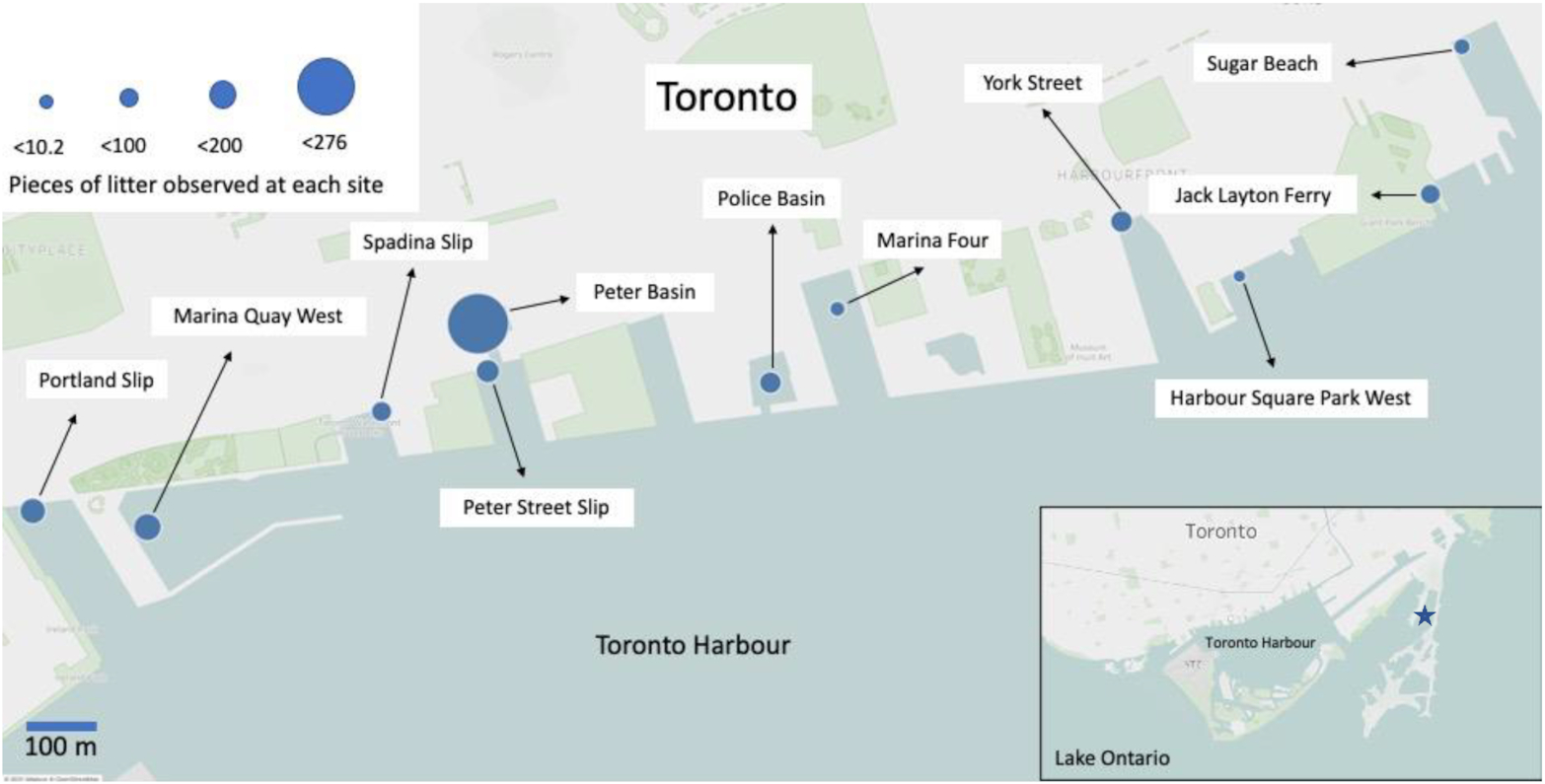
Three Seabins were installed in the Outer Harbour Marina in Toronto in 2020 (43°38′34.6″N, 79°19′30.8″W) (
Fig. 1). The location of these Seabins were chosen as a pilot to develop the methodology described below and to gauge the amount of maintenance needed to run a Seabin prior to choosing locations across the Toronto Waterfront. Two different methodologies were developed to quantify and characterize litter trapped by TCDs, using Seabins as a case study. The first is a “simple waste characterization,” intended to collect daily data in a simple and accessible manner that can be carried out by anyone maintaining the TCD. The second is a “detailed waste characterization” to collect data 5–10 times per year (aiming to capture variability by spreading out the data collection throughout each TCD season or year), where the content of each Seabin is meticulously sorted and characterized by material type. A season can be defined as that that the TCD is deployed annually. In Toronto, our TCD season typically occurs between May and October, which coincides with the ice-free season. Combined, these two methodologies provide a quantitative measure of the weight and count of anthropogenic debris diverted and information regarding what is collected to inform source-reduction upstream.
