Patient engagement in the SPOR Evidence Alliance: Reflection and learnings
Abstract
The Evidence Alliance (EA) is a Canada-wide multi-stakeholder organization providing national-level support in knowledge synthesis, clinical practice guidelines development, and knowledge translation. With a mandate to deliver the best available evidence to inform health policy and improve patient care, the EA involves patients and their caregivers in its governance, research priority setting and conduct, and capacity building. To reflect on the experiences of patient involvement in its first three years, the organization conducted a self-study with 17 actively involved patient partners. They answered the Patient Engagement in Research Scale 22-item short form (PEIRS-22) and open-ended questions. Of the 15 respondents, 12 were women with a mean age of 62.6 years (SD 10.1). The mean PEIRS-22 score was 82.1 (SD 15.9), indicating perceived meaningful engagement. Analysis of the free-text answers identified three themes: (i) communication: successes, changes, and improvements; (ii) a respectful and welcoming environment; and (iii) opportunities to learn and contribute. Patient partners noted the EA made genuine efforts to welcome them and value their contributions. They also identified a need for the organization to increase patient partner diversity. This self-study was perceived as rewarding as it provided a foundation for further growth in patient involvement within the organization.
Graphical Abstract
Introduction
Underpinning patient engagement in health research is the motto “Nothing About Us Without Us” (Staley 2009; United Nations n.d.). Support for involving community members in health research has grown considerably over the last two decades, with funding agencies recommending patient–researcher partnership as a means to improve research quality (Canadian Institutes of Health Research 2014; Patient-Centered Outcomes Research Institute 2016; Michael Smith Foundation for Health Research 2014; Boote et al. 2002). Patient engagement in research is broadly understood to occur when people with lived experience are actively involved in governance, priority setting, conducting research, or knowledge translation activities (Leese et al. 2018; Hamilton et al. 2018a). Hamilton et al. (2018a) described meaningful patient engagement as a planned approach that involves patients and their caregivers in the research process, with these individuals perceiving it as a rewarding and productive experience. Here we use the term “patient partners” to describe patients, their caregivers, and the general public who engage in health research activities in partnership with researchers.
It is generally recognized that involving patients as partners can improve relevance of health research and facilitate dissemination of results (Canadian Institutes of Health Research 2014; Boote et al. 2002). The literature has credited patient involvement with illuminating new areas of inquiry, increasing study enrolment, improvements to study protocols, sharing ownership of the research process, and knowledge translation (Rhodes et al. 2002; Kirwan et al. 2005; de Wit et al. 2013; Holmes 2014; Leese et al. 2018; Salsberg et al. 2017; Forsythe et al. 2016). Although most reports focus on the benefits of patient involvement in research, some studies identified challenges. For example, a case study led by patient partners in a Patient-Centered Outcomes Research Institute (PCORI) funded project documented challenges for fully integrating patients into research teams (Robbins et al. 2016). In another qualitative study of 50 patient partners and research stakeholders, Hahn et al. (2016) outlined characteristics of tokenism (i.e., researchers making perfunctory or uninformed gestures towards engaging with patients) and ways to minimize it. A scoping review also highlighted the potential for power imbalance between patients and researchers, and issues around adequately valuing patient partners in the research process (Bird et al. 2020). These studies highlighted the importance of matching partners with the “right” projects to better assure a successful partnership. In a qualitative study involving 22 people with arthritis who had previous experiences as patient partners ranging from 1 month to 10 years, Leese et al. (2018) addressed ways of minimizing challenges in partnerships, including: (i) negotiating the value of different ways of knowing (e.g., lived experience vs. objective fact) and (ii) having an environment that supports the physical and emotional impacts (e.g., fatigue, stress, uncertainty) of engaging in research as a person living with chronic illness.
In summary, current evidence suggests that the role of a patient partner can be both rewarding and burdensome. Increased efforts are often needed to support patients as partners in health research. The purpose of this paper is to offer an example of a patient–researcher partnership in a national research alliance and our learnings from the past three years. First, we describe how patient partners have been involved in the governance, priority setting, and research teams within the SPOR (Strategy for Patient-Oriented Research) Evidence Alliance (SPOR Evidence Alliance n.d.-b). Next, results of a self-study on the perceived quality of involvement of patient partners are reported. The paper ends with reflections on opportunities to advance the practice of involving patient partners in research within the SPOR Evidence Alliance.
