Introduction
One of the primary responses to the global decline in biodiversity in recent decades is the expansion of protected area (PA) networks. Targets developed under the post-2020 Global Biodiversity Framework have promoted a substantial increase in this expansion. One key target in this framework is to “protect and conserve through well connected and effective system of PAs and other effective area-based conservation measures at least 30 per cent of the planet with the focus on areas particularly important for biodiversity” (
Convention on Biological Diversity 2020). Canada has committed to this target as part of the High Ambition Coalition for Nature and People to “address the dual crises of biodiversity loss and climate change” (
Environment and Climate Change Canada 2021a). However, as of the end of 2020, Canada had only conserved about 12% of its land and freshwaters in formally designated PAs (
Environment and Climate Change Canada 2021b).
In addition to ambiguities in biodiversity response, there are other factors that make it difficult to draw firm conclusions about whether a particular PA is meeting conservation expectations. Often, no explicit conservation targets or indicators to assess progress have been identified or the identification process was not sufficiently precise (e.g., SMART – specific, measurable, ambitious, realistic, and time-bound) to allow for a meaningful evaluation (
Green et al. 2019;
Visconti et al. 2019). Because monitoring the effectiveness of PA management is critical to conserving biodiversity (
Graham et al. 2021), there has been a recent call to track progress in a more highly structured and defensible manner (
Adams et al. 2021).
Few efforts of this nature have been completed in Canada, although ongoing ecological condition monitoring in Canadian national parks is an important exception (
Woodley 2020). In addition,
Deguise and Kerr (2006) demonstrated that existing reserve networks in Canada rarely performed better than randomly selected areas and several included fewer endangered species than expected by chance.
Kerr and Cihlar (2004) proposed that one of the main reasons for this sort of result is that PAs in Canada have often not been established to protect biodiversity. They have in many cases been established for recreational benefits and the enjoyment of people and are often embedded in vast tracts of surrounding wilderness where threats to biodiversity are lower. However, this does not seem to be the case in Nova Scotia, with 71% of land in private ownership (
Government of Nova Scotia 2021) and a higher degree of landscape fragmentation than in many other parts of Canada. The more fragmented landscapes in Nova Scotia and the potential influence on successful biodiversity conservation likely heighten the importance of specific site selection choices for new PA designations relative to those in less developed parts of Canada.
There are no published studies that evaluate the biodiversity value of PAs relative to similar sites in the working landscape in Nova Scotia. However, recent unpublished studies suggest relative population declines in two flycatchers were less severe in PAs than in the broader landscape and that several classes of upland forest plants (e.g., orchids, sedges, and geophytes) are more likely to occur in PAs than in the surrounding working landscape (Robert Cameron, Dalhousie University, personal communication).
Nearly 13% of terrestrial lands have already been protected in Nova Scotia (
Environment and Climate Change Canada 2021a,
2021b), including more than 160 formally designated sites, and the Province has committed to protecting 20% of its terrestrial lands by 2030 as a target within the Environmental Goals and Climate Change Reduction Act of 2021 (
nslegislature.ca/legc/bills/64th_1st/1st_read/b057.htm). Because of limited effort to compare relative biodiversity value inside and outside of PAs in Nova Scotia and the current push for more quantitative evaluation of PAs from a biodiversity perspective (e.g.,
Adams et al. 2021), we undertook a study to examine the relative biodiversity value of sites inside and outside of Cloud Lake Wilderness Area (CLWA), one of the larger, formally designated PAs in Nova Scotia.
We selected birds as our focal biotic community to assess relative biodiversity values because forested wetlands support a rich diversity of avian species that occupy a wide variety of niches (e.g.,
Smith 1977;
Golet et al. 1993;
Morrissette et al. 2013;
Brazner and Achenbach 2020) and birds have long been known to be good indicators of forest condition (e.g.,
Welsh 1987;
Canterbury et al. 2000), including forested wetland–riparian condition (e.g.,
Croonquist and Brooks 1991;
Bryce et al. 2002). In addition, the home ranges of most forest songbirds (passerines) are well within 500 m of the nesting area (e.g.,
Mace and Harvey 1983;
Gnass-Giese et al. 2015), so the species we focus on here should be good indicators of relatively local forest conditions during the breeding season. However, birds do respond to habitat conditions at a variety of spatial scales (e.g.,
Mensing et al. 1998;
Crozier and Niemi 2003;
Cunningham and Johnson 2016), so also reflect conditions in the surrounding landscape. Birds are relatively easy to sample reliably during the breeding season in forested habitats (e.g.,
Howe et al. 1997) and there are many species with rapidly declining populations that are of special concern provincially (
Stewart et al. 2015), nationally (
Government of Canada 2022), and internationally (
Billerman et al. 2020).
