1. Introduction
The impacts of climate change on marine ecosystems are pervasive. Direct changes to the physical environment include changes in sea surface temperature (SST;
Cheung et al. 2009), acidification, and oxygen depletion (
Lotterhos et al. 2021). Indirect effects on ecological systems responding to these physical changes (
Weiskopf et al. 2020) include changes in productivity (
Sydeman et al. 2021), biodiversity (
Manes et al. 2021;
Worm and Lotze 2021;
Penn and Deutsch 2022), and altered ecosystem function (
Free et al. 2019), all of which are foundational elements of the marine environment. Mitigating and adapting to climate change will be integral to maintaining the health of our oceans and the continued provision of ecosystem services. Simultaneously, protecting marine ecosystems from other anthropogenic stressors, including overfishing and pollution, can enhance their resilience, potentially reducing the negative impacts of climate change (
Hughes et al. 2005;
Bryndum-Buchholz et al. 2022).
Following international commitments to the U.N. Sustainable Development Goals and the Convention on Biological Diversity, Canada has committed to protecting 30% of its coastal/marine areas by 2030. This will be achieved through the implementation of Marine Protected Areas (MPAs) and other effective area-based conservation measures (OECMs) (
DFO 2021a). Fisheries and Oceans Canada (DFO) is the primary government body leading the development of marine conservation areas and is the agency that administers formal Oceans Act “Marine Protected Areas” and Fisheries Act “Marine Refuges” (a form of OECM), which collectively comprise the majority of the Canadian Marine Conservation Network by area (∼80%;
Bryndum-Buchholz et al. 2022). MPAs in Canada are areas of the ocean that are legally protected from anthropogenic activities to help ensure the conservation of marine ecosystems and species. They may be designated due to the presence of special natural features (e.g., unique and productive habitats or diversity hotspots), concentrations of species at risk (e.g., endangered or threatened species under the Canadian Species at Risk Act), and/or environmental, cultural, or socioeconomic importance (
DFO 2018). Their boundaries are determined by the priorities of various ocean stakeholders (i.e., fisheries, aboriginal communities/organizations, ocean industries, federal and provincial government, conservation groups, coastal communities, etc.). Marine Refuges have been established to help to protect focal species or functional groups and their respective habitats (including corals and sponges) through closures of certain fisheries (
Schram et al. 2019;
DFO 2020) and in Canada are targeted for benthic habitats. The overarching goal of the Canadian Marine Conservation Network (a collection of MPAs and OECMs across the nation's marine bioregions) is to “provide long-term protection of marine biodiversity, ecosystem function and special natural features” with specific objectives developed according to the unique physical, ecological, and biological attributes of each area (
Government of Canada 2011). However, the dynamic impacts of climate change on marine ecosystems threaten the long-term effectiveness of these MPAs and OECMs, which are typically implemented as long-term fixed measures (
Tittensor et al. 2019). Climate-driven shifts in species geographic distributions (
Pinsky et al. 2013), timing of life history events (
Fuentes-Yaco et al. 2007), productivity (
Bryndum-Buchholz et al. 2020), and ecosystem structure (
Boyce et al. 2015) may necessitate changes to site-level management, boundaries, or the configuration of the conservation network. For this reason, there has been a recent movement to include climate change considerations in marine conservation planning in Canada, with workshops convened on the topic, recent publications outlining pertinent advice on how to ensure Canada's conservation network is climate resilient (e.g.,
Wilson et al. 2020;
Bryndum-Buchholz et al. 2022), and a new mandate to bring climate resilient conservation planning into the Canadian MPA Program (Minister of Fisheries, Oceans and the Canadian Coast Guard Mandate Letter (pm.gc.ca)).
Prevailing advice advocates using climate change vulnerability/risk assessments to promote climate resiliency within the design and management of conservation networks. Foundational to these assessments is understanding how species distributions will shift in response to changing environmental conditions (e.g.,
Shackell et al. 2014;
Stortini et al. 2015). The distribution of a species is, for the most part, constrained by the physical and environmental conditions (e.g., temperature and depth) required for survival (e.g.,
Guisan and Zimmermann 2000;
Sillero et al. 2021). When an organism is living at its upper or lower temperature limit, oxygen demand increases, often exceeding the organism's ability to maintain it, causing reduced performance in ventilation and circulation (
Pörtner 2001). In some cases, and over evolutionary time scales, adaptation to novel environmental conditions is possible. However, evidence suggests that the rapid environmental changes associated with climate change are already causing geographic shifts in distribution that track shifting distributions of their thermal niches (
Pinsky et al. 2013;
Crozier and Hutchings 2014;
Pinsky et al. 2021). In general, the magnitude of global surface warming will be a primary determinant of species extirpations from their historic habitats (
Penn and Deutsch 2022).
How a species interacts with its thermal environment plays a prominent role in determining its response to climate change, including geographic range shifts. For this reason, the thermal niche of the species is often used in vulnerability and risk assessments to assess the timing, location, and magnitude of climate impacts on that species (
Pacifici et al. 2015;
Stortini et al. 2015;
Stuart-Smith et al. 2015;
Foden et al. 2019;
Greenan et al. 2019;
Pinsky et al. 2019;
Boyce et al. 2022). The timing of a distributional response of a species is the product of its thermal niche and the chronology of environmental change (e.g., rate of warming). The “time of emergence” (ToE) or more specifically “time of thermal emergence” has been used to designate the expected timing of a biological response (e.g., increased stress, distributional shifts, or declining fitness) to temperature change, defined as the year in which SST exceeds the upper thermal tolerance of a species in a particular area of interest (
Henson et al. 2017;
Trisos et al. 2020). As species shift towards higher latitudes and into deeper, cooler waters (
Pinsky et al. 2021), species of conservation concern may eventually emerge from their thermal niche within MPAs that were initially designated for their protection. This emigration of a species from MPAs could co-occur with the immigration of new species from adjacent areas, potentially leading to new community compositional states not previously observed (
Lurgi et al. 2012).