Simple waste characterization
As regular maintenance and emptying will likely be carried out by a marina operator or municipal staff, the simple method was designed to be fast and accessible while accurately measuring the quantity of anthropogenic litter diverted from the local aquatic environment.
This protocol provides a direct measure of the mass of anthropogenic and organic material collected. To facilitate data collection, we designed a free application:
Data Trapper. The Data Trapper app is available for iOS and Android devices (links can be found in Supplementary material). It allows data to be collected on a local and global scale and is currently being used for several TCDs across North America. Metadata collected for each TCD include the type of TCD, location, weather conditions, date, time, amount of time the TCD was deployed, and whether a wet event occurred since the TCD was last emptied. The following example is for a Seabin, generally emptied daily, but has also been tested on a few other types of TCDs. After metadata are recorded, the catch bag of the Seabin is removed, the full bin is weighed using a luggage scale, and a picture of its contents in the catch bag is taken. The image is uploaded into the app, and the user provides the weight of the full catch bag and a qualitative estimate of how full the catch bag is. As we know the mass of an empty catch bag, we can calculate the mass of the diverted debris alone (see a video tutorial of the procedure in the Supplementary material). The catch bag is placed back into the Seabin, and the protocol will be repeated every 24 h. This process takes roughly 5 min per bin. If needed, paper datasheets are also available for download from our website with the full protocol (
https://oceanconservancy.org/trash-free-seas/international-coastal-cleanup/trash-trap-network/; link is also given in Supplementary material).
This protocol was designed to create a harmonized procedure to characterize and quantify anthropogenic debris collected in TCDs across space and time. Until our app is updated, data for this protocol are to be collected on a datasheet that can be found through the link below or in the Supplementary material.
Detailed waste characterization
During each detailed characterization, basic metadata are collected: type of TCD, location, weather conditions, date, time, amount of time the TCD was deployed, and whether a wet event (defined as precipitation over 10 mm) occurred since the TCD was last emptied. Like the simple protocol, the full catch bag is weighed using a luggage scale. The wet weight is recorded along with the qualitative measure of how full the catch bag is, meaning whether the catch bag is empty, quarter full, half full, or full. The contents of the bag are then placed on a tarp to be quantified and characterized.
Once placed on the tarp, the large anthropogenic debris (defined as >3 cm) is sorted first. To begin, any large anthropogenic debris is first separated from the organic material (e.g., macrophytes, woody debris). Next, each piece of large anthropogenic debris extracted is tallied and characterized to product type (e.g., plastic bag, cigarette butt, plastic wrapper). The large material is then weighed using a small battery-powered kitchen scale.
In addition to the large debris, we characterize the small anthropogenic debris (defined as anything >2 mm and less than 3 cm). In our study, much of the small anthropogenic debris is entangled in organic material. As such, we first need to extract small particles from the organic material. For this, two handfuls of plant material are placed into a 5gal bucket and rinsed with a hose at high pressure until the bucket is ¾ full. The bucket is then left to sit for roughly one minute to allow the small anthropogenic pieces to resurface. If large pieces of debris appear at the surface, they are removed and added to the tally of large anthropogenic pieces extracted previously. Next, a circular sieve that has a mesh size of ½in. is placed into the bucket and pushed down towards the bottom to separate the organic material from the small floating anthropogenic pieces. We call this the “French press technique.” While holding the sieve in the bucket, the contents of the bucket are poured into a 2 mm sieve. The extracted material consists of the small anthropogenic debris with some small pieces of organic material. This extraction step is then repeated twice, and the floating material is combined on the 2 mm sieve. This entire process is then repeated for the remaining plant material on the tarp. The organic material that remains at the bottom of the bucket after having gone through the French press technique should be disposed properly. In Toronto, we put this material in the garbage bin because it still contains some plastic debris and mostly comprised invasive plant species.