Our main objective for this study was to begin examining whether PAs in Nova Scotia are functioning to enhance the biodiversity value of forested wetland bird communities relative to those in the nearby working landscape using a large PA in the province and its associated surrounding landscape as an initial proxy for PAs elsewhere in the province. We evaluated biodiversity value using the approach described by
Brazner and MacKinnon (2020) where sites with higher richness and abundance of birds were considered to have higher biodiversity value, along with those that contained higher abundances or proportions of species of conservation concern (SOCC) and guilds of conservation concern (GOCC). Secondary objectives were to determine if the bird communities inside and outside of the PA were ecologically distinct, and which habitat and landscape factors were the best predictors of bird community characters and abundances across all sites.
Discussion
Our study reaffirms the idea that forested wetlands are diversity hotspots for breeding birds in Nova Scotia (
Brazner and MacKinnon 2020), with 79 species detected overall, including 12 SOCC and 4 species listed as at-risk either provincially or federally. The 79 species we observed are similar to richness levels reported by other studies of bird communities associated with forested wetlands in eastern Canada (
Brazner and Mackinnon 2020;
Calmé et al. 2002 – 102 species in 112 bogs in Quebec) and are notably higher than the bird species richness observed in similar studies further west in Canada and the northern United States (
Swift et al. 1984 – 46 species in 8 swamps;
Riffell et al. 2006 – 55 species in 25 swamps;
Morissette et al. 2013 – 55 species in 41 swamps and peatlands). As noted by
Brazner and Mackinnon (2020), it is difficult to make direct comparisons of species richness differences among studies due to design and effort disparities, but these results suggest forested wetlands in eastern Canada have atypically rich bird communities, and therefore may have heightened importance as avian diversity hotspots. It may be that the character (e.g., size, density, type, total area) of forested wetlands or the forest in general in eastern Canada is somehow different than those elsewhere (e.g., ecotonal between temperate and boreal landscapes), and as a result support greater avian species richness. Broad scale, comparative studies across North America would be required to definitively answer this question.
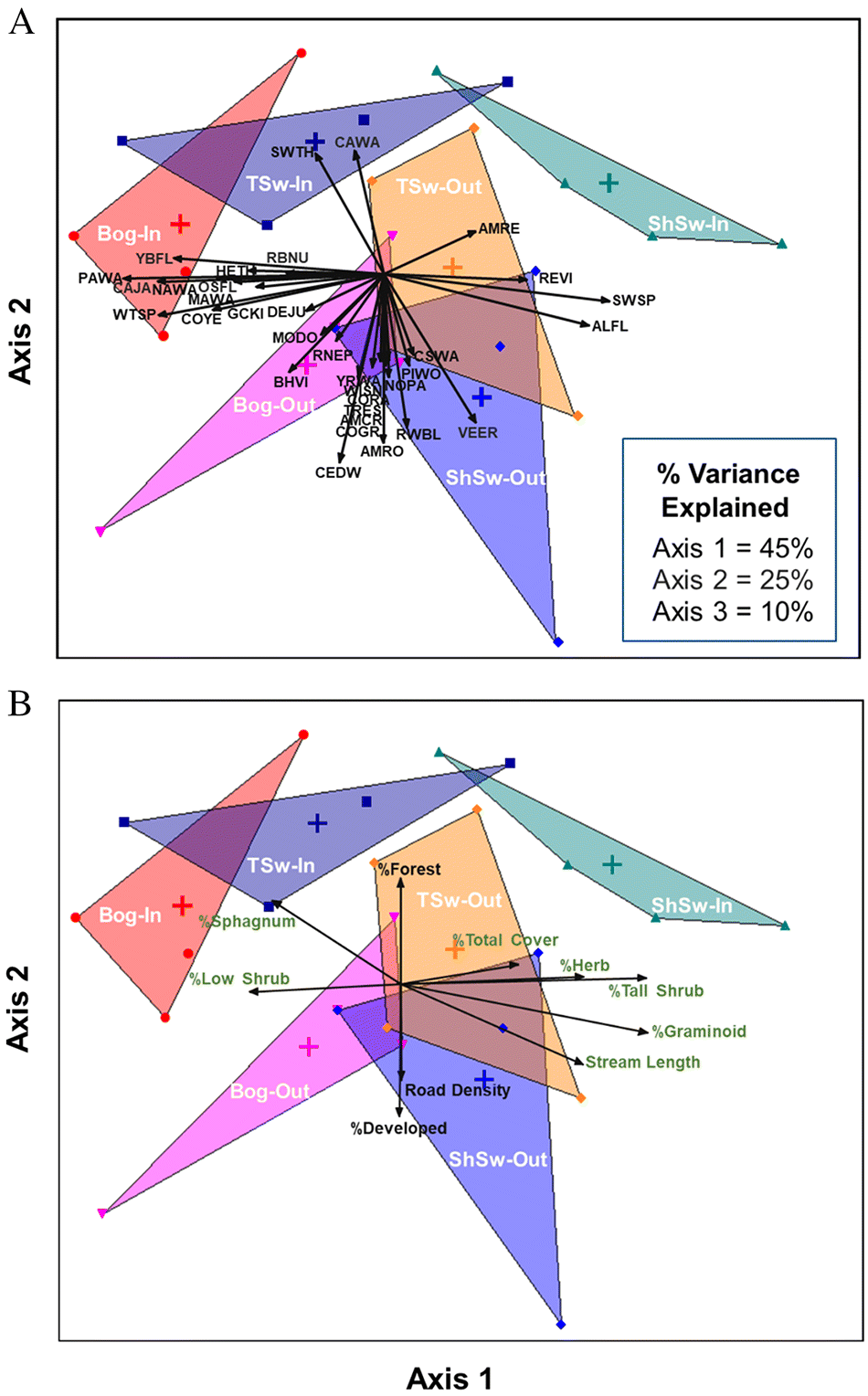
Another important finding was that the bird communities associated with forested wetlands inside and outside of CLWA were ecologically distinct, but differences among wetland types were only clear for sites inside CLWA. The fact that sites from different forested wetland types outside CLWA had large overlaps in ordination space suggests there has been a homogenization of these bird communities relative to those in minimally disturbed areas. Homogenization of biotic communities has been linked to landscape disturbance by numerous studies (e.g.,
Devictor et al. 2008;
Karp et al. 2018;