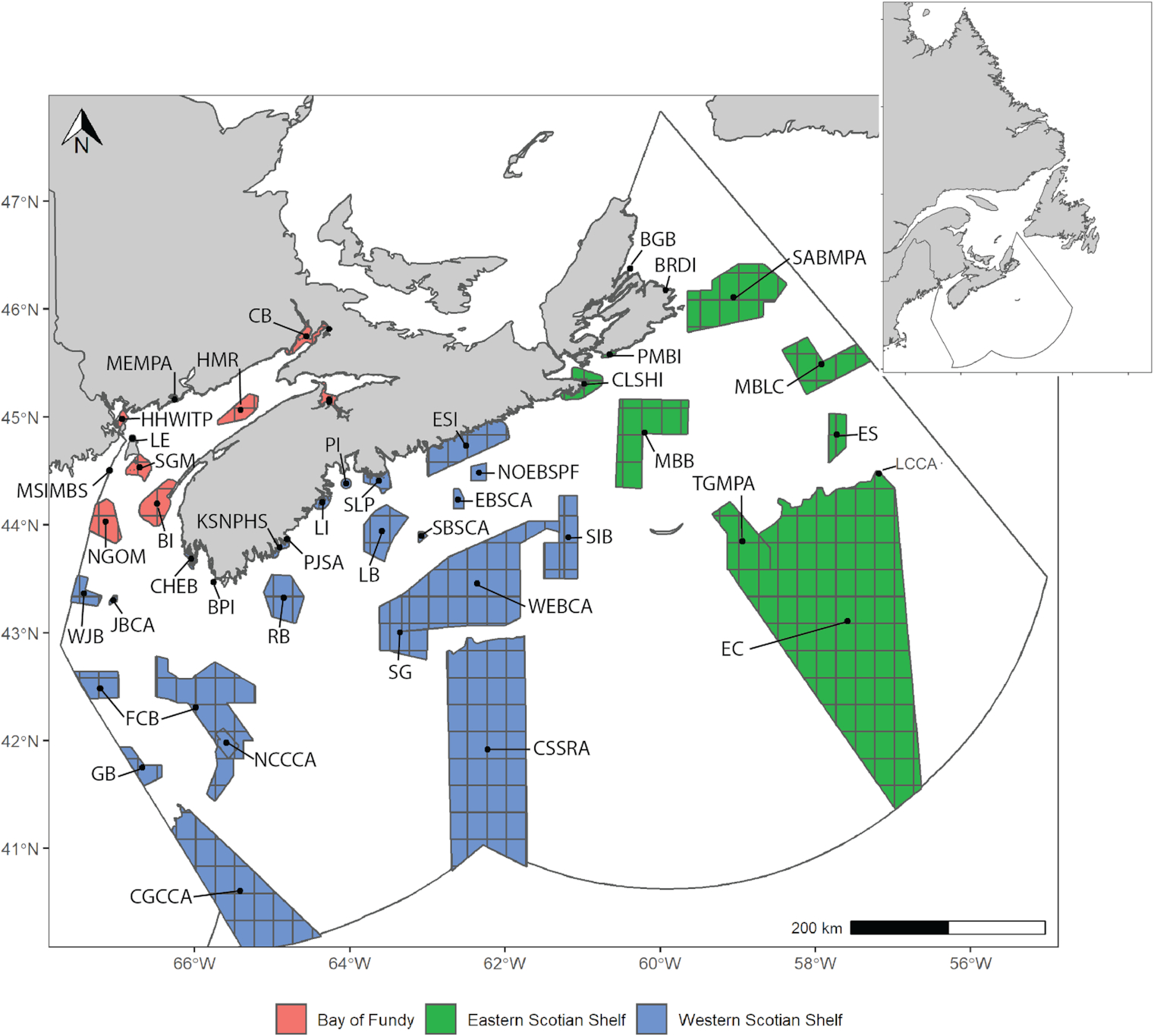
The regional network of MPAs and OECMs proposed for the Scotian Shelf, off Canada's eastern coast (
Fig. 1), was designed without explicit consideration for the impacts of climate change (
DFO 2018), and thus it remains unknown as to how climate change may impact this network’s efficacy, over the long term. The aim of this study was to estimate the ToE of 30 fish and invertebrate species of interest within each of the existing and proposed MPA/OECM site boundaries in the draft conservation network design for the Scotian Shelf-Bay of Fundy bioregion (
Fig. 1;
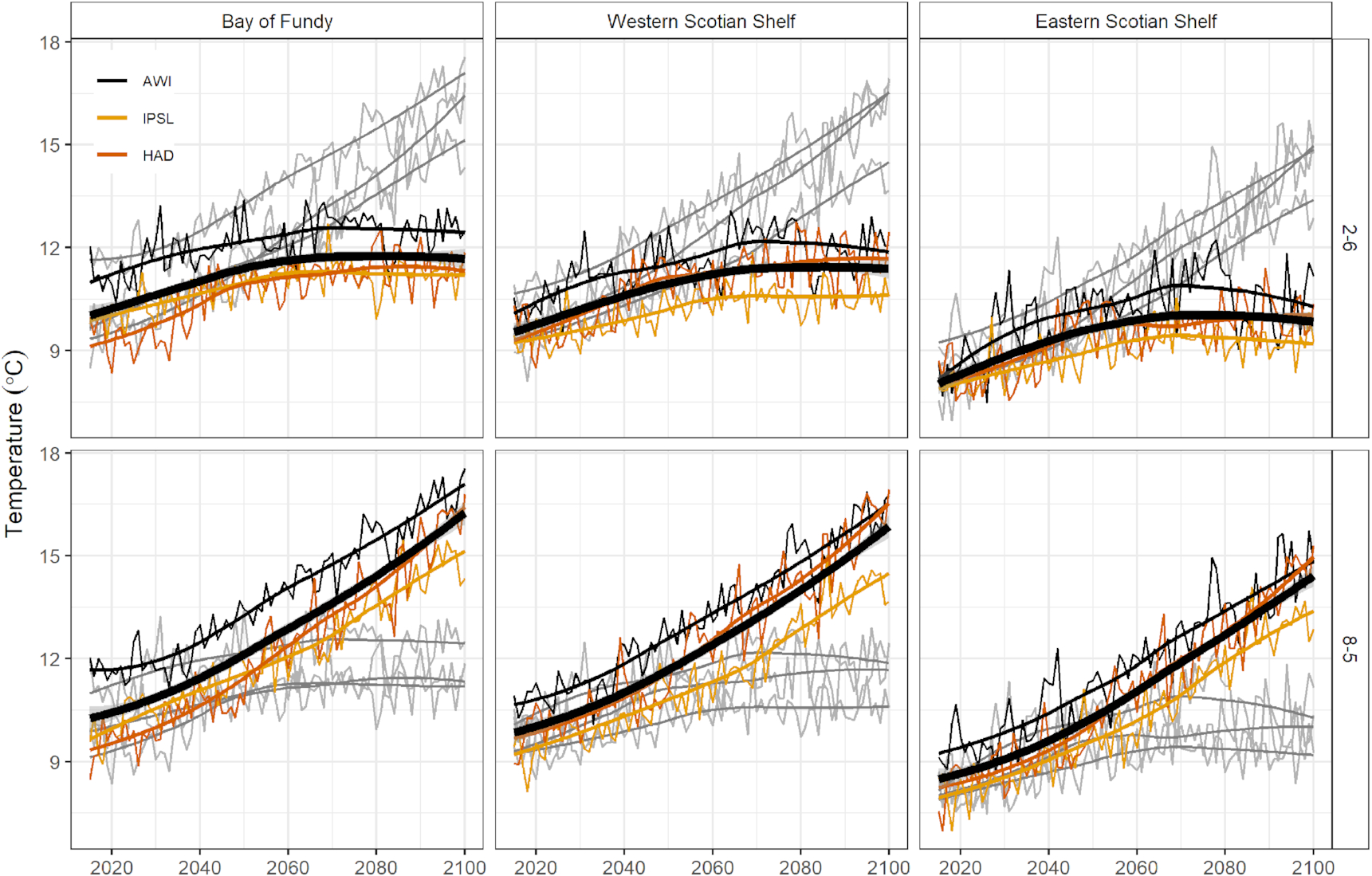
Table 1) under ensemble projections of future SST (two emission scenarios and three climate models). The ToE will vary by species and location, depending on the thermal niche of a species and the rate and magnitude of SST change at each site across the geographic range of the species (Table S1; Fig. S1). In general, species with more restricted thermal niches are more likely to experience habitat shifts and for those shifts to happen sooner.