Patient involvement in the SPOR Evidence Alliance
Established in September 2017 and fully operational as of April 2018, the SPOR Evidence Alliance is a Canada-wide alliance of researchers, trainees, patients, health care providers and organizations who use health research to inform decisions. The Evidence Alliance provides national-level support in knowledge synthesis, clinical practice guideline development, knowledge translation, and patient-oriented research. With a mandate to deliver the best available evidence to inform health policy and improve patient care, the Evidence Alliance has created an environment where patients and their caregivers are actively involved in its governance, research priority setting and conduct, and capacity building (SPOR Evidence Alliance 2020).
The Evidence Alliance draws on the guiding principles of the SPOR Patient Engagement Framework of Inclusiveness, Support, Mutual Respect, and Co-build (Canadian Institutes of Health Research 2014). Several patient partners participated in co-creating the funding proposal and the patient partner engagement strategy (SPOR Evidence Alliance 2020) through an iterative process. Over the years, the Evidence Alliance has expanded their membership by actively recruiting new patient partners across Canada. The Evidence Alliance considers all individuals, who are interested in participating in the organization’s activities in the capacity of a patient, as patient partners. To date it has 28 patient partners; of those, 17 have participated in the Alliance’s operation. The remaining individuals are new members who will start participating in activities when they feel ready.
Inclusiveness
Since inception, the Evidence Alliance has been striving to include a diverse representation of patient partners based on gender, geographic location, and official language. Business is conducted through six standing committees (International Advisory Committee, Steering Committee, Executive Committee, Partnerships Committee, Knowledge Translation Committee, and Training Committee) (SPOR Evidence Alliance n.d.-a). To ensure inclusiveness, each committee has reserved seats for patient partners and community members to serve as either co-chairs or general members with voting privileges and decision-making power. Of the 71 seats across the six committees, 15 (21% representation) are reserved for patient partners as individual voting members. Terms of Reference documents have been prepared for each committee and reviewed by all committee members to clarify roles and expectations.
Support
Historically, patient involvement in research has been deemed as a volunteer activity, and patient partners are expected to participate with little or no compensation (Richards et al. 2018). This viewpoint is, however, shifting in recent years with increasing recognition of the value of and respect for the unique perspectives of patient partners (Richards et al. 2018; Ludwig et al. 2020). Patients and their caregivers often bring important insights into research through their experiences with health conditions, their interactions with health professionals, and the sometimes complex process of navigating the health care system. In addition, they contribute through their life experiences, including their education, their occupational knowledge and skills, and their cultural insights.
Recognizing the distinctive contributions of individuals’ lived experiences with their health and health care, the Evidence Alliance has created a mechanism to promote patient partner involvement (SPOR Evidence Alliance 2019). In the last three years, four workshops on patient-oriented research were offered and attended by 74 patients and 77 researchers. Patient partners and researchers developed and delivered these workshops together. New patient partners are also offered opportunities to be mentored by experienced partners and to work closely with researchers with expertise in patient-oriented research. Currently, the Evidence Alliance is co-developing with patient partners a rapid review training curriculum. The pilot program will be tested with 20 patient partners.
Mutual respect
Patient partners involved in the Evidence Alliance’s activities are offered cash or near-cash honoraria in appreciation and recognition of their time and contribution (SPOR Evidence Alliance 2019). While the compensation policy is in line with organizations such as the CIHR (Canadian Institutes of Health Research 2019), PCORI (PCORI 2015), and INVOLVE (INVOLVE 2011), we recognize that the amount may not fully reflect the time, expertise, and lived experience contributed by patient partners. Within the standing committees and project teams, patient partners share the responsibility with researchers to foster mutual respect among members by creating a safe meeting space allowing for honest and well-balanced interactions. Emphasis is placed on consensus-building to ensure patient partners and other stakeholders are fully engaged in the discussions and related decision-making. Furthermore, training resources were available to committees and project teams to promote diversity, equity, and cultural competency (Intersectionality & Knowledge Translation 2020).
Co-build
Research priorities of patients sometimes differ from those set by researchers, policy-makers, and health care providers (Tallon et al. 2000; Domecq et al. 2014). To ensure patient priorities are considered, patients are invited to use an online portal to submit health research topics important to them for systematic reviews and guideline development. These topics are then prioritized by a steering panel consisting of patient partners, researchers, and other stakeholders using a modified James Lind Alliance (JLA) approach to priority setting (James Lind Alliance 2021). The JLA approach brings patients, health care providers, researchers, and policy makers together in an equal priority-setting partnership to identify and prioritize unanswered questions or areas of evidence uncertainties that the group collectively agrees to be the most important. Up to three priority research topics are fully funded by the Evidence Alliance to be co-developed by the nominated research teams with interested patient partners. This prioritization exercise takes place once a year, and any topics not considered in the first year were included in the following year for prioritization.
