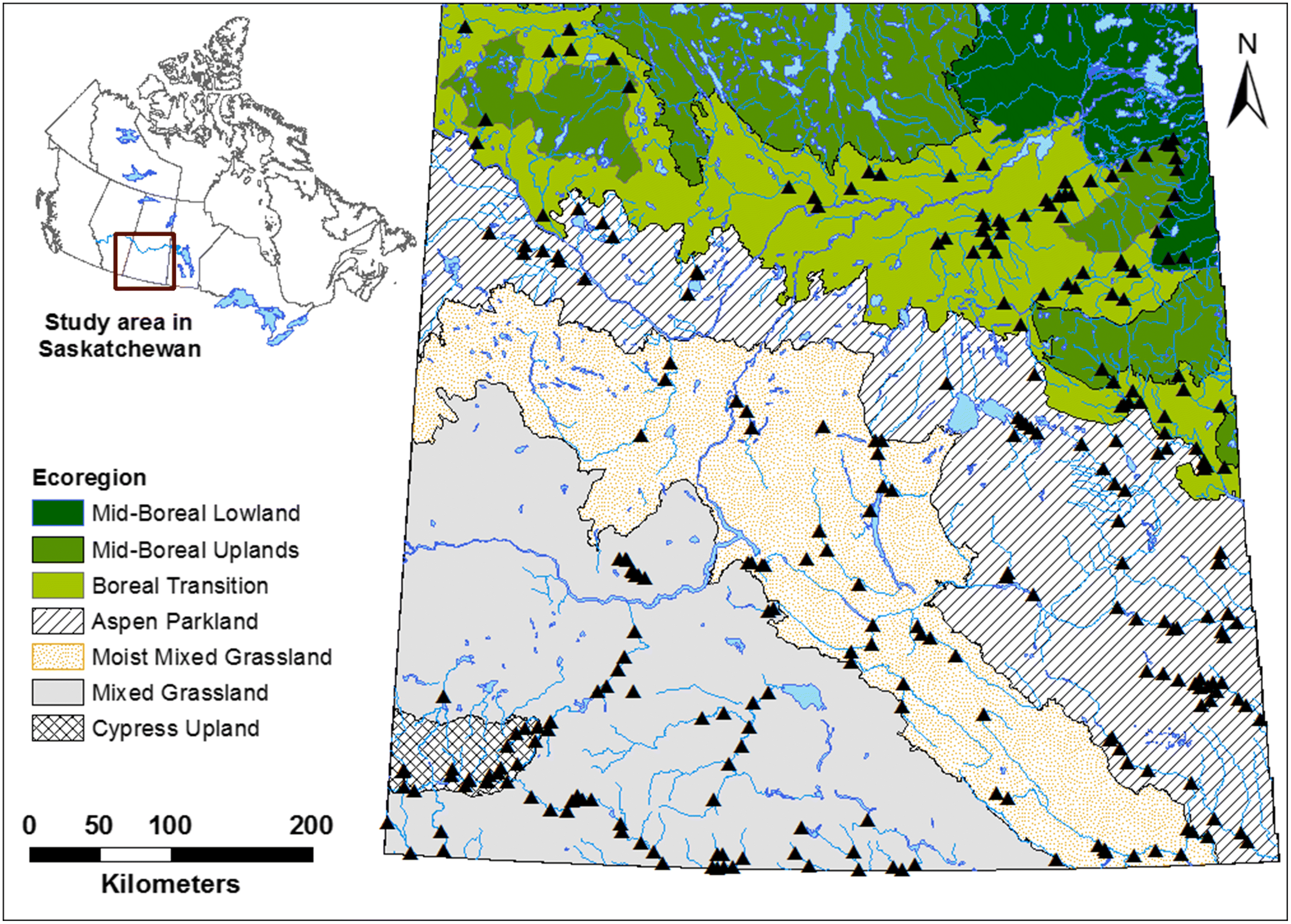
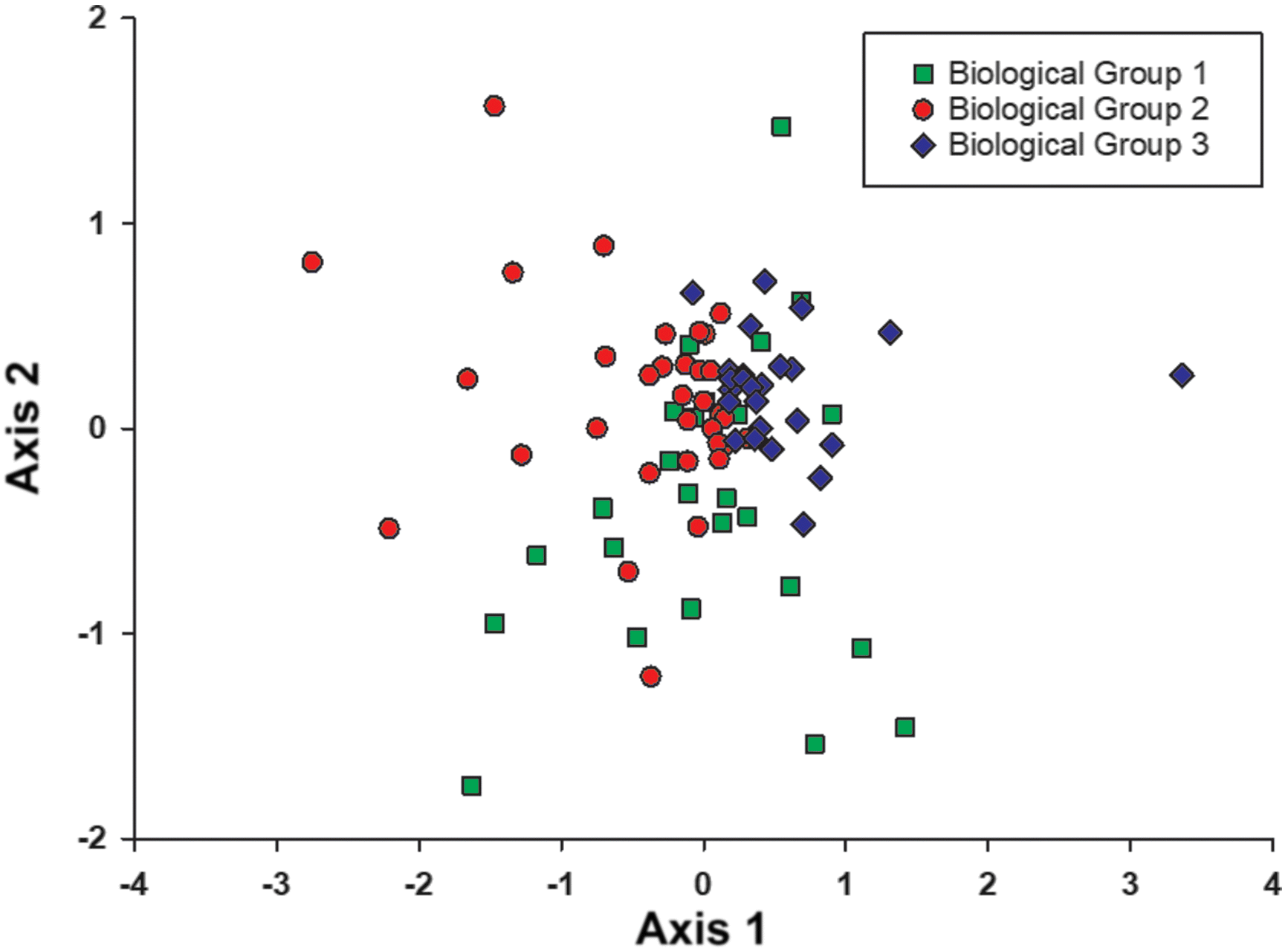
Metric sensitivity and evaluation of test site condition
In applying TSA to the 8 sites with known human activity, we found that two sites with upstream reservoirs in Biological Group 1 demonstrated moderately lower (possibly impaired) total abundance and number of species relative to reference (
Table 8); specifically, the site on the Avonlea Creek downstream of the Roleau Dam had a total abundance
p = 0.17, and number of species
p = 0.81, while the site downstream of the Rafferty Dam on the Souris River had a total abundance
p = 0.03 and number of species
p = 0.99. Further, the community of benthic macroinvertebrates (based on NMDS Axis 1) was possibly impaired relative to reference downstream of the Rafferty Dam on the Souris River (
p = 0.13), but not downstream of the smaller reservoir on Avonlea Creek (
p = 1.00,
Table 8). The third test site compared using TSA in Biological Group 1 had the distinct characteristic of its contributing watershed being completely dominated by cultivated cropland (
Table 8), and although the total abundance was possibly impaired (
p = 0.59), the other metrics are within reference (
Table 8). In this unique situation, all other stressors we measured are absent and we measured no change in the community (
p = 1.00,
Table 8).
