Sediment PCBs
Grant et al. (2011) described the geographic distribution and characteristics of surface sediment PCBs in the SoG. They found total PCBs to be negatively correlated with sedimentation rate.
Johannesssen et al. (2008) have shown that PCB concentrations in sediment cores collected in the SoG showed a peak at depth and a decreasing trend toward the sediment surface, consistent with the history of release. Thus, PCB concentrations in the top 2 cm surface of the grab sediment samples in areas without any expected bottom disturbance and low bioturbation would be expected to resemble current seston concentrations of PCBs. In our study, extremely elevated PCBs were found in industrialized harbours, particularly Victoria Harbour (see also
Hickie et al. 2007;
Johannesssen et al. 2008;
Grant et al. 2011;
Morales-Caselles et al. 2017), with values exceeding Canadian Sediment Quality Guidelines (SQG: 21,500 pg·g
−1dw) by 6–55×. Extremely high PCB levels are also still evident in sediments from moderate–heavily industrialized areas in the southern Salish Sea (Puget Sound:
Cullon et al. 2012;
West et al. 2017). Monitoring by King County in Washington State (
King County 2014;
eopugetsound.org/magazine/IS/atmospheric_deposition) suggests that an urban plume of PCBs from old construction materials, paints, transformers, and fluorescent light fixtures that can volatilize into the air before attaching to surfaces or particles on or near the ground, covers Seattle and Lake Washington, as described for San Francisco, Chicago, Camden (New Jersey) and Toronto, Canada.
These results suggest ongoing input of PCBs to urban sediments from land-based sources.
Yang et al. (2012) suggested that the inadvertent introduction of PCBs into marine areas adjacent to industrial zones may be related to e-waste from recycling of electronics off the coast of China, which may have resulted in higher sediment levels after the year 2000. This may be relevant in the Pacific northwest as well. Although there are no landfill sources near Vancouver Harbour, the most extreme sediment PCB values in Victoria Harbour may be related to ongoing inputs from contaminated fill and historical industrial practices in the near- and fore-shore. Several recent water quality samples from Victoria Harbour (November 2018) identified total PCBs ranging from 2.5 to 20.8 ng/L which is 20–200× above the short-term acute water quality guidelines (BC water quality objectives -
www2.gov.bc.ca/gov/content/environment/air-land-water/water/water-quality/water-quality-objectives). There is thus a need to identify previously undocumented sources of PCB contamination entering marine waters around the Salish Sea.
PCBs are typically hydrophobic and tend to bind strongly to organic carbon and particulates (
Cullon et al. 2012). PCBs in sediments in the present study were most correlated with organic flux and content (AVS, %TOC, less so with organic carbon or OC flux), as found by
Grant et al. (2011). Despite this, PCBs were modestly elevated in sediments near outfalls, suggesting that municipal wastewater is a minor source (
Johannesssen et al. 2008). The higher organic content in sediments near outfalls tends to be from relatively “newly fixed” (low N values—see
Burd et al. 2013) and recently deposited organic material and therefore is likely to have lower levels of the legacy PCBs. However, the high organic content and input (and therefore high AVS) in the shallow Victoria Harbour (
UMA and Morrow 2007) adsorbs the above-described continuing PCB input from land-based sources. The PCBs are then incorporated into pelagic and benthic algal and bacterial production, and ultimately ends up in sediments. This highlights the contamination hazard of any shallow, organically enriched harbour with surrounding historical input sources of PCBs.
In the current study, sediment %fines did not correlate with sediment PCBs. This has been found in other marine studies (
Yang et al. 2002;
Grant et al. 2011;
Li et al. 2012;
Gao et al. 2013). The lack of correlation between sediment PCBs and %fines may be related to the fact that organic matter tends to be associated with specific sediment size fractions rather than the finest particles, which are often inert clay. For example,
Zhao et al. (2010) found that sediment PCBs and %TOC were not associated with %fines but rather with different specific particle size ranges in different hydrographic conditions.