Liang et al. 2019), so the result is not surprising here given the differences in landscape character that were apparent between sites inside and outside of CLWA (e.g., higher development, road density, and recently cut forest in the working landscape).
However, given the biotic homogenization we observed, it was surprising that sites outside CLWA had higher species richness and diversity than inside sites. Results from other studies comparing species richness and diversity among sites inside and outside of PAs have not revealed a consistent pattern (e.g.,
Deguise and Kerr 2006;
Gaston et al. 2008;
Laurance et al. 2012;
Geldmann et al. 2013), but some of the more recent analyses strongly suggest that local biodiversity is typically higher inside terrestrial PAs worldwide (
Coetzee et al. 2014) even though neither rarefaction-based richness nor endemicity appears to differ significantly in many cases (
Gray et al. 2016). In some studies where sites inside PAs had the greatest species diversity, the distinctiveness and richness of species in sites outside of PAs were still found to contribute substantially to regional avifaunal diversity because they supported a complementary assemblage (
Gardiner et al. 2007;
Dahal et al. 2014;
Mönkkönen et al. 2014). We observed this sort of complementarity in our study as well, with 10 of 11 species that were only found in either inside or outside sites being found at outside sites. Our npANOVA results and biases in the frequency of detection (
Table S5) also reflect complementarity with respect to SOCC in that six SOCC were notably more abundant at outside sites (American bittern, American woodcock, common nighthawk, eastern wood-pewee, pine siskin, and veery) and our ordination results suggest that four other SOCC (Canada jay, Canada warbler, olive-sided flycatcher, and red-breasted nuthatch) were most strongly associated with inside sites.
It is important to consider the landscape context when trying to interpret the species richness and diversity patterns we observed. There have been many studies designed to determine the importance of landscape relative to local habitat context (e.g.,
Villard et al. 1999;
Driscoll et al. 2013;
Galitsky and Lawler 2015;
Häkkilä et al. 2018;
Basile et al. 2021;
Ramirez-Delgado et.al. 2022) since
Franklin (1993) first proposed it as a key need for biodiversity conservation across landscapes. The results, although mixed, are directly relevant to understanding the biodiversity differences that were apparent in our study. Given the high proportion of early successional-edge dwelling birds and community homogenization, the forested wetland bird communities we sampled outside of CLWA clearly reflect disturbance in the working landscape. Nevertheless, the high numbers of species we found in the working landscape overall and the presence of many SOCC suggest these sites may serve as important reservoirs of biodiversity and act as important refugia for many bird species. However, birds and other biota in disturbed or fragmented areas may experience reduced reproductive success (
Villard et al. 2012;
Betts et al. 2019) and increased risk of extirpation (e.g.,
Donovan et al. 1995;
Robinson et al. 1995;
Ramírez-Delgado et al. 2022), so more work would be needed to determine if these areas are acting as effective long-term refugia.