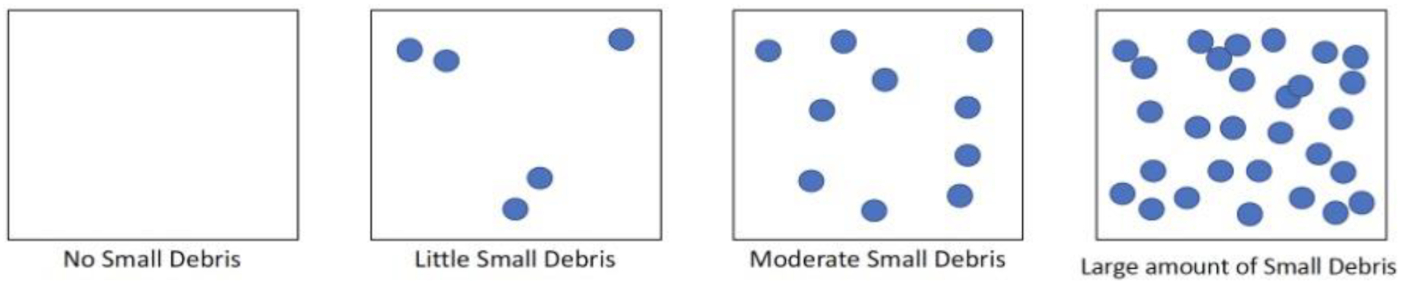
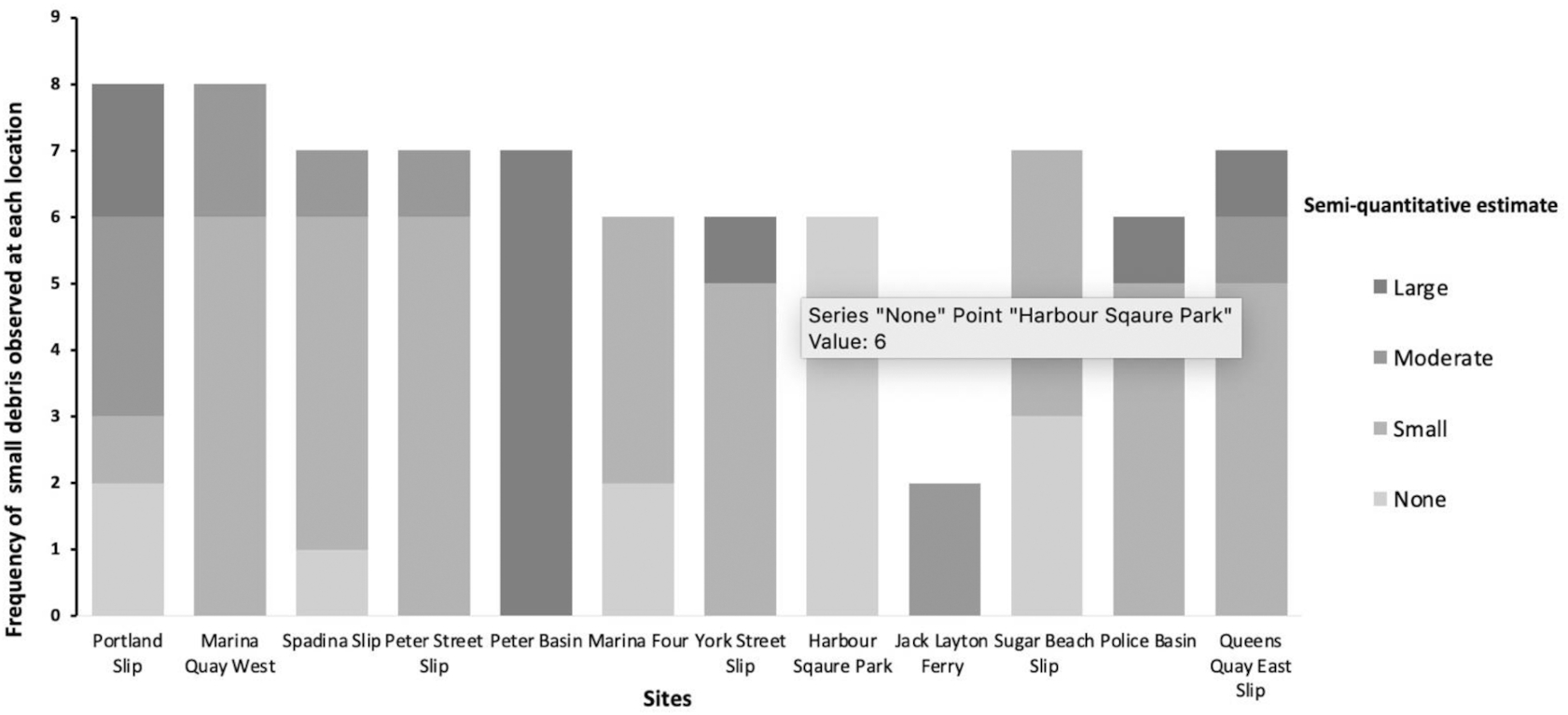
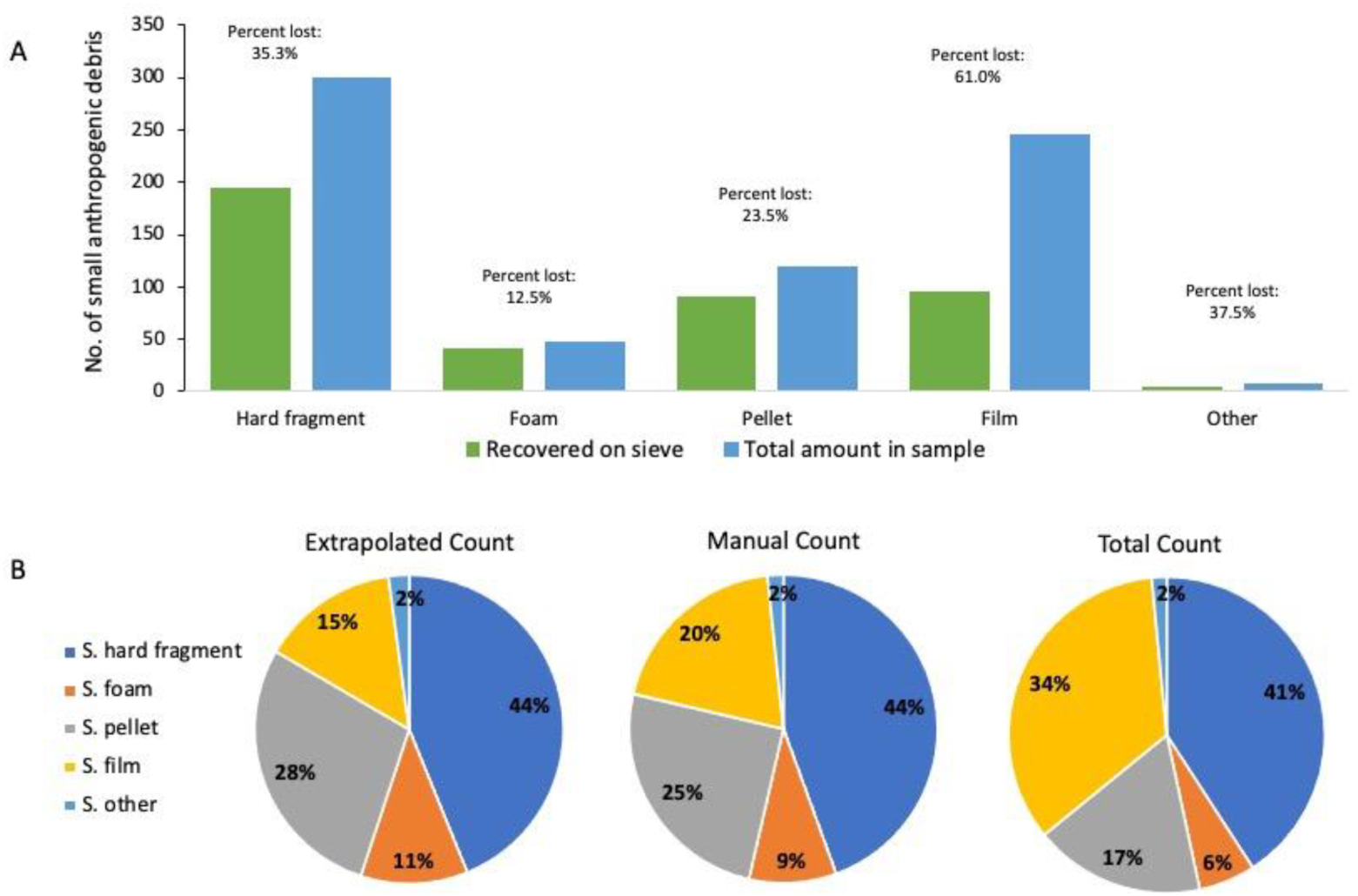
Next, the contents on the 2 mm sieve are examined. If there are less than 50–100 small pieces, then everything present on the sieve is quantified, weighed, and characterized to type (e.g., hard fragment, foam, pellets, film, and other; Table S1). If there appears to be more than 50–100 pieces of small anthropogenic debris present, the contents can be subsampled. For subsampling, all the debris on the sieve is scraped into one pile and then further split into four equal piles. Then, one pile is randomly picked for quantification and characterization. The counts and weight from this pile are then multiplied by four to estimate the small debris across the whole sample. The accuracy of the subsampling method was measured and is described below. The full protocol and datasheets are available on our website (
https://oceanconservancy.org/trash-free-seas/international-coastal-cleanup/trash-trap-network/), and a video tutorial of how to carry out the protocol can be found in the Supplementary material and on our website.