In addition to governance and prioritizing queries, patient partners also participated in developing systematic reviews (or other types of knowledge synthesis, such as rapid reviews and scoping reviews), guidelines, and a variety of knowledge translation activities. Patients were involved as full-voting members in each review panel for the Evidence Alliance’s annual seed grant competition. In Year 1, four patients participated as reviewers and each reviewed one application. The process was enhanced in Year 2 with 10 patient partners involved. Each application was reviewed by two patient partners.
Patient involvement experiences: a self-study
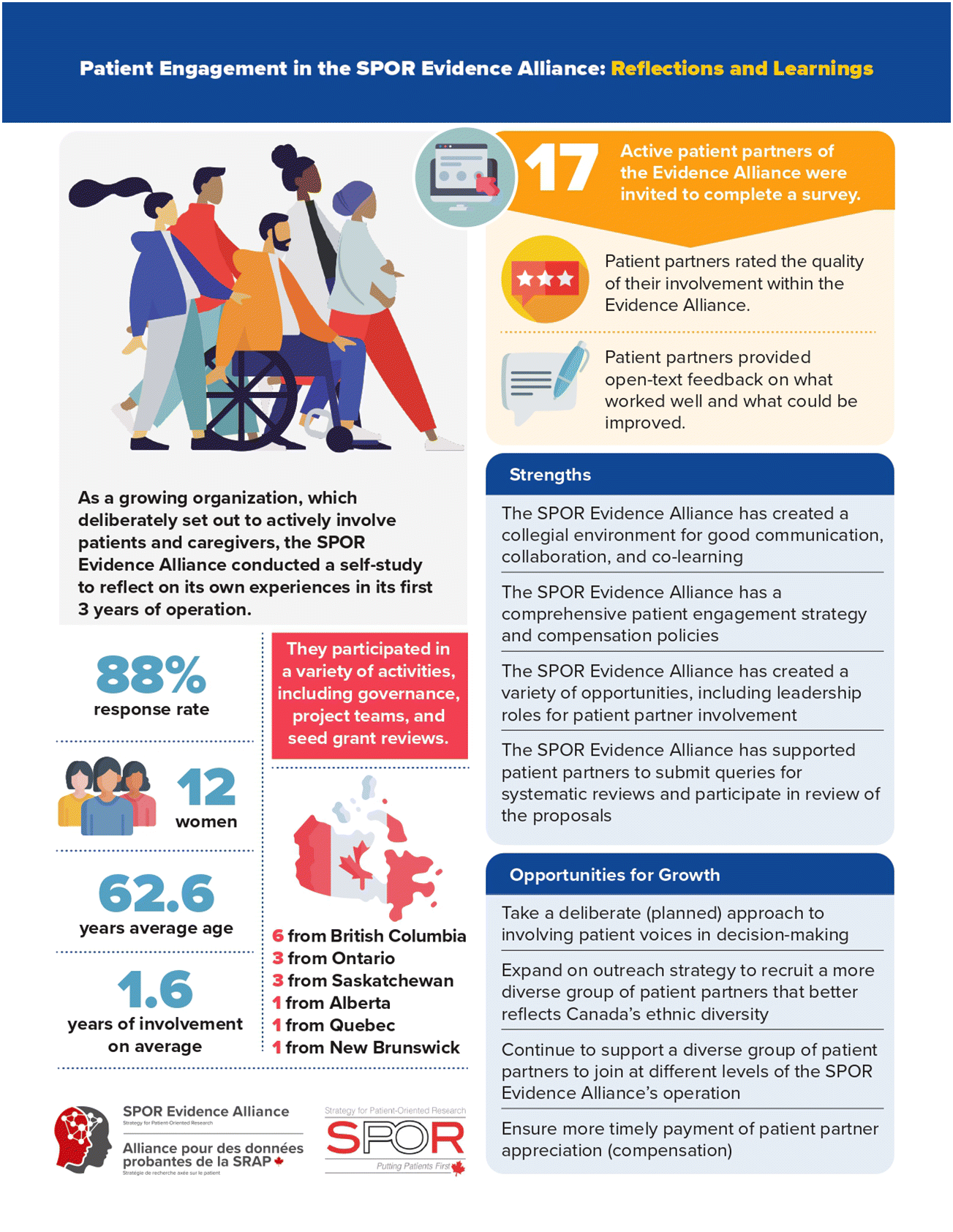
In 2020, the Evidence Alliance conducted a self-study on the experiences of this patient–researcher partnership from a patient partner perspective. An ad hoc team, consisting of five patient partners, three researchers, two trainees, and one research staff member from the Evidence Alliance developed the online questionnaire. A total of 17 patient partners, who had been actively involved in the Evidence Alliance’s activities, were invited to complete the survey.