The application of species-based estimated time(s) of emergence in the planning of MPA networks is novel and can be used to predict how the future effectiveness of MPAs and MPA network design configurations could change as species distributions and ecosystems shift in response to climate change. This information may help MPA managers to prioritize sites for immediate designation and to identify sites that may require more adaptive management strategies due to predicted shifts in priority species distributions.
4. Discussion
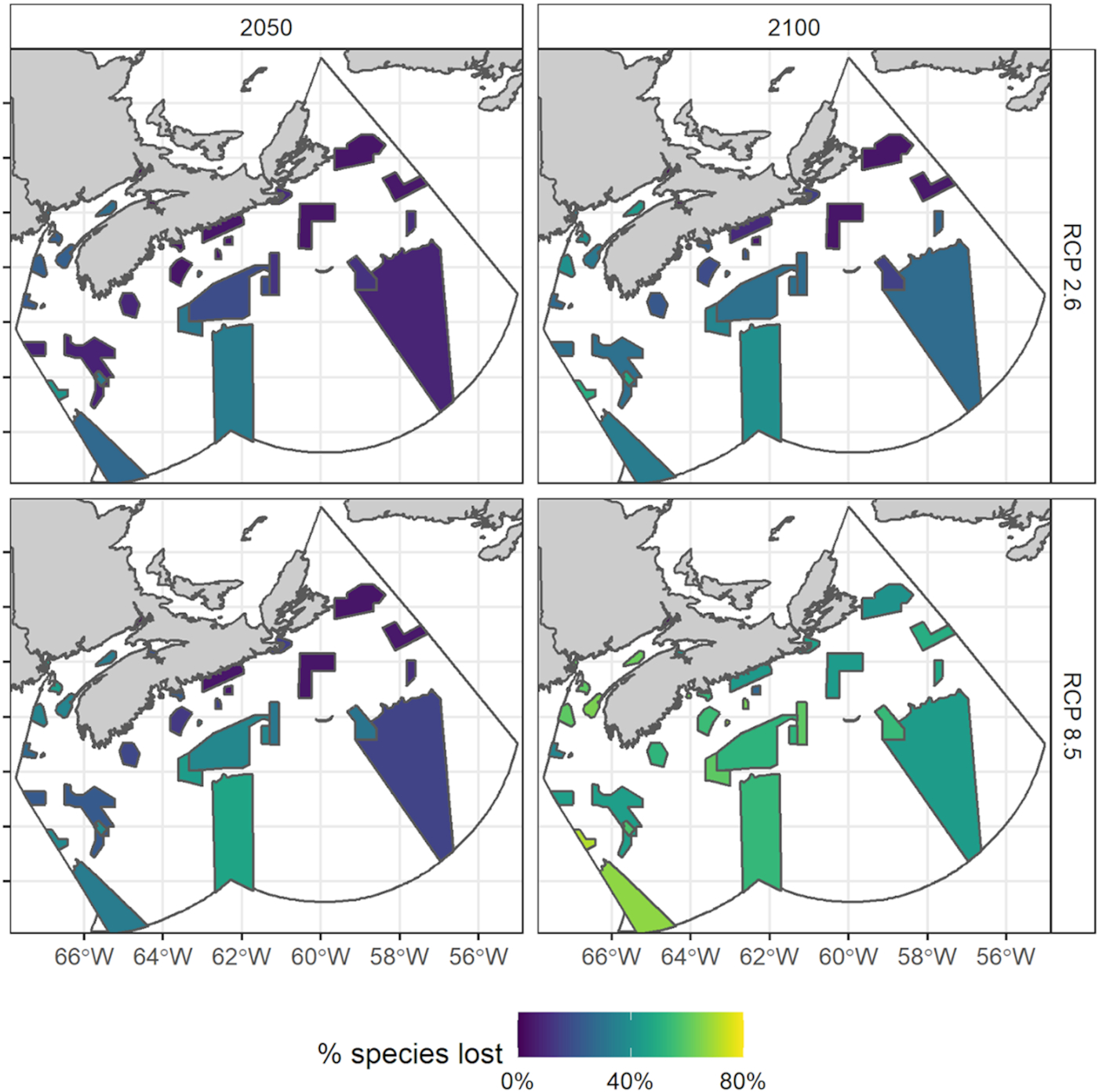
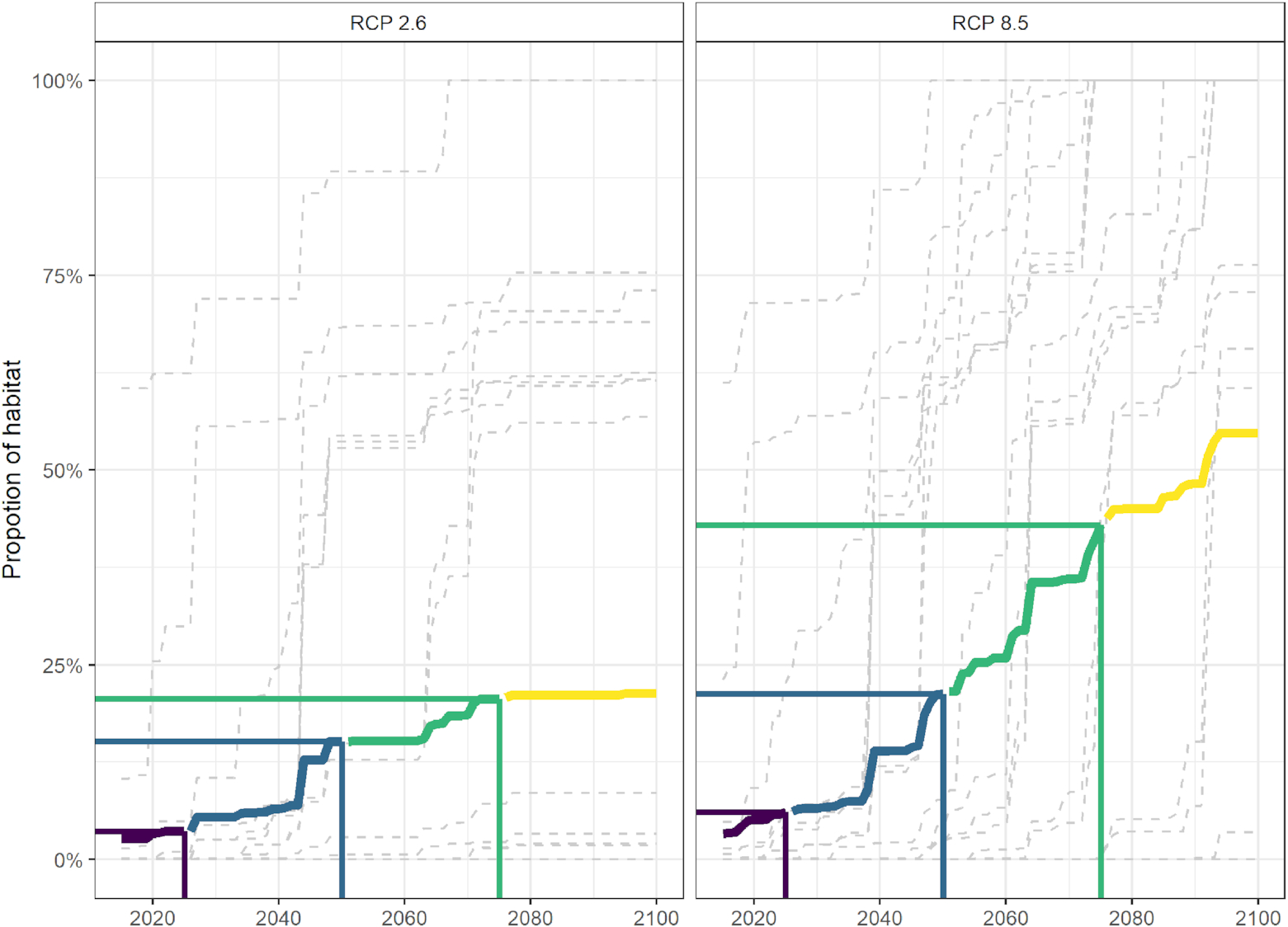
This study examined the ToE of 30 marine species within the MPA/OECM sites included in the Scotian Shelf-Bay of Fundy bioregion draft conservation network design. Under low emissions (RCP 2.6), the majority (>50%) of species are retained within the boundaries of the draft conservation network until at least the year 2100 (
Fig. 4). Conversely, under high emissions (RCP 8.5), 19 of 40 sites within this draft conservation network are predicted to lose more than 50% of the species considered in this study (
Fig. 4). This result is consistent with the IPCC's 2021 report and with several other studies indicating muted distribution shifts under low compared with high emissions (
Höhne et al. 2021;
IPCC 2021;
Lima et al. 2022;
Pielke et al. 2022), suggesting that, if targets for emissions reductions are met, the Scotian Shelf-Bay of Fundy bioregional conservation network, as it is currently designed, will be more effective in achieving its conservation goals and targets over the long term. However, recent studies have shown that global emissions are currently more comparable to that of the high emissions scenario (
Höhne et al. 2021). Under the high emissions scenario, species lost 32% more thermal habitat from the network by 2100 than under the low emissions scenario (
Fig. 5), indicating that the ability of the draft conservation network to achieve its conservation goals/targets over the mid- to long term may be impacted and should be continually evaluated.