Although the association of early successional-edge dwelling species with disturbance and fragmentation is well established (e.g.,
Rodewald and Yahner 2001;
Miller et al. 2007;
Gnass Giese et al. 2015), increased richness and diversity under these conditions are not. Greater bird species richness and diversity at sites in fragmented landscapes outside of PAs were observed in a study comparing fragmented to large contiguous forest patches in Missouri (
Howell et al. 2000). They attributed this to increased area of non-forested and edge habitats in fragmented landscapes that supported more early-successional and non-migratory and short-distance migrant species (similar to the pattern we observed in our study), suggesting disturbance and fragmentation may increase local species diversity in some circumstances (e.g.,
Noss 1983). However,
Halstead et al. (2019) caution that these sorts of results may be dependent on the landscape characterization approach. Their examination of the relative importance of habitat fragmentation and habitat loss on species richness found that a species-centered approach identified habitat configuration relationships obscured by land-cover based approaches, suggesting positive relationships between species richness and fragmentation may be an artifact of some study designs.
We do not think that is the case with our study, as we used similar patch and landscape metrics to those used by
Halstead et al. (2019) to assess landscape versus local habitat effects with our NPMR models. Their results suggest it was a combination of landscape and local habitat factors that were most important in driving differences in bird communities. In our study, community characteristics were mainly driven by landscape condition (e.g., mature forest cover and human disturbance factors such as distance to the nearest road and overall human disturbance), whereas SOCC abundances and GOCC proportions were driven by a combination of landscape and local habitat predictors. Landscape factors tended to be most important for SOCC (e.g., edge density and distance to the nearest road), but the response was highly species dependent. For example, olive-sided flycatcher abundance was strictly driven by local habitat factors (e.g., water table depth, herb, and shrub cover). With respect to GOCC, local habitat character (particularly shrub cover) had the strongest influence. Some recent studies (e.g.,
Lorenzón et al. 2016;
Halstead et al. 2019) have found local habitat to be most influential, but
Galitsky and Lawler (2015) found that the response was highly context specific with some species and guilds much more responsive to landscape factors and others to local factors. In contrast to our results related to bird diversity,
Galitsky and Lawler (2015) found that local factors explained four times more variance than landscape factors for overall species diversity. Other studies (e.g.,
Mazerolle and Villard 1999;
Häkkilä et al. 2018;
Basile et al. 2021) have found similarly context-dependent importance of local habitat and landscape matrix influences on bird community richness and diversity suggesting there is still much to be learned about what tips the balance in one direction or the other.
In direct comparisons of biodiversity inside and outside PAs, increased species richness and diversity are not typical of the sites outside PAs, but there are some notable exceptions, particularly in studies of marine PAs (e.g.,
Edgar et al. 2004;
Ashworth and Ormond 2005;
Monaco et al. 2007). One notable exception in relation to terrestrial PAs reported higher bird species richness at woodland sites outside of PAs compared to comparably structured sites inside of designated reserves in a long-term study in Australia (
Rayner et al. 2014). They concluded that both PAs and off-reserve conservation schemes have important roles to play in securing species populations and suggested that the conservation value of PAs is strongly influenced by the physical characteristics, as well as the landscape context, and can diminish with changes in surrounding land use over time.
Gardiner et al. (2007) in studies of multiple taxa (e.g., small mammals, birds, amphibians, butterflies) across a gradient of human disturbance from PAs to areas dominated by low intensity agriculture in East Africa found that species richness remained about the same across the gradient, but that there were distinctly different species in each taxon in the areas under different management regimes. This reinforces the idea of complementarity of species assemblages in working landscapes relative to those in PAs and highlights the importance of developing landscape-scale conservation strategies and a broader taxonomic or as
Caro et al. (2009) suggest, a functional approach to assessing changes over time.
