>300 beats min−1; (Kramer et al. 1993)), which is an additional possible source of variation. However, whilst frequency dependency of α1-AR stimulation has been demonstrated, greater positive inotropic effects are typically observed at low frequency, making this explanation unlikely. Considerable inter-strain variability has also been reported with regards to cardiac electrophysiology in mice (Waldeyer et al. 2009), which may also contribute to the distinction.
Wang et al. (2006) described prominent interventricular differences in response to α1-AR stimulation in the mouse heart. Whilst trabeculae of the left ventricle exhibit a positive inotropic response, trabeculae of the right ventricle have an overall negative inotropic response to α1-adrenergic stimulation (Wang et al. 2006). We were therefore interested to see if the APD response differed between ventricles. However, in both ventricles we observed an increase in APD of a similar magnitude response to α1-adrenergic stimulation and the effects were similar regardless of whether the adrenergic stimulus was phenylephrine or noradrenaline in the presence of propranolol (Fig. 5).
The ionic basis for the prolonging of APD that we report remains to be dissected. Although Nishimaru et al. (2001) observed a decrease in APD with α1-adrenergic stimulation, they reported an increased L-type Ca2+ current, which may be an effective means to increase APD in the mouse heart (Wang et al. 2019). The α1-adrenergic regulation of the L-type Ca2+ current in mammalian myocardium has proven to be particularly controversial (van der Heyden et al. 2005). Increased L-type Ca2+ current was initially suggested to be involved in the prolonged APD in several species (Miura et al. 1978; Brückner and Scholz 1984), but was subsequently largely discredited in favour of reduced outward currents (Apkon and Nerbonne 1988; Hartmann et al. 1988; Hescheler et al. 1988; Tohse et al. 1990; Endoh et al. 1991; Fedida and Bouchard 1992). Later work, however, indicated that the regulation of the L-type Ca2+ current is particularly sensitive to disturbances to intracellular milieu with conventional whole-cell patch-clamp techniques. With perforated patch-clamp, α-adrenergic stimulation has been convincingly shown to directly increase L-type Ca2+ current in rat ventricular myocytes (Liu and Kennedy 1998; Zhang et al. 1998, 2005; O-Uchi et al. 2005).
Recently, it was demonstrated that a transient prolonging of APD during sympathetic stimulation is optimised to increase calcium transient amplitude in the mouse heart (Wang et al. 2019). This effect was abolished by propranolol, suggesting that it is primarily mediated by β-ARs. However, this study only considered the effects of 1 min of sympathetic stimulation. The complex effects of α1-AR stimulation, however, develop more slowly than β-ARs (Baker 2014), perhaps because the receptors reside in the nucleus and hence require prior internalisation of the signal (O’Connell et al. 2014). Indeed, with α1-adrenergic stimulation of the mouse ventricle, during the first few minutes a negative inotropic effect dominates α-adrenergic stimulation, even when a positive inotropic effect ultimately transpires (Ross et al. 2003; Wang et al. 2006). Our data thus complement the study of Wang et al. (2019), and suggest that during tonic adrenergic stimulation, as may occur during more extended bouts of exercise, α1-ARs may reinforce and fine-tune the prolonging of APD.
Limitations
Optical mapping during Langendorff-perfusion is the only method that allows the measurement of APD and repolarization differences on the epicardial surface of the mouse heart. To reduce motion artefacts in the optical signals the heart was immobilized by administering blebbistatin. It has been proposed that blebbistatin affects intracellular Ca2+ homeostasis as it inhibits myosin ATPase-II, and the actin-myosin interaction relies on Ca2+. However, L-type Ca2+ current appears unaffected in the mouse ventricle in the presence of blebbistatin (Dou et al. 2007). In addition, in our experiments the heart was retrograde perfused at a constant rate. In the in vivo situation, α-adrenergic stimulation elicits profound effects on the vasculature, including arterial vasoconstriction and venoconstriction (Joyce and Wang 2020), which would also influence cardiac performance. However, we believe our strategy was well suited to studying the direct pharmacological effects of α1-adrenergic stimulation of the ventricle.