Under both emission scenarios, results indicated a clear southwest-to-northeast gradient in species times of emergence, with many species emerging from their thermal niche earlier in the southwest compared with the northeast (
Fig. 3). This gradient was most pronounced under high emissions near the end of the time series (
Fig. 3). Besides the Lophelia Coral Conservation Area (LCCA), which is a very small (14.7 km
2) Marine Refuge at the northeastern edge of the Scotian Shelf with only 13 of our species of interest present, the most vulnerable sites (predicted to have temperatures outside the tolerance limits of more than 25% of the species assessed here by 2025 under both emission scenarios) were all located in the southwestern part of the bioregion. These sites included Central Scotian Slope, Rise and Abyss (CSSRA), Horse Mussel Reef (HMR), and Long eddy (LE) (
Fig. 4). CSSRA is positioned where warm waters derived from the Gulf Stream flow into the Gulf of Maine (
Brickman et al. 2018,
2021), and both HMR and LE are located in the Bay of Fundy, which is presently the warmest part of the bioregion and predicted to warm the most rapidly due to its connection with the Gulf of Maine (
Brickman et al. 2018,
2021). This result is consistent with many studies documenting the steep temperature gradient, and consequent gradient in genetic structure and species assemblages, that characterize the Scotian Shelf-Bay of Fundy bioregion (
Stanley et al. 2018;
Stortini et al. 2021;
O'Brien et al. 2022). This is also consistent with studies that have indicated the southern range limit of many of the Scotian Shelf's resident species occurs around the southwestern reaches of the bioregion, such that northward distribution shifts lead to extirpation from this end of the region first (
Shackell et al. 2014;
Brennan et al. 2016).
The anticipation of rapid (as early as 2025) emergence of resident species from southwestern conservation areas should not be justification for the deprioritization of sites that were designed to capture representative examples of habitat features (
DFO 2018). Rather, these sites will provide safe havens for new emerging ecological communities, such that the biodiversity of the area may be protected from other anthropogenic influences as inevitable climate-driven ecological shifts occur (e.g.,
Matthews and Wynes 2022). In these cases, particular care should be taken to monitor climate-related changes and re-evaluate conservation priorities where the greatest changes are predicted to occur. On the other hand, areas designed to protect particular species or species groups may require re-evaluation if the species of interest are predicted to become severely thermally stressed in the near term (e.g., ToE ≤ 2050). Results identifying rapidly emerging/shifting species will also have implications for fisheries; such species will require enhanced monitoring, the inclusion of climate change considerations in stock assessment processes, and adaptation of the industry to new species or occupations.