Quality of partnership was assessed with the Patient Engagement In Research Scale 22-item short form (PEIRS-22) (Hamilton et al. 2021). The original PEIRS (37 items) was developed based on an empirical conceptual framework and a literature review (Hamilton et al. 2018c). Each item of the 22-item measure is rated on a 5-point Likert scale to evaluate meaningful engagement in research (internal consistency α = 0.96). PEIRS-22 showed acceptable floor and ceiling effects (<15%) and test–retest reliability (intraclass correlation = 0.86). The overall possible scores range from 0 to 100, with a score under 70.1 meaning a low-to-moderate level of meaningful engagement, and above 92 meaning extremely meaningful engagement from a patient perspective (Hamilton et al. 2021). In addition, the patient partners were asked to elaborate on their ratings on the PEIRS-22 and how their involvement had changed because of the COVID-19 pandemic.
To maintain confidentiality and reduce bias, patient partners’ PEIRS-22 scores and demographic information were summarized in descriptive statistics by a biostatistician who was not an Evidence Alliance member. For the free text data, a thematic analysis was conducted by the two PhD trainees who were not involved in the day-to-day operations of the Evidence Alliance (JL, TO). Both have been trained in qualitative research and analysis, and one trainee has been involved as a patient partner in a research setting in British Columbia prior to beginning her research training (TO). Initial open coding (i.e., assigning conceptual labels to content elicited during the sessions) was followed by clustering the labels into thematic categories. Each researcher read and coded the free text independently. The codes were then compared and discussed to achieve a broad initial coding scheme. Preliminary themes were presented and discussed with the patient partners in the self-study team to enhance rigour.
Self-study findings
Between October and December 2020, 15 (88%) patient partners completed the questionnaire (Table 1). The majority (n = 12; 80%) were women with a mean age of 62.6 years (SD = 10.1). Forty percent of patient partners were from the province of British Columbia, and 73% had a university degree or trades certificate. The mean duration engaging with the Evidence Alliance was 1.6 years (SD = 1.1). Patient partners participated in a variety of activities, including governance, project teams, and seed grant reviews (Table 2).
Table 1.
| Characteristic | Patient partners (n = 15) |
|---|---|
| Women, n (%) | 12 (80) |
| Age, year (SD) | 62.6 (10.1) |
| University degree or trades certificate, n (%) | 11 (73) |
| Role in the Evidence Alliance, n (%) | |
| Patient partner | 11 (73) |
| Family or caregiver partner | 2 (13) |
| Public member partner | 1 (7) |
| All of the above | 1 (7) |
| Duration of involving in the SPOR Evidence Alliance, year (SD) | 1.6 (1.1) |
| Province, n (%) | |
| British Columbia | 6 (40) |
| Alberta | 1 (7) |
| Saskatchewan | 3 (20) |
| Ontario | 3 (20) |
| Quebec | 1 (7) |
| New Brunswick | 1 (7) |
| Race/ethnicity, n (%) | |
| White | 12 (80) |
| Asian | 1 (7) |
| Prefer not to answer | 2 (13) |
| Number of comorbid conditions, median (25th, 75th percentile) | 4 (1, 4) |
| Patient Engagement in Research Scale -22-item, mean (SD) | 82.1 (15.9) |
Table 2.
| Patient partners (n = 15), n (%) | |
|---|---|
| Governance, e.g., committee co-chair or member | 8 (53) |
| Systematic review/research team | 4 (27) |
| Submitting a research query | 3 (20) |
| Prioritizing exercise for review queries | 1 (7) |
| Reviewing seed grants | 9 (60) |
| Newsletter submission | 5 (33) |
| Co-developing and reviewing policies and procedures | 3 (20) |
| Taking part in learning opportunities | 4 (27) |
| Other | 3 (20) |
*
Individuals might be involved in multiple activities.
The mean PEIRS-22 score was 82.1 (SD = 15.9). Our analysis of the free text identified three broad themes: (i) communication: successes, changes, and improvements; (ii) a respectful and welcoming environment; and (iii) opportunities to learn and contribute.