Adeyinka and Moodley (2019) found that the sorption of PCB congeners onto particulates was dependent on the amount of organic matter, particle surface area, pH, and pore size distribution.
The source of organic matter may also influence PCB association with sediment grain size.
Kuzyk et al. 2010 found elevated ∑PCB concentrations and more highly chlorinated PCB signatures in surface sediments underlying eutrophic regions and lower PCB concentrations and weathered signatures in oligotrophic regions dominated by “old” marine organic matter in Hudson’s Bay, Canada. Similarly,
Grant et al. (2011) found that sediment surface PCBs were highest in “older” organic sediments in the SoG.
Staniszewska et al. (2011) suggested that black carbon seems to be more important for PCB absorption in sediments than %TOC, which is why charcoal is often used for remediation (for review of methods see
Gomes et al. 2013;
Galran et al. 2015). The structure of non-natural inorganic particles in sediments may affect PCB adsorption.
Parks et al (2014) indicated that nanotubules may be effective remediators as they adsorb well to PCBs, and also reduce bioavailability to benthic organisms. These studies highlight the importance of understanding not only organic content of sediments relative to PCB distributions, but also the type, source, and age of organic material (see also
Frouin et al. 2013).
In more remote areas far from source, there is rarely a close association between sediment PCBs and sediment organic matter or structure.
Ameur et al. (2011) found no significant correlation between total PCB concentrations and organic carbon content or sediment %fines (<40 um), suggesting that sediment properties did not play an important role in controlling the PCB levels in sediments off Tunisia.
Hong et al. (2012) studied the spatial distribution and potential source of PCBs in surface sediments from Bering Sea, Chukchi Sea, and Canada Basin. They found that sedimentary properties including grain size, water content, loss on ignition, TOC, and black carbon showed no apparent relationships with sediment PCBs. This indicated that the distribution of PCBs away from source is controlled mainly by atmospheric transport and deposition, sedimentation rates, mixing, partitioning, and sorption in the water column and sediments. This may have relevance in some of the background sample locations far from source in the current study.
Other important factors affecting sediment PCB levels include sediment resuspension due to tides, waves, dredging, anchor dragging, bottom trawling, or bioturbation.
Joseffsson et al. (2011) described a study in which the sediment-to-water flux of PCBs was inversely related to the burial depth (2–10 cm) and inversely related to the hydrophobicity of the congener. The flux was therefore most pronounced for less hydrophobic contaminants and was linked to the bio-irrigating behaviour of deep burrowing polychaetes. Contaminants previously considered buried at a “safe” depth can thereby be remobilized to surface sediments and (or) the overlying water column.
In the shallow Victoria Harbour, considerable sediment disturbance is caused by dredging, anchor chains, and prop wash, all of which can remobilize buried PCBs and other contaminants (
UMI and Morrow 2007;
Morales-Caselles et al. 2017) from remnant-contaminated sediment “pockets” (
Goossens and Zwolsman 1996;
National Research Council 2007). This is likely a factor influencing Victoria and Vancouver Harbour sediments. Monitoring studies suggest that recurrent natural disturbances such as tides and waves may cause the majority of contaminant release from sediments in shallower marine environments (
Roberts 2012). This may explain why persistent bioaccumulation of organic contaminants can occur despite dredging efforts targeted at removing contaminated sediments (
Voie et al. 2002), such as those in certain areas of Victoria Harbour.
Tissue PCBs relative to sediments
The sediment dry-weight PCB concentrations were considerably lower (>10×) than tissue concentrations across all taxa for background, harbours, and outfalls. Therefore, the sediment-dependent food chain in the Salish Sea still represents a significant and refractory repository of PCBs.