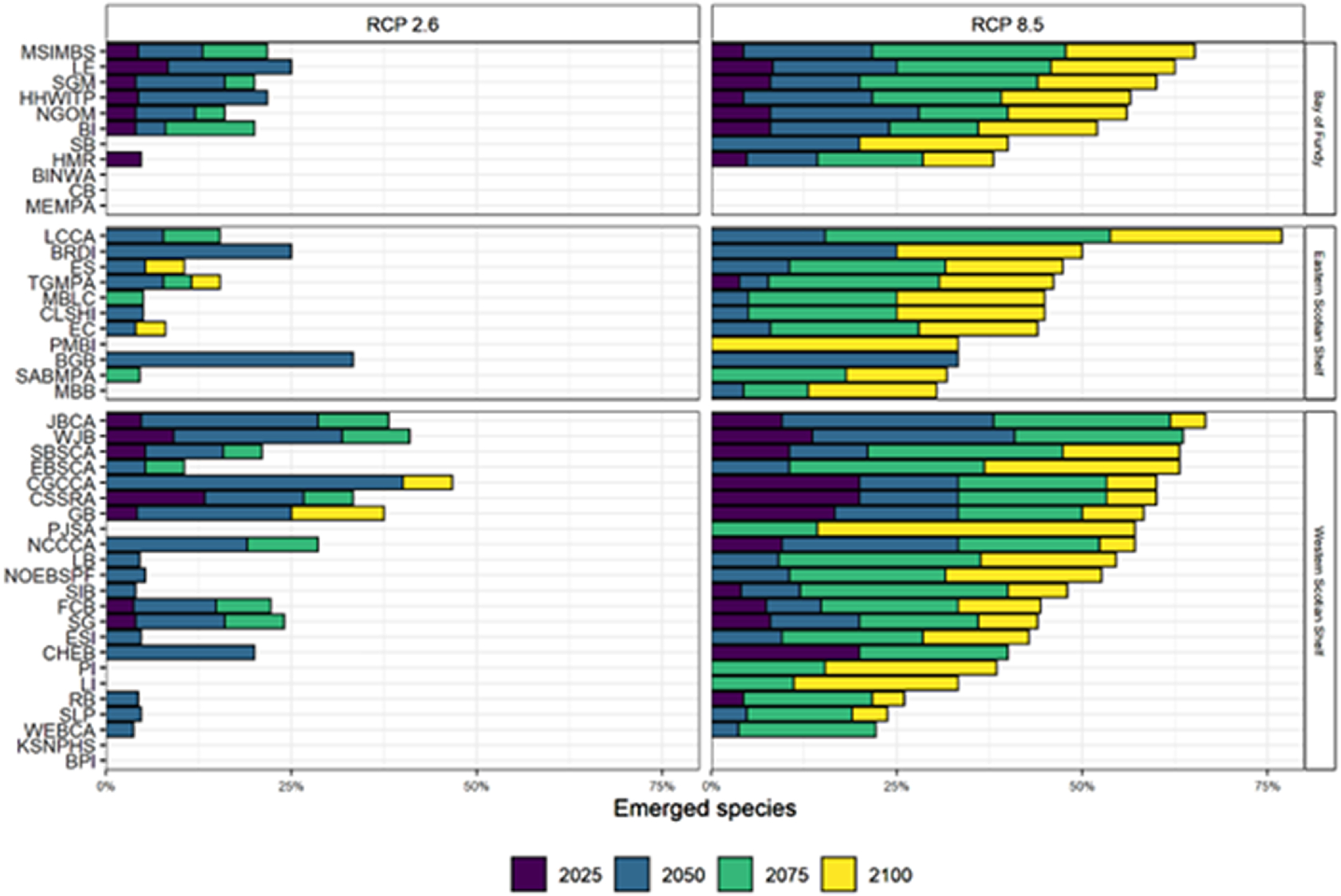
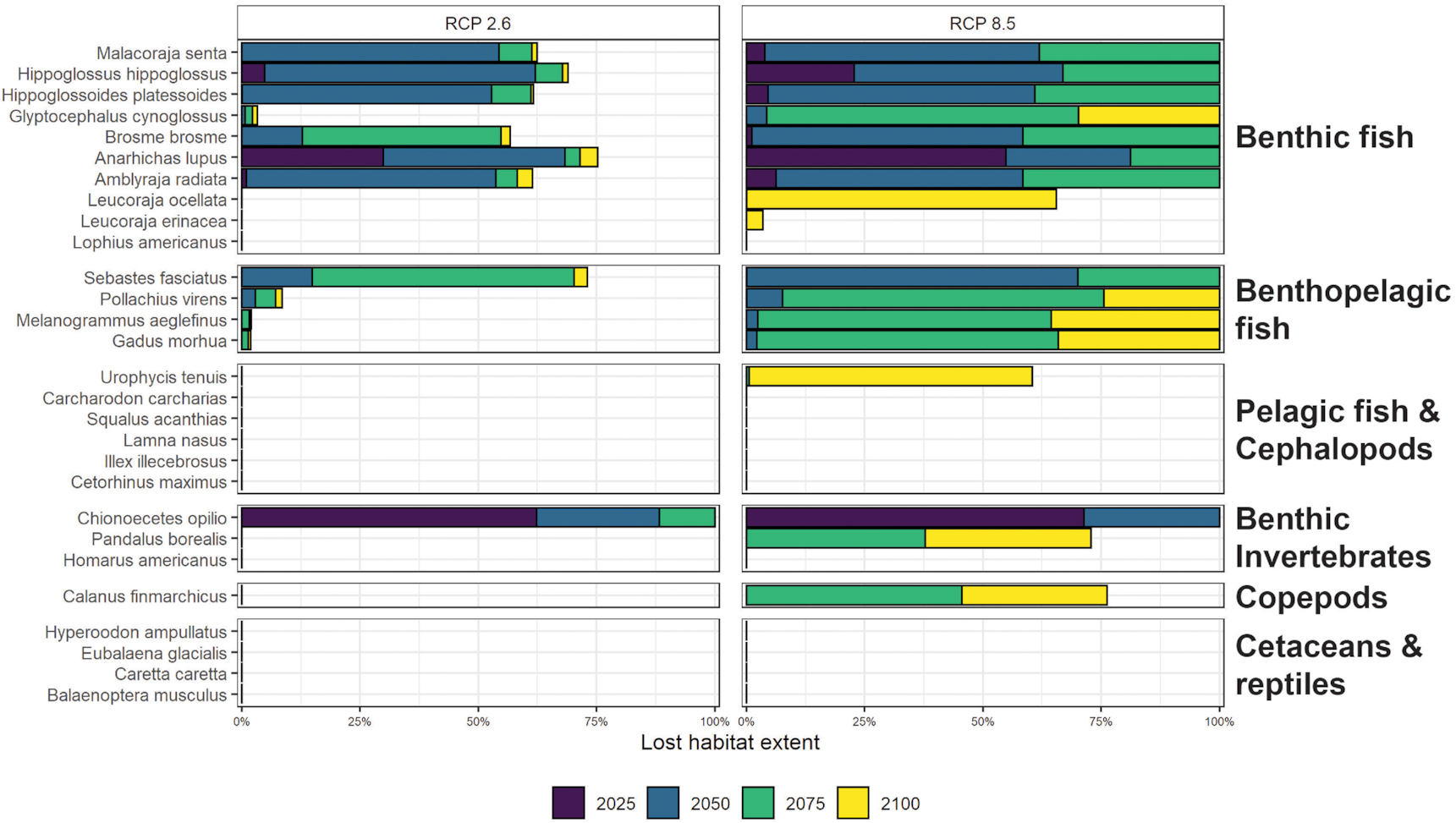
Our results indicate that Snow crab (
Chionoecetes opilio), a highly valuable commercially harvested invertebrate species (
DFO 2021b), is most rapidly emerging from the Scotian Shelf-Bay of Fundy bioregion. Snow crab was often the first species to become extirpated from network sites, with site-level ToEs as early as 2015 in some sites, indicating that, although this species has previously been observed, it is unlikely that it currently occupies any southwestern sites in any significant abundance. It was also projected to lose 100% of its preferred habitat from the entire draft conservation network in this region by 2050 under the high emissions future climate scenario (and 100% by 2075 under the low emissions scenario;
Fig. 5). This species has the smallest thermal range of the selected invertebrates and is already occupying the southern-most extreme of its spatial distribution in the northwest Atlantic (
DFO 2021b). Snow crab supports the third most profitable fishery in Nova Scotia (
NS Department of Fisheries and Aquaculture 2014). Recent studies indicate decreases in the amount of Snow crab landings in the western portion of the study region and marked rapid responses to warming events (
Zisserson et al. 2018). As an important scavenger and predator species (
Boudreau and Worm 2012), the extirpation of Snow crab will likely have implications for the structure of benthic invertebrate communities throughout the bioregion as well. Reduced predator presence has been documented to lead to either compensation by other predators (
Shackell and Frank 2007) or prey release resulting in reduced body size diversity, increased beta diversity (
Ellingsen et al. 2015;
Stortini et al. 2018), and increases in both spatial and temporal community turnover (
Alonso et al. 2015;
Stortini et al. 2021). Reduction in Snow crab abundance within the benthic invertebrate communities of the ESS (the Snow crab's current last stronghold in the bioregion) over the next 30–50 years (
Fig. 5) will likely result in some of these community-level responses, which could have implications for the conservation sites in this part of the bioregion. Monitoring and the potential for adaptive management will be imperative to ensure these sites continue to meet their ecological conservation objectives.