Future directions
Due to the nature of our protocol involving sequential drug administrations, we were restricted to making a single measurement of APD 10 min after the initiation of each treatment. This was intended to allow us to focus on the effects of the drug treatments once they reached steady state (Ross et al. 2003; Wang et al. 2006). However, in doing so we neglected the early stages of α1-AR signalling, which may be relevant during brief periods of adrenergic stimulation. Multiphasic contractile responses are known to occur in the mouse heart during α-adrenergic stimulation in the initial phases (Ross et al. 2003; Wang et al. 2006), so it may prove fruitful for future work to study the time course in greater temporal resolution to reveal how APD may change in the mouse heart during immediate and sustained stimulation. In addition, unravelling the mechanistic basis for the increased APD in both ventricles of the mouse heart reported here may provide clarification on the complex role of L-type Ca2+ current in the myocardium of mammals (van der Heyden et al. 2005). α1-AR signalling is known to activate protein kinase C (Talosi and Kranias 1992) and Ca2+/calmodulin-dependent PK II (CaMKII) (O-Uchi et al. 2005), which in turn will catalyse the phosphorylation of a host of proteins that may directly or indirectly affect APD, and could be identified by future work. The APD prolongation may also depend on stimulation of phosphoinositide hydrolysis (Endoh et al. 1991).
Conclusions
Our data show that epicardial action potentials prolong in response to α-AR stimulation in the intact mouse heart. This suggests the electrophysiological response to α-AR stimulation in the mouse heart could share more similarities with other mammals than previously acknowledged, consolidating it as a model to advance our understanding of general α-adrenergic control.
Acknowledgements
This study was supported by a Dutch Heart Foundation grant (2016T047) to BJB. WJ is supported by a Novo Nordisk Foundation grant (NNF19OC0055842) and TW is supported by the Independent Research Fund Denmark (Natur og Univers, Det Frie Forskningsråd, Grant number: 7014-00375B). We thank Joost Offerhaus for assisting with part of the experiments and two anonymous referees for providing constructive comments that helped improve the manuscript.
References
Apkon M, and Nerbonne JM. 1988. α1-Adrenergic agonists selectively suppress voltage-dependent K+ current in rat ventricular myocytes. Proceedings of the National Academy of Sciences of the United States of America, 85(22): 8756–8760.
Baker AJ. 2014. Adrenergic signaling in heart failure: a balance of toxic and protective effects. Pflugers Archiv: European Journal of Physiology, 466(6): 1139–1150.
Bondarenko VE, and Rasmusson RL. 2010. Transmural heterogeneity of repolarization and Ca2+ handling in a model of mouse ventricular tissue. American Journal of Physiology—Heart and Circulatory Physiology, 299(2): H454–H469.
Boukens BJ, Hoogendijk MG, Verkerk AO, Linnenbank A, van Dam P, Remme C-A, et al. 2013. Early repolarization in mice causes overestimation of ventricular activation time by the QRS duration. Cardiovascular Research, 97(1): 182–191.
Boukens BJ, Rivaud MR, Rentschler S, and Coronel R. 2014. Misinterpretation of the mouse ECG: ‘musing the waves of Mus musculus’. The Journal of Physiology, 592(21): 4613–4626.
Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, et al. 1982. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. The New England Journal of Medicine, 307(4): 205–211.
Brückner R, and Scholz H. 1984. Effects of α-adrenoceptor stimulation with phenylephrine in the presence of propranolol on force of contraction, slow inward current and cyclic AMP content in the bovine heart. British Journal of Pharmacology, 82(1): 223–232.
Brückner R, Hackbarth I, Meinertz T, Schmelzle B, and Scholz H. 1978. The positive inotropic effect of phenylephrine in the presence of propranolol. Increase in time to peak force and in relaxation time without increase in c-AMP. Naunyn-Schmiedeberg’s Archives of Pharmacology, 303(3): 205–211.
Brückner R, Mugge A, and Scholz H. 1985. Existence and functional role of alpha1-adrenoceptors in the mammalian heart. Journal of Molecular and Cellular Cardiology, 17(7): 639–645.