Desforges et al. (2014) indicated that phytoplankton levels of legacy PCBs were uniform across sites in southern BC, suggesting that biotic uptake has become spatially uniform in coastal BC due to recycling over time. In the present study, salmon, intertidal bivalves, scallops, and horse mussels had the lowest lipid-weight PCBs. Since these are pelagic feeders, it suggests lower availability of water column PCBs (particulate-bound) than sediment availability in the SoG. Trophic level bio-magnification of PCBs is likely, as a result of either the pelagic or benthic food chain transfer, since lipid-weight concentrations were highest in seal blubber, as well as in echinoderms and polychaetes from background areas. These organisms showed an average apparent bio-magnification relative to whole benthos at a rate of 2–3×. This magnification rate would be even higher for a pelagic food chain.
In this study, dry weight PCBs in sediment dwellers were poorly related to sediment PCB levels (
Fig. 3a and
3b,
Table 4;
Kuzyk et al. 2005;
deBruyn et al. 2009). The disconnect between dry-weight tissue and sediment PCBs suggests at first that fauna are no longer taking up PCBs from sediments but just recycling them within the food chain. However, the highest tissue PCBs (dry weight or lipid weight) were found in a few Victoria Harbour whole infaunal community samples, with values much higher than in local sediments, crab tissues, and
Mytilus. Since the infauna are typically annuals, this suggests that there is still considerable and rapid uptake and bio-concentration of PCBs directly into the food chain from these extremely contaminated, organically enriched, and chronically disturbed sediments. In addition, lipid-weight PCBs were more clearly related to sediment PCBs, reflecting the strong lipid-affinity of these chemicals. This also suggests that the long-term storage of PCBs is primarily associated with body fats. Most marine organisms lose considerable lipids during reproduction (typically annually). For example, the lipid-rich reproductive byproducts of benthic infauna and epifauna typically rise to the ocean surface, where they become part of the pelagic food chain. However, ultimately most of the PCBs would settle back to sediments since they are strongly bound to organic particulates. The only way to remove them from the food chain is to bury them with clean sediments too deeply for bioturbation or other disturbances to remobilize.
The multiple regression model illustrates that sediment PCBs are the primary predictor of tissue lipid PCB levels, but that this relationship is positively enhanced by increasing sediment %fines. Since sediment PCB levels were not as strongly related to %fines as tissue lipid levels were, this may suggest that areas with high fines are more susceptible to sediment resuspension (
Grant et al. 2011). Multi-species mesocosm experiments have shown that contaminants remobilised by burrowers and irrigators are bioavailable to co-occurring organisms, leading to greater body burdens of contaminants in nearby infaunal species (
Roberts 2012). Since elevated sediment %fines are an indicator of depositional environments, this results in higher tissue PCBs in general in the SoG relative to Juan de Fuca Strait (see
Burd et al. 2019).
The ratio of lipid/sediment PCBs (uptake rate) declined with increasing sediment PCBs and increasing AVS or reducing conditions in sediments (
Fig. 4). These patterns imply that in very high sediment concentrations, some of the PCBs are not bio-available, possibly because they are bound to nonlabile particles (such as black carbon or nano-plastics—see next section). PCB uptake rate is further diminished by reducing conditions in sediments, which is related to increased or pulsed organic input to sediments. Although the geochemical data collected in this study were insufficient to show how this uptake dynamic might work, pulsed or seasonal input of organics from algal blooms are particularly prevalent in the extremely high PCB content, organically enriched, shallow Victoria Harbour sediments, whereas organic input is more constant and different in source and composition near the primary or untreated wastewater outfalls. It is impossible from the current study to know how the seasonality and composition of such inputs could affect sediment organism uptake of contaminants, particularly in light of the changing metabolic requirements surrounding reproductive cycles. There are several possible explanations that can by hypothesized for uptake patterns, one of which is that
Matturro et al. (2016) point out that the only known way to dechlorinate PCBs in marine sediments to less toxic and bio-accumulative congenors is via anaerobic bacteria in reducing conditions (see also
Quensen et al. 1988;
Abramowicz et al. 1995) such as those found in Victoria Harbour and around the outfalls. Although the data were not collected to test this hypothesis, it is possible that the most bio-accumulative PCBs (penta, hexa, hepta), which dominated all taxa in the current study, are rapidly being broken down in anaerobic sediments with high PCB levels, making them less available for uptake by sediment-feeding fauna.