Communication: successes, changes, and improvements
Many patient partners highlighted that good communication helped their involvement as a partner in the Evidence Alliance. They shared examples of what good communication meant to them, including frequent email updates, the newsletter, quick responses to queries, and invitations to participate in projects, surveys, research query submissions, reviewing policies, and annual meetings/conferences. One patient partner appreciated that email communication came from one consistent source,
“It helps that the communications come from a consistent source. That makes it easy to find info from the past, and to organize it.”—Patient Partner (PP) 9
During the COVID-19 pandemic, 2 patient partners highlighted they had attended more virtual meetings. Their perspectives on these virtual meetings were varied. While one favoured meeting virtually because it eliminated challenges related to travel for “those of us living with disabilities”, another “missed the in-person meetings and the learning these entail”. Nine participants indicated their experience as a partner had not changed substantially because of the pandemic.
Some patient partners were pleased with their experiences and did not feel anything could be done to improve their involvement in the Evidence Alliance activities. One commented,
“I can’t think of anything. What can be better than handwritten notes of appreciation from our hardworking principal investigator!”—PP10
On the other hand, others highlighted that “more communication” would improve their experience as a partner. For example, improvements in communication could involve more interaction or “real life” participation among Evidence Alliance members, as one patient partner commented “I would like to know more about my partner members” (PP8). Another individual would have appreciated knowing the date of the final review of grant applications sooner, because it would have helped her to coordinate important priorities she was juggling in her daily life.
A respectful and welcoming environment
Patient partners commented how feeling welcome and confident that their contributions would be taken seriously was helpful for their involvement in the Evidence Alliance. One individual credited,
“Support from other [Evidence] Alliance members and being comfortable and confident enough to share my perspective without feeling that my thoughts would be minimized.”—PP5
Another appreciated the supportive environment created by the research leadership,
“I was amazed at how welcoming the principal investigators were when I first met them and how they took care to talk with everyone. This friendly, supportive approach has been echoed in all my dealings with staff, researchers, and other patient partners in the EA [Evidence Alliance]. It has enabled me to grow into the role of public partner, a role I knew nothing about at the beginning, and has helped me develop confidence I have something to contribute.”—PP10
Opportunities to learn and contribute
Many patient partners highlighted they had a positive learning experience in the Evidence Alliance. Two commented they appreciated their involvement as an experience “to learn and grow” in a new area “which typically does not involve patients and caregivers”. They also valued opportunities to contribute to the health care system in a way they felt was important and looked forward to continuing in this role.
To better support opportunities for partners to contribute to the Evidence Alliance, two participants highlighted that improvements could be made to foster a sense of collaborative decision-making within committees and project teams. One commented “that you are only there to legitimize management’s predetermined decisions”. Another suggested that training opportunities might be helpful to support patient partners to follow the “logic and language” of decision-making processes. One participant observed she had contributed to “a lot of administrative activities for a small amount of project work” (PP16).
Finally, to better recognize partners’ contributions, one individual highlighted that “more timely compensation disbursement” would improve the experience as a partner in the Alliance activities.
Reflection from the self-study
The SPOR Evidence Alliance was established with a goal to support and advance patient-oriented research. As such, it has invested heavily in supporting patient partner involvement in the research process since inception. This has led to the success of recruiting individuals from across Canada. This self-study reveals that our patient partners have been actively involved in a variety of roles and activities. On average, individuals felt that their engagement with the Alliance has been meaningful, as reflected in the mean PEIRS-22 score. The mean score was 82.1 (SD = 15.9), which is above the cut-point of low-to-moderate level of meaningful engagement (70.1) and below that of extremely meaningful engagement from a patient perspective (92). Findings from the free text analysis, further revealed strengths, as well as opportunities for improvement from the viewpoints of patient partners.
In general, the patient partners credited the Evidence Alliance with supporting their involvement in a variety of roles and activities. While there was agreement that the Evidence Alliance has created a collegial environment for collaboration and co-learning, some experienced situations whereby a more deliberate process could have been helpful to involve patient voices in decision-making. One approach to improve in this area could be for committee chairs and project leads to periodically check if the ground rules for decision-making are addressing the needs and expectations of patient partners. To this end, tools such as the Patient Engagement In Research Plan workbook (Hamilton et al. 2018b) could be useful to facilitate open conversations. For complex or potentially controversial decisions that require deeper discussions, consensus techniques such as deliberative dialogue may be considered for reaching a thoughtful decision (Acosta et al. 2017). Since this technique performs better when applied with an experienced facilitator, this might be an area for the Evidence Alliance to build training capacity.