The only species not predicted to experience habitat loss in the region were either large migratory species with global distribution ranges or species with a known preference for warmer water (
Fig. 6). These species are in the minority, but these results imply that they may gain suitable habitat along a latitudinal gradient (southwest to northeast) as warming trajectory continues. The focus of our study was to identify areas and timelines for change relative to the baseline on which the network was developed. We limited our examination to area lost instead of the potential for gain within the conservation network due to the assumptions that would have to be made about species dispersal processes and species interactions that underpin expansion, but there have already been cases where warming has manifested spatially variant responses by species (gains and losses). For example, American lobster (
Homarus americanus) has boomed as a result of warming in the Gulf of Maine with continued increases in suitable habitat anticipated (
Tanaka et al. 2019), and this species is predicted to benefit from increases in the extent of suitable habitat throughout the WSS (
Greenan et al. 2019). Spiny dogfish (
Squalus acanthias) are typically found only on the WSS and known to expand northeastward only when temperatures warm (e.g.,
Shepherd et al. 2002;
Shackell and Frank 2007), suggesting this species may become more prevalent on the ESS as waters warm. However, increased prevalence of diseases (
Castro et al. 2006;
Glenn and Pugh 2006;
Wahle et al. 2009), increased frequency of extreme heat waves (
Oliver et al. 2018), and shifts in prey species distributions (
Carloni et al. 2018) have been noted as potential indirect impacts of climate change that may negatively impact even those species not predicted to lose thermal habitat (
Greenan et al. 2019).
Our results indicate that key species at the base of the food chain (
Calanus finmarchicus and
Pandalus borealis) may lose substantial amounts (>50%) of habitat throughout the draft network design over the next 80 years under the high emissions scenario. This result is consistent with other studies that have predicted substantial loss of
Calanus finmarchicus from the Gulf of Maine and Scotian Shelf regions over the next 50–80 years (
Reygondeau and Beaugrand 2011;
Grieve et al. 2017) and a continued trajectory of reduced productivity resulting from losses and shifting size composition at low trophic levels (
Boyce and Worm 2015;
Boyce et al. 2015;
Britten et al. 2016;
Bernier et al. 2018;
Bryndum-Buchholz et al. 2020). Decreased prey availability may lead to further loss of priority species, even those with wider temperature tolerance ranges (e.g., North Atlantic Right whale;
Pendleton et al. 2012;
Meyer-Gutbrod et al. 2015). These changes will likely lead to shifts in the composition of marine communities throughout the region, particularly as species losses will inevitably coincide with, be followed by, or be preceded by a northeastward progression of invasions (or northward expansion) of new, warm water-tolerant species (
Cheung et al. 2009;
Brennan et al. 2016) or warm-adapted populations (
Stanley et al. 2018). In fact, recent studies have suggested that the expansion of warm-water species distributions towards the poles may outpace the shift of cold-water species, leading to shifts in ecosystem structure (
Pound et al. 2020) and changes to ecosystem functions (e.g.,
Bianchi et al. 2021). In other words, the Scotian Shelf-Bay of Fundy ecosystem may undergo “deborealization” and “tropicalization” simultaneously over time, starting within the next 50 years (
Vergés et al. 2014;
McLean et al. 2021;
Osland et al. 2021). Evidence suggests that some key warm water species have already begun invading the Scotian Shelf-Bay of Fundy bioregion, such as Cory's Shearwaters (
Gjerdrum et al. 2018). Novel communities that emerge could prompt the re-evaluation of conservation priorities and the spatial arrangement of sites. The network must therefore be monitored regularly to ensure ecological conservation objectives are being met (
Edgar et al. 2014).
4.1. Implications for conservation network design and management
To ensure the long-term effectiveness of the Maritimes Region conservation network in terms of its ability to safeguard biodiversity, southwestern and Bay-of-Fundy sites predicted in this study to experience rapid change in species composition (e.g., CSSRA, HMR, and LE;
Fig. 1;
Table 1) should be evaluated to determine whether their contributions to species-specific conservation objectives are key to the network design (i.e., are they key areas for species-based targets). If species-based targets in the design are covered by these sites currently, then network design modifications might be required. Alternatively, the prospective conservation priorities for these sites should be refocused to features that are not anticipated to degrade over time (e.g., the prioritization of representative habitat types in the initial design of the Maritimes Region conservation network;
DFO 2018). Prioritization of monitoring effort in these sites should also be applied to ensure conservation priorities are re-evaluated periodically, so that, for example, incoming vulnerable species may be appropriately protected from human activities.