A further comparison was made between mixed tissue lipid uptake ratios and limited matched data available for infaunal production and biomass (from
Burd et al. 2012a,
2012b;
2013). This limited comparison is suggestive that increasing infaunal biomass turnover (ratio of production to biomass (P/B)) may reduce uptake rate of tissue PCBs in local taxa (
Fig. 6). This makes sense if we assume that high P/B implies that smaller, more rapid turnover fauna dominate benthos. These fauna have a short time to accumulate sediment contaminants before they become part of the food chain or are recycled in sediments. Rapid biomass turnover promotes a higher rate of bacterial breakdown of organic matter in sediments, which would contribute to more rapid metabolization and dechlorination of PCBs. This is most likely to occur in areas of high organic input (oxygen-reducing) or frequently disturbed sediments. Therefore, the concurrent patterns of increasing biomass turnover, sediment AVS and PCBs collectively result in reduced PCB uptake rates into benthos.
The filter-feeding
Mytilus tended to higher tissue PCBs similar to direct sediment feeders but higher than other filter-feeding bivalves.
Mytilus lives in high current, rocky areas likely to have considerable sediment resuspension, making PCBs more bio-available (
Roberts 2012). Sediment resuspension in intertidal rocky areas is likely to be intense due to wave and tidal action and can enhance the growth of water column bacteria and protozoa through release of nutrients. Mobilised organic contaminants may be accumulated by these microorganisms and subsequently taken up by nearby filter-feeding organisms (
Latimer et al. 1999;
Zarull et al. 1999;
Eggleton and Thomas 2004). Similarly, bioaccumulation studies by
Voie et al. (2002) showed that PCB concentrations in mussels and lipid-containing semipermeable membrane devices transplanted 1 m above a contaminated sediment site increased during remediation dredging and up to 6 months after dredging activities had ceased.
As the correlations, multiple regressions, and figures in this study suggest (see
Figs. 4,
6;
Tables 4 and
5), the relationship between sediment conditions and PCB uptake in sediment feeders is complex. Sediment homologue profiles are affected by source and recent input of historical deposits (for which we have no real data) and by sediment geochemistry. Uptake rate seems to be related to sediment homologue composition, resuspension, sediment structure (fines), long-term oxygen conditions (AVS), biotic community functioning (P/B ratios which reflect complex trophic and biomass interactions), and other possible unknown factors that are beyond the scope of this paper. With the few samples and limited goechemical and biophysical data available it is only possible to show uptake patterns and hypothesize about reasons for them.
Trophic accumulation
West et al. (2017) found that PCBs in Puget Sound (southern Salish Sea) herring and English sole declined after 1997 in undeveloped areas, but not in the moderate to heavily industrialized basins. Some PCB increases in English sole were noted after that date, and tissue levels were much higher than for conspecific sole from densely populated areas of the Baltic Sea. Notably, the southern SoG PCB (see stations on the US side near the Canadian/US border of the Salish Sea—
Fig. 1) values for English sole described in
West et al. (2017) were similar to that for a single specimen of Midshipman, as well as the English Sole from the current study. As in Puget Sound, values in the current study were much higher for whole bottom fish and muscle tissue near outfalls than in background areas. However, the salmon tissue levels in the current study were <1/5 those of pelagic fish measured in Puget Sound (
West et al. 2017). These results collectively suggest that source control of PCBs in Puget Sound has not been particularly effective, and levels of concern are still evident in Puget Sound’s pelagic prey base.