A strength of the Evidence Alliance is the creation of a comprehensive patient engagement strategy (SPOR Evidence Alliance 2020) and compensation policies (SPOR Evidence Alliance 2019), acknowledging the unique and important contributions of patient partners. The self-study, however, revealed an opportunity to improve the speed for processing compensation for the partners. Fair and timely compensation is essential for showing respect to the patient partners and that their contributions are valued (Richards et al. 2018). While the processing time for compensation claims in academic and research institutions varies, a streamlined protocol will likely be helpful to ensure timely payments.
This self-study has also uncovered areas for further work to advance the practice of patient involvement in the Evidence Alliance. Among those who completed the survey, the majority self-identified as women, white, and having a university degree or training in a trade. These findings resonate with an observation that research teams tend to involve patient partners who are selected and in a position to contribute their perspectives (Leese et al. 2018). It has been suggested that sometimes the criteria used to identify patient partners might have contributed to the homogeneous characteristics in research groups (Canfield 2018). Ideas generated from these selected groups, however, may not represent those from larger target populations, when some members are not able to add their voices because of age, race/ethnicity, sex/gender, disability, occupation, education level, socioeconomic status, and where they live. To this end, we consider this an opportunity to expand on the Evidence Alliance’s patient partner outreach and recruitment strategy to include a more diverse group of partners to join at different levels of the Alliance’s operation.
The self-study has several limitations. First, since it was a co-learning opportunity for patient partners and researchers, the survey was designed for a quality improvement purpose rather than seeking answers that are generalizable to other research partnerships or settings. Second, the qualitative data were analysed by the research trainees who were not involved in the day-to-day activities of the Evidence Alliance. Specifically, this was highlighted by one patient partner during reviews of this paper that the free text data coding and thematic analysis was done by the trainees with patients involved only at the feedback level to ensure the findings resonate with their experiences, once the preliminary themes had been identified. While this process could result in missing some nuances in the responses, this was necessary for protecting the identity of patient partners who contributed their thoughts. We have balanced this approach by involving a research trainee with experience as a patient partner to add a critical lens to this analysis. Finally, this study centered only on the experiences of patient partners. Given that patient–researcher partnership is a complex and dynamic process, we recognize that further evaluation from the perspectives of researchers and other stakeholders will help to enrich our learning.
In conclusion, this self-study has offered a dedicated opportunity for the Evidence Alliance members to pause and listen to patient partners and identify areas for growth and further evaluation. Overall, our patient partners commanded about the respectful environment within the Evidence Alliance and identified opportunities for growth. The use of a standardized questionnaire on meaningful patient engagement with open-ended questions allowed us to gain a deeper understanding of our current practice, which was rewarding. We encourage research teams and networks to consider this approach when pursuing future self-studies of their own.
Acknowledgements
We thank all the patient partners who have contributed to the development, operation, and growth of the SPOR Evidence Alliance. We also thank Leo Lu for providing statistical support for the self-study.
References
Acosta A, Oelke N, and Lima M. 2017. Theoretical Considerations of Deliberative Dialogue: Contributions for Nursing Practice, Policy and Research. Texto & Contexto - Enfermeria, 26(4): e0520017.
Bird M, Ouellette C, Whitmore C, Li L, Nair K, McGillion MH, et al. 2020. Preparing for patient partnership: A scoping review of patient partner engagement and evaluation in research. Health Expectations, 23(3): 523–539.
Boote J, Telford R, and Cooper C. 2002. Consumer involvement in health research: A review and research agenda. Health Policy, 61(2): 213–236.
Canadian Institutes of Health Research. 2014. Strategy for Patient-Oriented Research - Patient Engagement framework [online]: Available from cihr-irsc.gc.ca/e/48413.html
Canadian Institutes of Health Research. 2019. Considerations when paying patient partners in research [online]: Available from cihr-irsc.gc.ca/e/51466.html.
Canfield C. 2018. The capacity for patient engagement: What patient experiences tell US about What’s ahead. Healthcare Quarterly, 21(Special Issue): 68–72.
de Wit M, Abma T, Koelewijn-Van Loon M, Collins S, and Kirwan J. 2013. Facilitating and inhibiting factors for long-term involvement of patients at outcome conferences—lessons learnt from a decade of collaboration in OMERACT: A qualitative study. BMJ Open, 3(8): e003311.
Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. 2014. Patient engagement in research: A systematic review. BMC Health Services Research, 14(1): 89.
Entwistle VA, Renfrew MJ, Yearley S, Forrester J, and Lamont T. 1998. Lay Perspectives: Advantages for Health Research. British Medical Journal, 316(7129): 463–466 [online]: Available from bmj.com/content/316/7129/463.