Furthermore, there was no relationship between % cropland cover and any of the metrics applied in our stressor gradient analysis for Biological Group 1 (
Table 9). The metric % detritivores had a weak negative relationship with the number of landfill/lagoon/road crossings in the contributing watershed (
R2 = 0.18,
p < 0.01;
Table 9). Although the metric %
Hyalella azteca was not included in the four metrics selected for this biological group (as it was correlated with total abundance in Biological Group 1 reference sites), it was also positively correlated with landfill/lagoon/road crossings (
R2 = 0.24,
p < 0.001;
Table 9). Percent EPT was also weakly correlated with forest cover in Group 1 (
R2 = 0.10,
p < 0.01;
Table 9).
In Biological Group 2, TSA provided that the Wascana River downstream of the Regina Waste Water Treatment Plant (WWTP) had a significantly lower Log
(n+1) number of Coleoptera and significantly different NMDS Axis 1 relative to reference sites (
p = 0.01 and
p < 0.001, respectively;
Table 10). The Moose Jaw River site possessing high levels of PAH contamination demonstrated significantly lower Log
(n+1) number of Coleoptera (
p = 0.01) and potentially stressed NMDS Axis 1 metric (
p = 0.51;
Table 10). The Frenchman River near Eastend, downstream of a reservoir, and the Wascana River in Regina downstream of high urban cover but upstream of the WWTP both had ecosystem health metrics not significantly different from reference sites (
Table 10).
The gradient analysis for Biological Group 2 indicated that %
Hyalella azteca had a weak relationship with the number of landfill/lagoons/road crossings (
R2 = 0.18,
p < 0.01) and % cropland upstream of sites (
R2 = 0.11,
p < 0.01;
Table 9). Total abundance had a stronger positive relationship with the amount of uncultivated land upstream of sites (
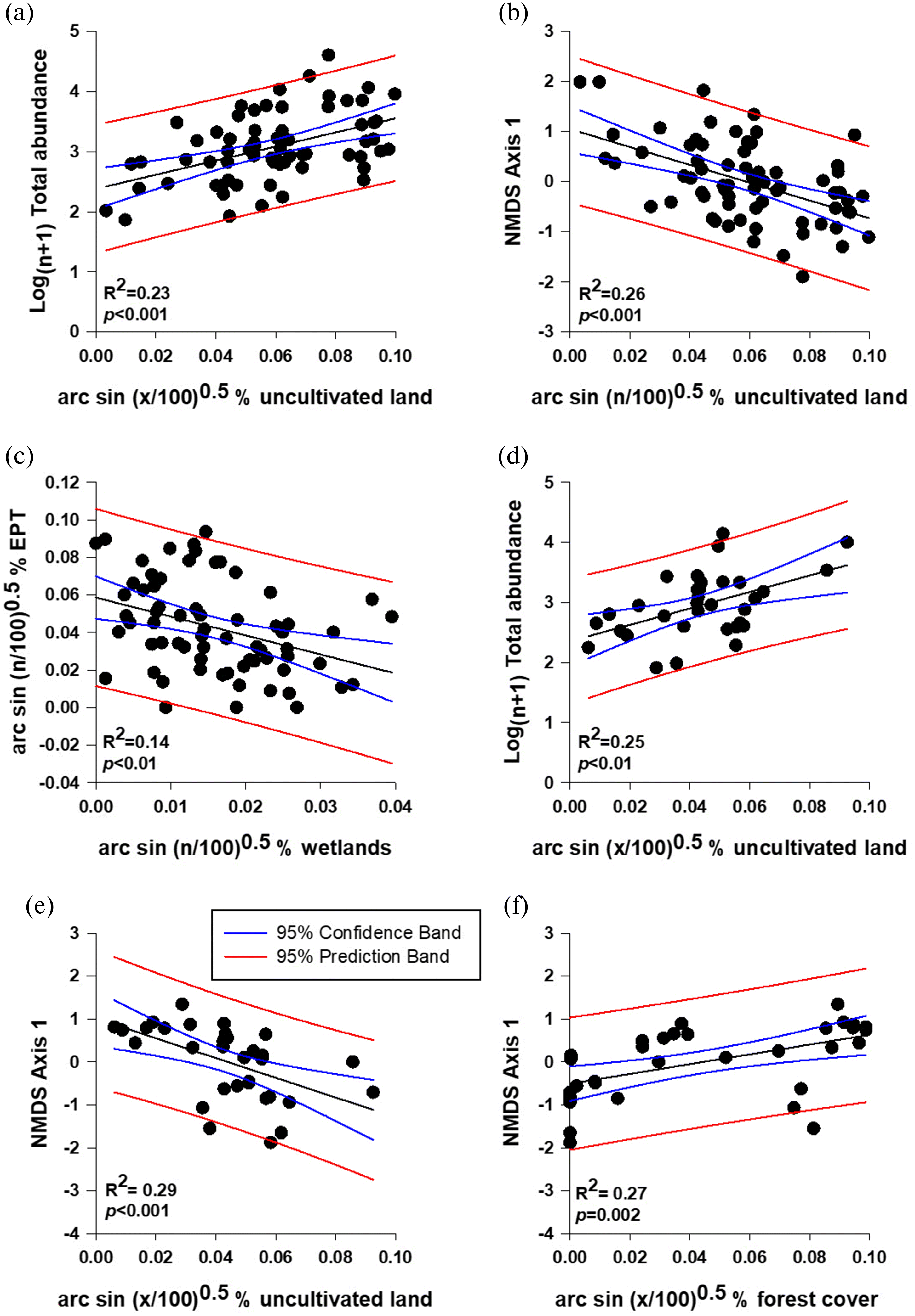
Fig. 5a,
Table 9). NMDS Axis 1 however, demonstrated negative relationship with % uncultivated (
R2 = 0.26,
p < 0.001;
Fig. 5b) and % wetlands (
R2 = 0.08,
p < 0.05), and positive relationships with % forest (
R2 = 0.12,
p < 0.05;
Table 9). The alternative metrics for Biological Group 2 showed that % shredders had a negative relationship with the % uncultivated land in the upstream watershed (
R2 = 0.15,
p < 0.001), while % detritivores had a negative relationship with % forest cover in the upstream watershed (
R2 = 0.06,
p < 0.05;
Table 9). Finally, % EPT demonstrated a weak negative relationship with the number of landfill/lagoons/road crossings (
R2 = 0.10,
p < 0.01), % uncultivated (
R2 = 0.15,
p < 0.001) and % wetlands (
R2 = 0.14,
p < 0.01;