Hickie et al. (2007) predicted it would take 14–57 years for PCB concentrations to fall below an effects threshold of 17 mg total PCBs/kg blubber lipids (= 17000 pg/g lipid-weight) for southern resident killer whales in Puget Sound. Lipid-based PCB concentrations in all faunal tissues except intertidal bivalves in the current study were much higher than the effects threshold, so 50–60 years may be more realistic.
Lachmuth et al. (2010) did extensive model simulations with recommendations of a protective sediment PCB limit of 200 pg/g dry wt (about 1/4 mean background levels measured in the current study) and suggested that disposal of dredged material from highly contaminated harbour sediments will likely add to the body burden of southern resident killer whales in critical habitats. Current CCME marine sediment PELs are about 16,000 pg/g dry wt (CCME factsheet:
st-ts.ccme.ca/en/index.html?factsheet = 173), which is only exceeded in harbour and a few near-outfall sediment samples in the current study.
Congenor patterns
Highly chlorinated or hydrophobic PCBs tend to adsorb to suspended particulate matter and deposit close to the source (
Gao et al. 2013;
Hong et al. 2003). These tend to desorb from sediment particulates slowly, in the order of years (
Eggleton and Thomas 2004), and become more resistant to desorbtion over time (
Chen et al. 1999). The lighter PCB congeners (particularly di- and tri-CBs) disperse more readily through atmospheric and oceanographic processes (
Ross et al. 2004;
Grant et al. 2011). The fact that a lighter congenor mix doesn’t show up in the sediment homologue composition for Victoria Harbour samples suggests that there is a fairly constant supply of legacy PCBs with the classic (heavier) industrial configuration, entering the marine system. In summary, proximity to source and duration in sediments influences the PCB mixture to which marine fauna are exposed (
Hong et al. 2012).
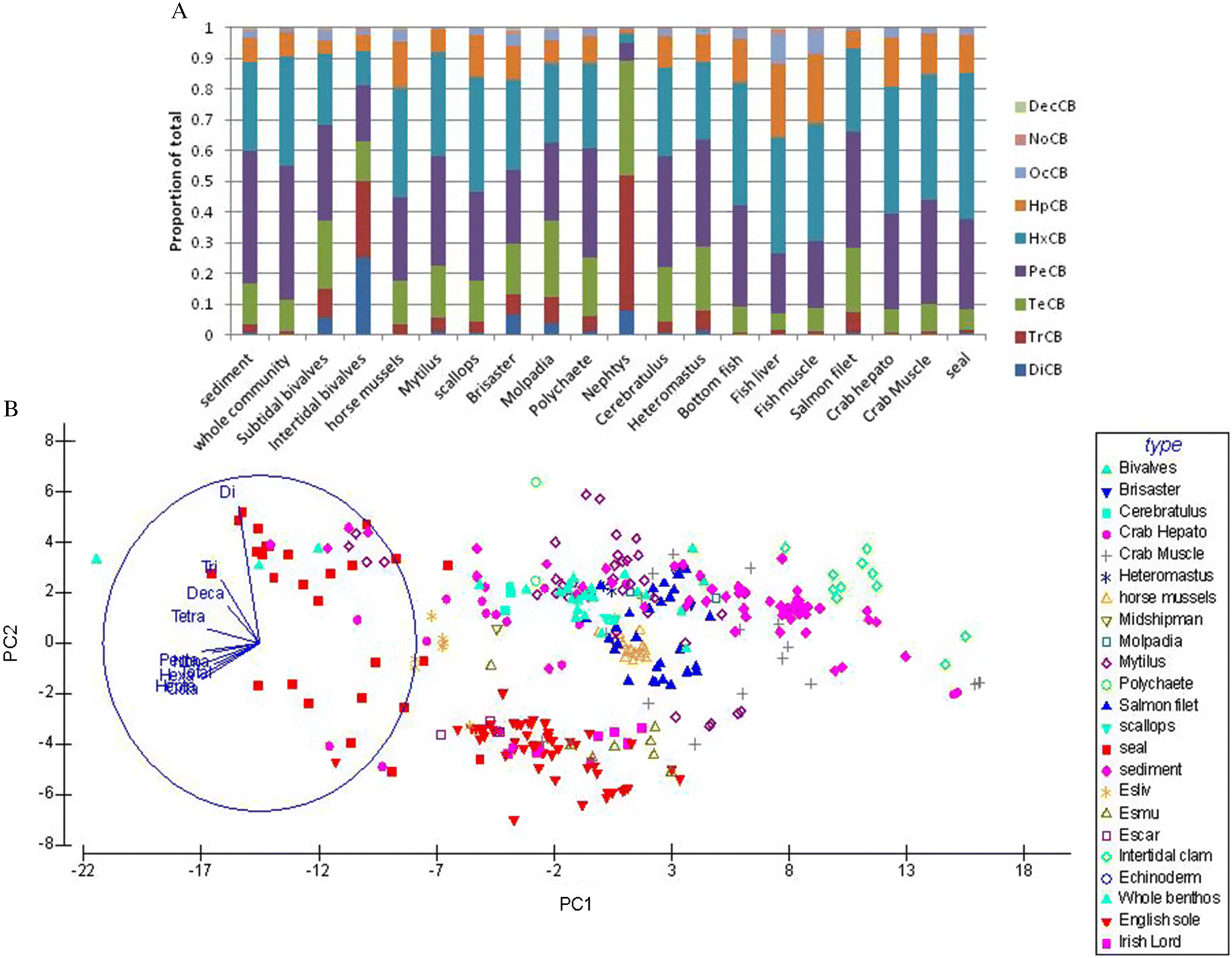
In the present study the congenor group patterns were very similar between all taxa and sediments (see also
Burd et al. 2014), with the exception of one
Nephtys/polychaete sample from near the Fraser River discharge. The lighter PCB homologues predominant in the remote intertidal filter-feeding bivalves are assumed to reflect the untransformed parent congenor patterns from sediments (
Porte and Albaiges 1993), which are likely less chlorinated far from source due to the long residence time of legacy PCBs with no recent input source (
Grant et al. 2011). Unfortunately, no matching sediment PCB data were available for these distant clam samples. In addition, these clams had the lowest tissue PCB levels of any samples in the study.