Forsythe LP, Ellis LE, Edmundson L, Sabharwal R, Rein A, Konopka K, et al. 2016. Patient and stakeholder engagement in the PCORI Pilot Projects: Description and lessons learned. Journal of General Internal Medicine, 31(1): 13–21.
Hahn DL, Hoffmann AE, Felzien M, LeMaster JW, Xu J, and Fagnan LJ. 2016. Tokenism in patient engagement. Family Practice, 34(3): 290–295.
Hamilton C, Hoens AM, Backman CL, McKinnon AM, McQuitty S, English K, et al. 2018a. An empirically based conceptual framework for fostering meaningful patient engagement in research. Health Expect, 21(1): 396–406.
Hamilton CB, Hoens AM, Backman CL, English K, McKinnon AM, McQuitty S, et al. 2018b. Workbook to Guide the Development of a Patient Engagement in Research (PEIR) Plan. Arthritis Research Canada. [online]: Available from: arthritisresearch.ca/wp-content/uploads/2018/06/PEIR-Plan-Guide.pdf.
Hamilton CB, Hoens AM, McKinnon AM, McQuitty S, English K, Hawke LD, et al. 2021. Shortening and validation of the Patient Engagement In Research Scale (PEIRS) for measuring meaningful patient and family caregiver engagement. Health Expectations, 00: 1–17.
Hamilton CB, Hoens AM, McQuitty S, McKinnon AM, English K, Backman CL, et al. 2018c. Development and pre-testing of the Patient Engagement In Research Scale (PEIRS) to assess the quality of engagement from a patient perspective. PLoS ONE, 13(11): e0206588.
Holmes BMC. 2014. How can research help us get it right? [online]: Available from msfhr.org/news/spark-bc-health-research-blog/patient-engagement-how-can-research-help-us-get-it-right.
Intersectionality & Knowledge Translation. 2020. Intersectionality guide. Knowledge translation program [online]: Available from knowledgetranslation.net/wp-content/uploads/2020/08/Intersectionality_KT_Guide_20200317_FD.pdf.
INVOLVE. 2011. What you need to know about payment: An introductory guide for members of the public who are considering active involvement in NHS, public health or soical care research. INVOLVE. 20 p.
James Lind Alliance. 2021. The James lind alliance Guidebook. National Institute for Health Research [online]: Available from jla.nihr.ac.uk/jla-guidebook/.
Kirwan JR, Hewlett SE, Heiberg T, Hughes RA, Carr M, Hehir M, et al. 2005. Incorporating the patient perspective into outcome assessment in rheumatoid arthritis–progress at OMERACT 7. The Journal of Rheumatology, 32(11): 2250–2256.
Leese J, Macdonald G, Kerr S, Gulka L, Hoens AM, Lum W, et al. 2018. ‘Adding another spinning plate to an already busy life’. Benefits and risks in patient partner–researcher relationships: A qualitative study of patient partners’ experiences in a Canadian health research setting. BMJ Open, 8(8): e022154.
Ludwig C, Graham ID, Gifford W, Lavoie J, and Stacey D. 2020. Partnering with frail or seriously ill patients in research: A systematic review. Research Involvement and Engagement, 6(1): 52.
Michael Smith Foundation for Health Research. 2014. Patient engagement: How can research help us get it right? [online]: Available from msfhr.org/1/news_article/patient-engagement-how-can-research-help-us-get-it-right.
Patient-Centered Outcomes Research Institute. 2016. Engagement rubric for applicants [online]: Available from pcori.org/sites/default/files/Engagement-Rubric.pdf.
PCORI. 2015. Financial compensation of patients, caregivers, and patient/caregiver organizations engaged in PCORI-funded research as engaged research partners. PCORI. 2 p.
Rhodes P, Nocon A, Booth M, Chowdrey MY, Fabian A, Lambert N, et al. 2002. A service users’ research advisory group from the perspectives of both service users and researchers. Health & Social Care in the Community, 10(5): 402–409.
Richards DP, Jordan I, Strain K, and Press Z. 2018. Patient partner compensation in research and health care: The patient perspective on why and how. Patient Experience Journal, 5(3): 2.
Robbins M, Tufte J, and Hsu C. 2016. Learning to “Swim” with the Experts: Experiences of Two Patient Co-Investigators for a Project Funded by the Patient-Centered Outcomes Research Institute. The Permanente Journal, 20(2): 85–88.
Salsberg J, Macridis S, Garcia Bengoechea E, Macaulay AC, Moore S, and Committee ObotKSTP. 2017. The shifting dynamics of social roles and project ownership over the lifecycle of a community-based participatory research project. Family Practice, 34(3): 305–312.