It should also be noted that invading nonlocal species can also have a direct negative impact on fragile ecosystems found within the bioregional conservation network sites through competitive, predatory, or parasitic species dynamics (
Beal and Kraus 2002;
Carver et al. 2003), or habitat destruction (e.g., green crab;
Howard et al. 2019;
Bricknell et al. 2020;
Lyons et al. 2020;
Wilson and Garbary 2020;
Richards and Hunter 2021). Sites containing a high proportion of species with early emergence may be particularly vulnerable to the establishment of invasive species (
Chaffin et al. 2016). This is because, where species are predicted to emerge from their thermal niche, small populations may still be present, but at higher risk to other stressors including invasive species, habitat destruction, and fishing due to physiological stress (
Sylvester 1972;
Pörtner 2001;
Dulvy et al. 2003;
Pörtner and Farrell 2008;
Whiteley and Mackenzie 2016). Long-term protection of biodiversity and native species will require regular monitoring of these high-change/early emergence sites for the presence of harmful invasive species, and, when detected, prompt action.
Further, as remnant, less resilient individuals are left behind in “emerged” sites, other individuals will be shifting in distribution (e.g.,
Stanley et al. 2018;
Cote et al. 2021), avoiding thermally stressful environments and encountering new, potentially suboptimal environments (due to the presence of other stressors and new ecosystem structures) along the way. It is for this reason that implementing a portfolio of conservation efforts (i.e., ecosystem-based approaches for fisheries management (EBFM);
Link et al. 2011), in addition to static conservation network sites (MPAs), is imperative to ensure healthy populations and ecosystems are maintained through periods of transition. Even with strong emission mitigation projected changes in temperature will drive ecosystem responses at various spatial and temporal scales. It is therefore imperative that conservation planning pre-emptively considers how the desired conservation objectives can be best achieved through conservation measures, planning for this change as opposed to trying to design around it.
4.2. Caveats
It is important to note that, while we evaluated climate impacts on species via temperature changes, additional factors may also influence the fitness and distribution of the species assessed here. Ocean acidification can also have a substantial negative impact on a species, especially crustaceans and other invertebrates that require calcium carbonate to make their shells or tests (
Whiteley 2011;
Kroeker et al. 2013;
Jones et al. 2017). Climate-driven changes in ocean circulation have been implicated as a factor influencing larval dispersal capacity in fish (
Raventos et al. 2021) and invertebrates (
Quinn 2017), which in turn can influence recruitment patterns and gene flow. Recent evidence also suggests that intensifying low-oxygen events may have a more severe impact on marine species fitness and distributions than warming (
Sampaio et al. 2021). These additional factors could lead to earlier ToEs of some of the resident species evaluated here, particularly those with shells or tests (e.g., American lobster;
Keppel et al. 2012;
Niemisto et al. 2021) and those with a preference for deep, hypoxifying basins (e.g., Atlantic wolffish;
Bianucci et al. 2016). For this reason, further study of additional and interconnected components of the impacts of climate change could be modelled to expand upon the results found here. However, temperature change is primarily the first environmental factor contributing to species distribution shifts (
Pinsky et al. 2013). Therefore, our results should be considered as warnings of potential initial responses to climate change within the bioregional conservation network in the coming decades.
5. Conclusions
MPAs and conservation networks are tremendously important tools with which to safeguard marine biodiversity and ecosystems, and the numerous functions and services they provide. MPAs, particularly those with strong protections put in place (“highly protected areas”), have a strong potential to complement tools put in place for climate mitigation, helping to safeguard biodiversity and associated ecosystem function (
Jacquemont et al. 2022). However, the influence of climate change will be pervasive, irrespective of whether an area is spatially managed for biodiversity conservation. We demonstrate how climate change will have a variable influence on the effectiveness of a draft network of conservation sites in protecting 40 species of direct and indirect conservation importance, depending on location and our societal approach to emissions reduction. Our results indicate that, within the southwestern portion of the Scotian Shelf-Bay of Fundy region, conservation planners should consider implementing climate change adaptations within network design, management, and monitoring programs, particularly under a scenario of very limited emissions reduction. These adaptations can include setting conservation goals and objectives that can be adapted as necessary, prioritizing enduring features such as habitat features over rapidly shifting species (to support biodiversity, regardless of community composition), supplementing area-based conservation with other management efforts outside of MPA boundaries (i.e., EBFM;
Link et al. 2011), performing climate vulnerability or risk assessments (to evaluate how the sites could be further impacted by climate change in the future), including sites that capture projected future habitat for the high-risk species identified here, implementing strong protective measures within new and existing sites (sensu
Jacquemont et al. 2022), and/or prioritized monitoring for anticipated changes (to identify new threats such as invasive species, or any needs for re-evaluation of conservation priorities and associated activity restrictions).
As Canada continues towards the conservation objective of 30% by 2030 (30by30 initiative—Global Ocean Alliance 2022) under the U.N. Convention on Biological Diversity (15th Conference of the Parties, 2021), the time is now to build climate change directly to the design and monitoring of MPAs networks (
O'Regan et al. 2021;
Bryndum-Buchholz et al. 2022). Sites projected to experience a rapid change in species composition could benefit from such a flexible approach to boundary design, or the design of the network itself may need to change to reflect that efficacy based on the contemporary distribution of conservation priority species will not translate into the efficacy in the long term (
Tittensor et al. 2019). Implementing predictions of how climate change may influence priority species within the Scotian Shelf-Bay of Fundy bioregion can help to improve the design and ultimately help “future-proof” the network design as best as possible; however, as our results corroborate, the success of conservation efforts may ultimately be predicated on the ability to implement strong climate action through reduced emissions (
Penn and Deutsch 2022).