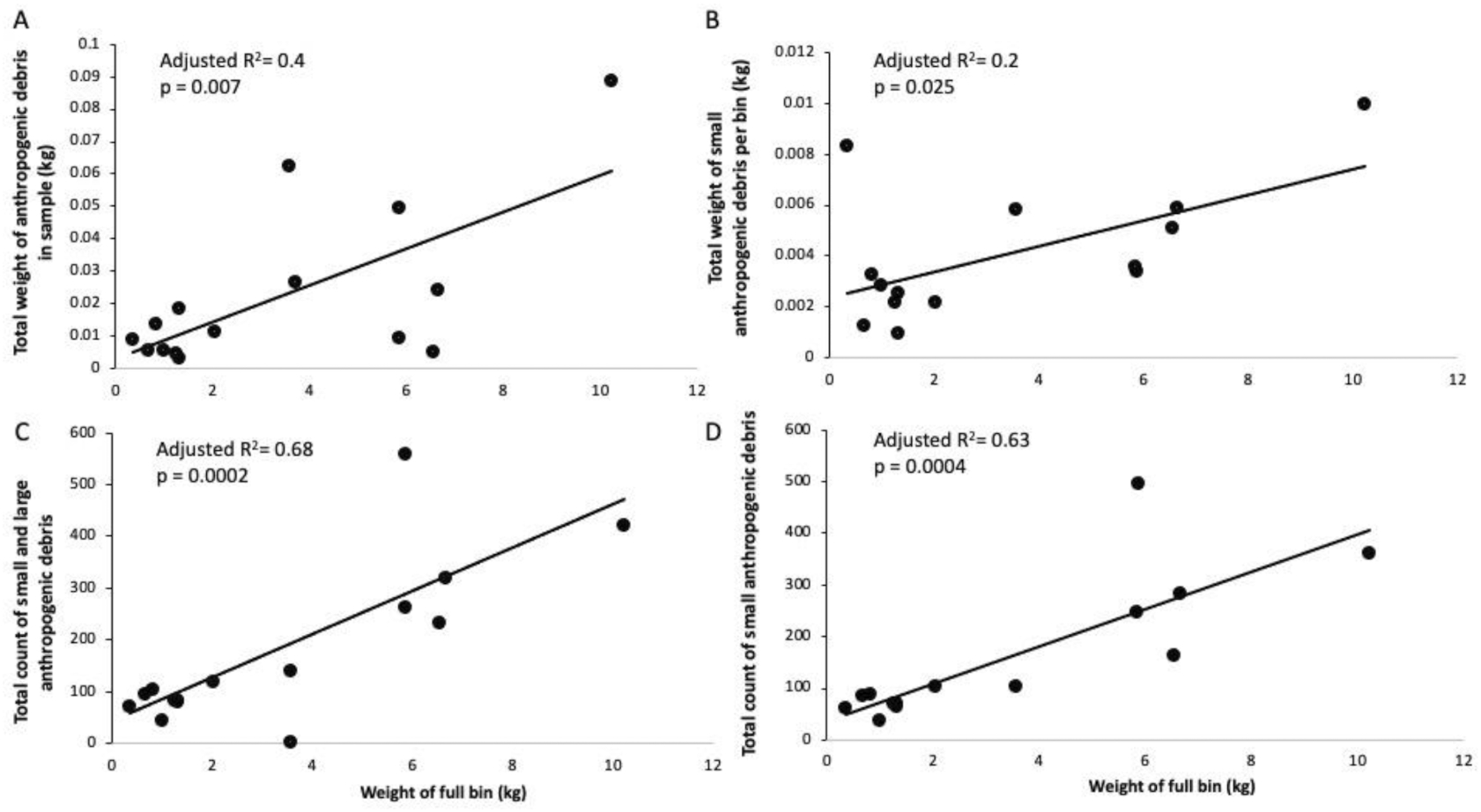
To test these methods and validate it on the field, we quantified and categorized debris from three individual Seabins from July to October 2020. In total, we characterized 27 samples, which equate to 10 from Seabin 1, 10 from Seabin 2, and 7 from Seabin 3.