Chu C, Thai K, Park KW, Wang P, Makwana O, Lovett DH, et al. 2013. Intraventricular and interventricular cellular heterogeneity of inotropic responses to α1-adrenergic stimulation. American Journal of Physiology—Heart and Circulatory Physiology, 304(7): H946–H953.
Dou Y, Arlock P, and Arner A. 2007. Blebbistatin specifically inhibits actin-myosin interaction in mouse cardiac muscle. American Journal of Physiology—Cell Physiology, 293(3): C1148–1153.
Endoh M. 2016. Cardiac α1-adrenoceptors and inotropy: myofilament Ca2+ sensitivity, intracellular Ca2+ mobilization, signaling pathway, and pathophysiological relevance. Circulation Research, 119(5): 587–590.
Endoh M, Hiramoto T, Ishihata A, Takanashi M, and Inui J. 1991. Myocardial α1-adrenoceptors mediate positive inotropic effect and changes in phosphatidylinositol metabolism. Species differences in receptor distribution and the intracellular coupling process in mammalian ventricular myocardium. Circulation Research, 68(5): 1179–1190.
Fedida D, and Bouchard RA. 1992. Mechanisms for the positive inotropic effect of α1-adrenoceptor stimulation in rat cardiac myocytes. Circulation Research, 71(3): 673–688.
González-Espinosa C, González-Espinosa D, Romero-Avila MT, and García-Sáinz JA. 1999. Inverse α1A and α1D adrenoceptor mRNA expression during isolation of hepatocytes. European Journal of Pharmacology, 384(2–3): 231–237.
Hartmann HA, Mazzocca NJ, Kleiman RB, and Houser SR. 1988. Effects of phenylephrine on calcium current and contractility of feline ventricular myocytes. American Journal of Physiology—Heart and Circulatory Physiology, 255(5 Pt 2): H1173–1180.
Hescheler J, Nawrath H, Tang M, and Trautwein W. 1988. Adrenoceptor-mediated changes of excitation and contraction in ventricular heart muscle from guinea-pigs and rabbits. The Journal of Physiology, 397(1): 657–670.
Janssen PML, Canan BD, Kilic A, Whitson BA, and Baker AJ. 2018. Human myocardium has a robust α1A-subtype adrenergic receptor inotropic response. Journal of Cardiovascular Pharmacology, 72(3): 136–142.
Jensen BC, O’Connell TD, and Simpson PC. 2011. Alpha-1-adrenergic receptors: targets for agonist drugs to treat heart failure. Journal of Molecular and Cellular Cardiology, 51(4): 518–528.
Jensen BC, O’Connell TD, and Simpson PC. 2014. Alpha-1-adrenergic receptors in heart failure: the adaptive arm of the cardiac response to chronic catecholamine stimulation. Journal of Cardiovascular Pharmacology, 63(4): 291–301.
Joyce W, and Wang T. 2020. What determines systemic blood flow in vertebrates? The Journal of Experimental Biology, 223(4): jeb215335.
Koch WJ, Lefkowitz RJ, and Rockman HA. 2000. Functional consequences of altering myocardial adrenergic receptor signaling. Annual Review of Physiology, 62: 237–260.
Kramer K, van Acker SABE, Voss H-P, Grimbergen JA, van der Vijgh WJF, and Bast A. 1993. Use of telemetry to record electrocardiogram and heart rate in freely moving mice. Journal of Pharmacological and Toxicological Methods, 30(4): 209–215.
Landzberg JS, Parker JD, Gauthier DF, and Colucci WS. 1991. Effects of myocardial α1-adrenergic receptor stimulation and blockade on contractility in humans. Circulation, 84(4): 1608–1614.
Liu SJ, and Kennedy RH. 1998. α1-Adrenergic activation of L-type Ca current in rat ventricular myocytes: perforated patch-clamp recordings. American Journal of Physiology—Heart and Circulatory Physiology, 274(6): H2203–H2207.
Lymperopoulos A, Rengo G, and Koch WJ. 2013. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circulation Research, 113(6): 739–753.