Fig. 5c), and a positive relationship with the % forest in the upstream watershed (
R2 = 0.17,
p < 0.001;
Table 9).
In summary, linear regression showed that for Group 2 the % uncultivated land in the contributing watershed had a significant relationship with total abundance (R
2 = 0.23,
p < 0.001;
Fig. 5a) and community structure (NMDS Axis 1;
Fig. 5b; R
2 = 0.26,
p < 0.001), while there was a significant negative relationship between % EPT and the number of wetlands upstream of a site (R
2 = 0.14,
p < 0.01;
Fig. 5c).
The benthic macroinvertebrate metrics of the Whitesand River site downstream of Theodore Reservoir in Biological Group 3 were not significantly different from reference sites (
Table 11). However, the gradient analysis in Biological Group 3 showed that % EPT is weakly positively related to the % forest upstream of sites (
R2 = 0.14,
p < 0.05;
Table 9). The % shredders and % detritivores had no significant relationship with any of the abiotic variables analyzed here (
Table 9). Total abundance was found to be positively related to the number of landfill/lagoons/road crossings (
R2 = 0.12,
p < 0.05), % uncultivated (
R2 = 0.25,
p < 0.01;
Fig. 5d), and % forest (
R2 = 0.16,
p < 0.01;
Table 9); however, Log
(n+1) number of Coleoptera demonstrated the opposite response to % EPT, having a significant positive relationship with % uncultivated (
R2 = 0.25,
p < 0.01) and negative relationship with % forest in the upstream watershed (
R2 = 0.19,
p < 0.01;
Table 9). NMDS Axis 1 had a negative relationship with % uncultivated (
R2 = 0.29,
p < 0.001;
Fig. 5e) and positive relationship with % forest in the upstream watershed (
R2 = 0.27,
p = 0.002;
Fig. 5f;
Table 9). In the alternative metrics, %
Hyalella azteca had a positive relationship with % uncultivated cover (
R2 = 0.20,
p < 0.01), but a negative relationship with % forest in the upstream watershed (
R2 = 0.18,
p < 0.0;
Table 9).
There were strong significant correlations with individual taxa along the uncultivated and forest gradients in Group 3. We found the crawling water beetle Haliplus apicalis Thomson, 1868 (Coleoptera: Haliplidae) to have a strong negative correlation with the % forest cover (r = −0.674, p < 0.001), as well as Hyalella azteca (r = −0.427, p < 0.05), and the elmid riffle beetles (Coleoptera: Elmidae, r = −0.584, p < 0.01). Further, we found the uncultivated gradient to be characterized by positive correlations with the small square-gilled mayflies Caenis latipennis Banks, 1907 (Ephemeroptera, Caenidae, r = 0.711, p < 0.05), Haliplus apicalis (r = 0.624, p < 0.001), and Hyalella azteca (r = 0.516, <0.05).