SPOR Evidence Alliance. 2019. Patient partner appreciation policy and protocol [online]: Available from sporevidencealliance.ca/wp-content/uploads/2019/08/SPOR-EA_Patient-Partner-Appreciation-Policy-and-Procedure.pdf.
SPOR Evidence Alliance. 2020. Patient Partner Engagement Strategy. SPOR Evidence Alliance.
SPOR Evidence Alliance. n.d.-a. Our governance [online]: Available from sporevidencealliance.ca/about/governance-structure/.
SPOR Evidence Alliance. n.d.-b. SPOR evidence alliance [online]: Available from sporevidencealliance.ca
Staley K. 2009. Exploring impact: Public involvement in NHS, public health and social care research 116 p.
Tallon D, Chard J, and Dieppe P. 2000. Exploring the priorities of patients with osteoarthritis of the knee. Arthritis Care Research, 13(5): 312–319.
United Nations. n.d. International day of disabled persons 2004: “Nothing about Us, without Us”. [online]: Available from un.org/development/desa/disabilities/international-day-of-persons-with-disabilities-3-december/international-day-of-disabled-persons-2004-nothing-about-us-without-us.html.
Information & Authors
Information
Published In

FACETS
Volume 7 • Number 1 • January 2022
Pages: 126 - 138
Editor: Debra Clendinneng
History
Received: 30 August 2021
Accepted: 9 November 2021
Version of record online: 3 February 2022
Notes
This paper is part of a collection titled “Strategy for Patient Oriented Research (SPOR) Evidence Alliance: A Canadian Model to Build Rapid-learning Health Systems”.
Copyright
© 2022 Li et al. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Data Availability Statement
The data and materials used and (or) analysed during this study are available from the corresponding author on reasonable request.
Key Words
Sections
Subjects
Plain Language Summary
What we have learnt from involving patients as partners in a health research network
Authors
Author Contributions
LCL, WZ, and ACT developed the initial outline and wrote the manuscript and WZ helped manage the Evidence Alliance and develop the operational procedures.
LCL, LW, WZ, and ACT obtained funding, conceptualized the research initiative, and provided guidance on the operation and direction of the initiative.
LCL, AMH, LW, VB, EP, AM, JL, TO, CBH, WZ, and ACT (including all five patient partners) contributed to the survey development.
JL and TO analysed the free text data and CBH analysed the PEIRS-22 data.
LCL, AMH, LW, VB, EP, AM, JL, TO, CBH, WZ, and ACT contributed to the interpretation and narrative of the paper, reviewed and revised the content, approved the final version, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work.
Competing Interests
LCL is Co-Principal Investigator and member of the Executive Committee for the Evidence Alliance (EA). AMH, VB, EPJ, and AM are Patient Partners for the EA. LW is Co-Principal Knowledge User and Patient Partner for the EA. JL is Executive Committee Trainee Member for the EA. TO has no competing interests. CBH is Co-Investigator for the EA. WZ is Research Manager of the EA. ACT is Nominated Principal Investigator and Chair of the Executive Committee for the EA.
Funding Information
This self-study was conducted through the Strategy for Patient-Oriented Research Evidence Alliance (SPOR EA), which is supported by the Canadian Institutes of Health Research (CIHR) under the Strategy for Patient-Oriented Research (SPOR) initiative (grant number GSR-154442), and the generosity of partners from 41 public and not-for-profit sectors across Canada. JL and TO received trainee stipends from this Evidence Alliance self-study. LCL is funded by a Tier 2 Canada Research Chair in Patient-oriented Knowledge Translation and holds the Harold Robinson/Arthritis Society Chair in Arthritic Diseases. ACT is funded by a Tier 2 Canada Research Chair in Knowledge Synthesis.
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Linda C. Li, Alison M. Hoens, Linda Wilhelm, Vikram Bubber, Elliot PausJenssen, Annette McKinnon, Jenny Leese, Thalia Otamendi, Clayon B. Hamilton, Wasifa Zarin, Andrea C. Tricco, and for the SPOR Evidence Alliance. 2022. Patient engagement in the SPOR Evidence Alliance: Reflection and learnings. FACETS.
7(): 126-138. https://doi.org/10.1139/facets-2021-0133
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
Cited by
1. Introducing the Strategy for Patient Oriented Research (SPOR) Evidence Alliance: a partnership between researchers, patients and health system decision-makers to support rapid-learning and responsive health systems in Canada and beyond
2. A Canadian model for providing high-quality, timely and relevant evidence to meet health system decision-maker needs: the SPOR Evidence Alliance