Abramowicz et al. (1995) suggested that the lower chlorinated PCBs may be less bio-accumulative.
The tetra-, penta- and hexa-CB congenor groups dominated all other samples (tissues and sediments), similar to the configuration reported elsewhere (
Porte and Albaiges 1993), where deposition of land-based PCBs is still proximate. This suggests considerable spatial sorting and distribution of PCBs over time throughout the BC coast, as well as remarkable stability in congenor composition in sediments and the sediment-based food chain. Homologue composition does not seem to shift notably at higher trophic levels, suggesting that very little PCB metabolization or selective uptake occurs at any trophic level.
The differences in historical usage of PBDEs and PCBs and their divergent distributions in marine sediments in the SoG are discussed in detail in
Johannesssen et al. (2008). Sediment PBDEs are still predominantly entering marine systems through wastewater outfalls and combined sewer overflows. In contrast, the legacy PCBs have dispersed much more broadly, but also appear to still be entering marine systems from historical sources in industrially developed harbours.
Figure 7 shows the matched total PCB and PBDE dry weight sediment and tissue values from this study and from
Burd et al. (2019). There is a reasonable log/log relationship (
R2 = 0.57,
n = 283;
p < 0.001), reflecting similar long-term dispersion patterns of these contaminants in southern BC. Despite the longer legacy period, PCB tissue levels were considerably higher (up to 100×) than PBDE levels in many samples. The ratio of PCBs/PBDEs was almost always >1 in harbours, lower near outfalls than in other areas, and mixed for background and near-river samples. Ratios in sediment, whole benthos and
Mytilus were highest overall in all background salmon and some crab muscle and hepatopancreas samples (>10), and always >1 in background scallops, seal blubber and bottom fish. PCBs therefore appear to be more persistent than PBDEs in higher trophic levels. The notably high ratios in salmon suggest a much stronger persistence of PCBs than PBDEs in the pelagic food chain. The opposite appears to be true for intertidal clams taken from remote areas. Reduced ratios near outfalls are in line with findings from previous research (
Johannesssen et al. 2008;
Burd et al. 2019), indicating that PBDEs are still being discharged from wastewater outfalls in the southern SoG, whereas historical sediment PCBs are being progressively diluted by wastewater outfall deposition.