Miura Y, Inui J, and Imamura H. 1978. Alpha-adrenoceptor-mediated restoration of calcium-dependent potential in the partially depolarized rabbit papillary muscle. Naunyn-Schmiedeberg’s Archives of Pharmacology, 301(3): 201–205.
Mohl MC, Iismaa SE, Xiao X-H, Friedrich O, Wagner S, Nikolova-Krstevski V, et al. 2011. Regulation of murine cardiac contractility by activation of α1A-adrenergic receptor-operated Ca2+ entry. Cardiovascular Research, 91(2): 310–319.
Montgomery DE, Wolska BM, Pyle WG, Roman BB, Dowell JC, Buttrick PM, et al. 2002. α-Adrenergic response and myofilament activity in mouse hearts lacking PKC phosphorylation sites on cardiac TnI. American Journal of Physiology—Heart and Circulatory Physiology, 282(6): H2397–H2405.
Myagmar B-E, Flynn JM, Cowley PM, Swigart PM, Montgomery MD, Thai K, et al. 2017. Adrenergic receptors in individual ventricular myocytes: the beta-1 and alpha-1B are in all cells, the alpha-1A is in a subpopulation, and the beta-2 and beta-3 are mostly absent. Circulation Research, 120(7): 1103–1115.
Nishimaru K, Kobayashi M, Matsuda T, Tanaka Y, Tanaka H, and Shigenobu K. 2001. α-Adrenoceptor stimulation-mediated negative inotropism and enhanced Na+/Ca2+ exchange in mouse ventricle. American Journal of Physiology—Heart and Circulatory Physiology, 280(1): H132–H141.
O’Connell TD, Jensen BC, Baker AJ, and Simpson PC. 2014. Cardiac alpha1-adrenergic receptors: novel aspects of expression, signaling mechanisms, physiologic function, and clinical importance. Pharmacological Reviews, 66(1): 308–333.
O-Uchi J, Komukai K, Kusakari Y, Obata T, Hongo K, Sasaki H, et al. 2005. α1-Adrenoceptor stimulation potentiates L-type Ca2+ current through Ca2+/calmodulin-dependent PK II (CaMKII) activation in rat ventricular myocytes. Proceedings of the National Academy of Sciences of the United States of America, 102(26): 9400–9405.
Perez DM, and Doze VA. 2011. Cardiac and neuroprotection regulated by α(1)-adrenergic receptor subtypes. Journal of Receptor and Signal Transduction Research, 31(2): 98–110.
Petrashevskaya NN, Bodi I, Koch SE, Akhter SA, and Schwartz A. 2004. Effects of alpha1-adrenergic stimulation on normal and hypertrophied mouse hearts. Relation to caveolin-3 expression. Cardiovascular Research, 63(3): 561–572.
Ross SA, Rorabaugh BR, Chalothorn D, Yun J, Gonzalez-Cabrera PJ, McCune DF, et al. 2003. The α1B-adrenergic receptor decreases the inotropic response in the mouse Langendorff heart model. Cardiovascular Research, 60(3): 598–607.
Sjaastad I, Schiander I, Sjetnan A, Qvigstad E, Bøkenes J, Sandnes D, et al. 2003. Increased contribution of α1- vs. β-adrenoceptor-mediated inotropic response in rats with congestive heart failure. Acta Physiologica Scandinavica, 177(4): 449–458.
Skomedal T, Borthne K, Aass H, Geiran O, and Osnes JB. 1997. Comparison between alpha-1 adrenoceptor-mediated and beta adrenoceptor-mediated inotropic components elicited by norepinephrine in failing human ventricular muscle. The Journal of Pharmacology and Experimental Therapeutics, 280(2): 721–729.
Talosi L, and Kranias EG. 1992. Effect of alpha-adrenergic stimulation on activation of protein kinase C and phosphorylation of proteins in intact rabbit hearts. Circulation Research, 70(4): 670–678.