Studies that have taken a more functional approach to assessing bird community responses to fragmentation (e.g.,
Flather and Sauer 1996;
Howell et al. 2000;
Keller and Yahner 2007;
Brown et al. 2019) report a higher percentage of long-distance migrants in large contiguous forest patches, just as insectivores seem to be more common (
Greve et al. 2011;
Timmers et al. 2022) in PAs compared to those in more fragmented landscapes. Large contiguous forest patches, even if outside of a formally designated PA, can function in a similar manner to those typical of PAs like CLWA in our study and may explain why we found higher proportions of both long-distance migrants and insectivorous birds at sites inside CLWA. In their meta-analysis of a global data set including 2000 bird species in 741 forest fragments that varied in size and protection status,
Timmers et al. (2022) found that the occurrence of all bird foraging guilds strongly declines with decreasing fragment size (particularly below 50 ha) and that declines were especially pronounced for forest-dependent species, insectivores, carnivores, and more dispersive species. In a long-term study at a 26 ha temperate forest in a highly fragmented New Jersey, USA landscape,
Brown et al. (2019) found that nearly half the species found in the forest at the time of initial protection 40 years ago are now extirpated and that long-distance migrants and ground nesters were most likely to be extirpated. Both the Timmers et al. and Brown et al. studies indicate that PAs are effective for maintaining bird species if they are larger and highlight the importance of large intact forests for conserving avian diversity. These results also support the conclusion of
Gray et al. (2016) that the positive effects of protection are strongly attributable to differences in land use between protected and unprotected sites, but Gray et al. also noted that protection does not consistently benefit all species or guilds (e.g., low mobility guilds) or increase the variety of ecological niches for functional groups to exploit. Our study provides support for the idea that certain species and guilds (e.g., Canada warbler and long-distance migrants) will benefit from being in PAs, while other species, like woodcock, benefit from intact wetlands within a matrix of other habitat types – landscape context is clearly important. Our study also highlights the importance of broadening the focus to habitats and landscapes outside of PAs to provide more effective and comprehensive conservation for many avian species.
Management implications and conclusions
Although our study was limited in scope and should only be considered a necessary first step in testing the effectiveness of PAs throughout the province for conserving biodiversity, it does highlight the fact that there are many aspects of biodiversity conservation in PAs that we still do not fully understand. Why, in some instances, biodiversity is lower in PAs than in similar habitats in the working landscape is an important question that deserves greater attention. Based on their meta-analyses,
Gaston et al. (2008) suggested that greater richness or abundance outside rather than within PAs could arise because PAs were originally designated in areas of lower richness or abundance, because of competing interests for resource exploitation (e.g., forest harvesting), insufficient consideration of biological criteria as part of formal designation, or if there were significant spatial mismatches in the richness and abundance of features for which the protected places were primarily designated. It might also be due to expanding human land use on lands surrounding PAs, resulting in changes in ecological function and biodiversity within PAs (e.g.,
Hansen and DeFries 2007;
Rayner et al. 2014;
Mönkkönen et al. 2014;
Betts et al. 2019). Determining whether the intensity of harvesting or other development is substantially higher in the boundary immediately adjacent to CLWA relative to the landscape in general would be one way of investigating this possibility in relation to our study. However, the results we observed may also be at least partially due to the effects of the “ghost of land-use past” (
Harding et al. 1998). The area within CLWA may still be recovering from historic land use in the study area (e.g., forest harvesting and agriculture) or past large-scale disturbances (e.g., wildfire or blowdowns) that continue to affect species richness and abundance (
Bernes et al. 2015). Regardless of the specific reason, it has become increasingly clear that additional research and better monitoring are required to improve our understanding of factors affecting the capacity of PAs for maintaining biodiversity through time and whether PAs are meeting conservation expectations and targets (
Adams et al. 2021;
Graham et al. 2021).
The fact that bird species richness and the abundance of SOCC in forested wetlands inside CLWA were lower than at sites in the working landscape outside CLWA suggests that forested wetlands outside CLWA may be acting as important refugia for birds despite the higher intensity of human disturbance adjacent to these sites. Our results also support the idea that these ecosystems merit special protection provided by the new silvicultural guidelines which prescribe no harvesting of trees in wet deciduous, wet coniferous, and floodplain forests on Crown lands in Nova Scotia (
McGrath et al. 2021). In addition, our results also suggest that there is potential for biodiversity gains through investments in conservation schemes outside of PAs and on private lands (e.g.,
Lindenmayer and Franklin 2002;
Cox and Underwood 2011) and highlights the need for working lands conservation. The challenge of shifting from managing working lands solely for profit to conservation of working lands is substantial, but there are clear paths toward larger-scale integration of this approach (
Kremen and Merenlender 2018). Only time will tell, but the recommended move toward ecological forestry in Nova Scotia (
Lahey 2018), which prioritizes biodiversity conservation over other forest values, seems like a sensible first step in that direction and worth careful evaluation in the coming years.