Tissue PBDEs and PCBs both increased in a predictable way with sediment values, but otherwise responded differently to geochemical conditions in sediments (
Burd et al. 2019), reflecting similar particle affinities of both classes of contaminants but different source concentrations.
Whole benthos tissue PBDEs relative to sediment levels reflect more efficient contaminant uptake from ongoing, fresh organic input from urban waste, as well as rapid biomass turnover (
Burd et al. 2013). By contrast, the legacy PCBs are recycling through the ecosystem absorbed to existing, refractory or “old” carbon sources, with “new” supplies disconnected from fresh organic food resources, coming instead from fine, silty river runoff or stormwater runoff through industrial harbours.
The uptake rate (tissue dry wt/sediment dry wt) of total PCBs and PBDEs showed a similar range (about 0–100+ times). Tissue/sediment PBDEs and PCBs (rate of uptake) both declined with increasing sediment contaminant levels, suggesting that in extremely contaminated areas, not all sediment contaminants are bio-available. This could be due to adsorbtion of contaminants to black carbon or other refractory particles such as microplastics or engineered nanotubules (
Parks et al. 2014). In fact, these types of materials have been shown to significantly reduce bioaccumulation of PCBs in marine polychaetes (
Janssen et al. 2010;
Beckingham and Ghosh 2017) and are thus useful for remediation of contaminated marine sediments. With PBDEs, bio-dilution (ratio <1) occurred at extreme sediment PBDE concentrations (>10,000 pg/g dry wt) in urban harbours. PCBs did not show bio-dilution, with tissue levels always at least 10× higher than sediment levels. This further supports the finding described above that PCBs (particularly penta-, hexa- and hepta-) are much more bio-accumulative in marine tissues than PBDEs.
Uptake rate of PBDEs decreased with increasing %fines (
Burd et al. 2019). In contrast, uptake rate of PCBs declined relative to increasing sediment AVS, less so organic carbon, but not %fines. As discussed above, this is likely related to the more rapid dechlorination of heavier, more toxic, and strongly organic-bound PCB congenors, by anaerobic microbes in reducing sediments.
There is clearly a high initial accumulation of both PBDEs and PCBs from sediment to infaunal whole benthos, despite low tissue lipid content. A profound change to PBDE congener composition occurs at the point of sediment uptake (
Burd et al. 2019), a pattern seen in primary consumer zooplankton as well (
Frouin et al. 2013). However, tissue PCBs tend to be very similar in composition to surrounding sediments (
Kobayashi et al. 2010). Considerable accumulation of dry-weight PBDEs and PCBs both occurred in the higher trophic level organisms, but the lipid-normalized accumulation in these animals relative to direct sediment feeders was very low for PBDEs (ratio close to 1), suggesting no bio-magnification (rather lipid accumulation). In contrast, the PCBs showed modest bio-magnification relative to the background whole infauna in some larger benthic taxa (echinoderms, large polychaetes) and higher magnification in seal blubber (2-3×).
Accumulation patterns for both contaminant groups could be interpreted very differently depending on the relative importance of benthic versus pelagic food chains, since the pelagic feeders typically had the lowest tissue PCB and PBDE levels. Added to this complexity, understanding the PCB and PBDE trophic accumulation patterns will continue to be confounded by the difficulty in determining whole-body burdens and the lack of research on temporal accumulation in larger, long-lived organisms.