Tohse N, Hattori Y, Nakaya H, and Kanno M. 1987. Effects of alpha-adrenoceptor stimulation on electrophysiological properties and mechanics in rat papillary muscle. General Pharmacology, 18(5): 539–546.
Tohse N, Nakaya H, Hattori Y, Endou M, and Kanno M. 1990. Inhibitory effect mediated by alpha1-adrenoceptors on transient outward current in isolated rat ventricular cells. Pflugers Archiv: European Journal of Physiology, 415(5): 575–581.
Tohse N, Nakaya H, and Kanno M. 1992. Alpha1-adrenoceptor stimulation enhances the delayed rectifier K+ current of guinea pig ventricular cells through the activation of protein kinase C. Circulation Research, 71(6): 1441–1446.
Turnbull L, McCloskey DT, O’Connell TD, Simpson PC, and Baker AJ. 2003. A1-Adrenergic receptor responses in α1AB-AR knockout mouse hearts suggest the presence of α1D-AR. American Journal of Physiology—Heart and Circulatory Physiology, 284(4): H1104–H1109.
van der Heyden M, Wijnhoven T, and Opthof T. 2005. Molecular aspects of adrenergic modulation of cardiac L-type Ca channels. Cardiovascular Research, 65(1): 28–39.
Wagner J, and Brodde O-E. 1978. On the presence and distribution of α-adrenoceptors in the heart of various mammalian species. Naunyn-Schmiedeberg’s Archives of Pharmacology, 302(3): 239–254.
Waldeyer C, Fabritz L, Fortmueller L, Gerss J, Damke D, Blana A, et al. 2009. Regional, age-dependent, and genotype-dependent differences in ventricular action potential duration and activation time in 410 Langendorff-perfused mouse hearts. Basic Research in Cardiology, 104(5): 523–533.
Wang G-Y, McCloskey DT, Turcato S, Swigart PM, Simpson PC, and Baker AJ. 2006. Contrasting inotropic responses to alpha1-adrenergic receptor stimulation in left versus right ventricular myocardium. American Journal of Physiology—Heart and Circulatory Physiology, 291(4): H2013–H2017.
Wang L, Morotti S, Tapa S, Francis Stuart SD, Jiang Y, Wang Z, et al. 2019. Different paths, same destination: divergent action potential responses produce conserved cardiac fight-or-flight response in mouse and rabbit hearts. The Journal of Physiology, 597(15): 3867–3883.
Zhang S, Hiraoka M, and Hirano Y. 1998. Effects of α1-adrenergic stimulation on L-type Ca2+ current in rat ventricular myocytes. Journal of Molecular and Cellular Cardiology, 30(10): 1955–1965.
Zhang S, Lin J, Hirano Y, and Hiraoka M. 2005. Modulation of ICa-L by α1-adrenergic stimulation in rat ventricular myocytes. Canadian Journal of Physiology and Pharmacology, 83(11): 1015–1024.
Supplementary material
Supplementary Material 1 (PDF / 242 KB)
- Download
- 242.93 KB
Information & Authors
Information
Published In

FACETS
Volume 6 • Number 1 • January 2021
Pages: 823 - 836
Editor: Trevor A. Day
History
Received: 17 September 2020
Accepted: 21 January 2021
Version of record online: 20 May 2021
Copyright
© 2021 Joyce et al. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Data Availability Statement
All relevant data are within the paper and in the Supplementary Material.
Key Words
Sections
Subjects
Authors
Author Contributions
WJ and BJB conceived and designed the study.
WJ, KTS, and BJB performed the experiments/collected the data.
WJ and BJB analyzed and interpreted the data.
KTS, BJ, TW, and BJB contributed resources.
All drafted or revised the manuscript.
Competing Interests
The authors have declared that no competing interests exist.
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
William Joyce, Koen T. Scholman, Bjarke Jensen, Tobias Wang, and Bastiaan J. Boukens. 2021. α1-adrenergic stimulation increases ventricular action potential duration in the intact mouse heart. FACETS.
6(): 823-836. https://doi.org/10.1139/facets-2020-0081
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
Cited by
1. Incorrectly corrected? QT interval analysis in rats and mice
