Study area and design
A regional community-driven research network (
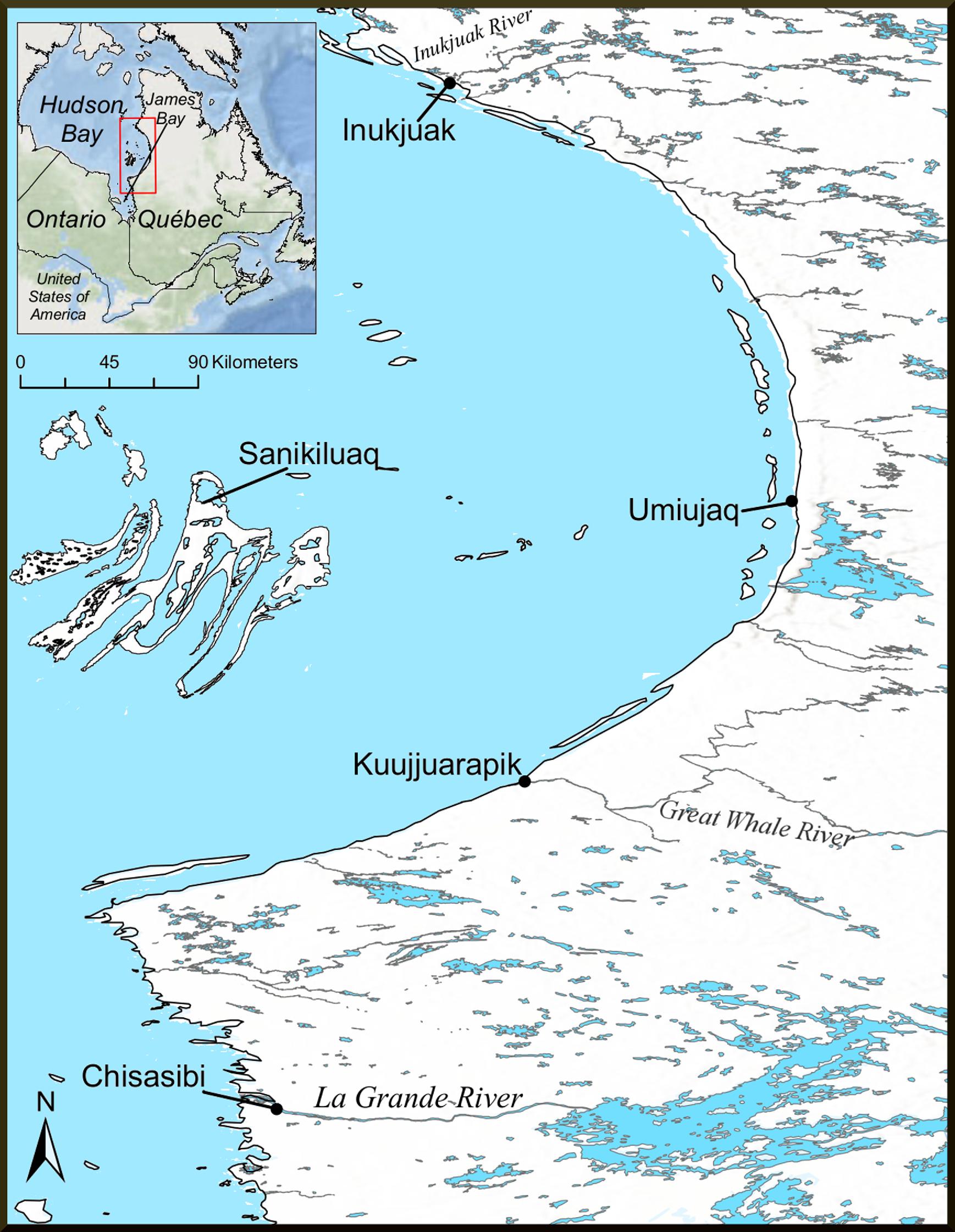
Arctic Eider Society 2024) participated in a study of metal concentrations in coastal food webs of four communities on east Hudson Bay (Sanikiluaq, Kuujjuarapik, Umiujaq, and Inukjuak) and the community of Chisasibi on east James Bay (
Fig. 1). The southern portion of the study area is within Eeyou Istchee, the traditional territory of the Cree of Quebec, while the northern portion comprises Inuit homelands of Nunavik and Nunavut. Hudson Bay and James Bay are polar marine ecosystems with winter ice cover and ice-adapted marine animals typical of more northern latitudes including polar bear, Arctic cod (
Boreogadus saida), and ringed seal (
Stewart and Barber 2010). The region is also important for migratory birds, which use coastal habitats during the spring and summer seasons. More details about the environmental characteristics of the Hudson Bay complex can be found in
Stewart and Lockhart (2005),
Stewart and Barber (2010), and
Keller et al. (2014).
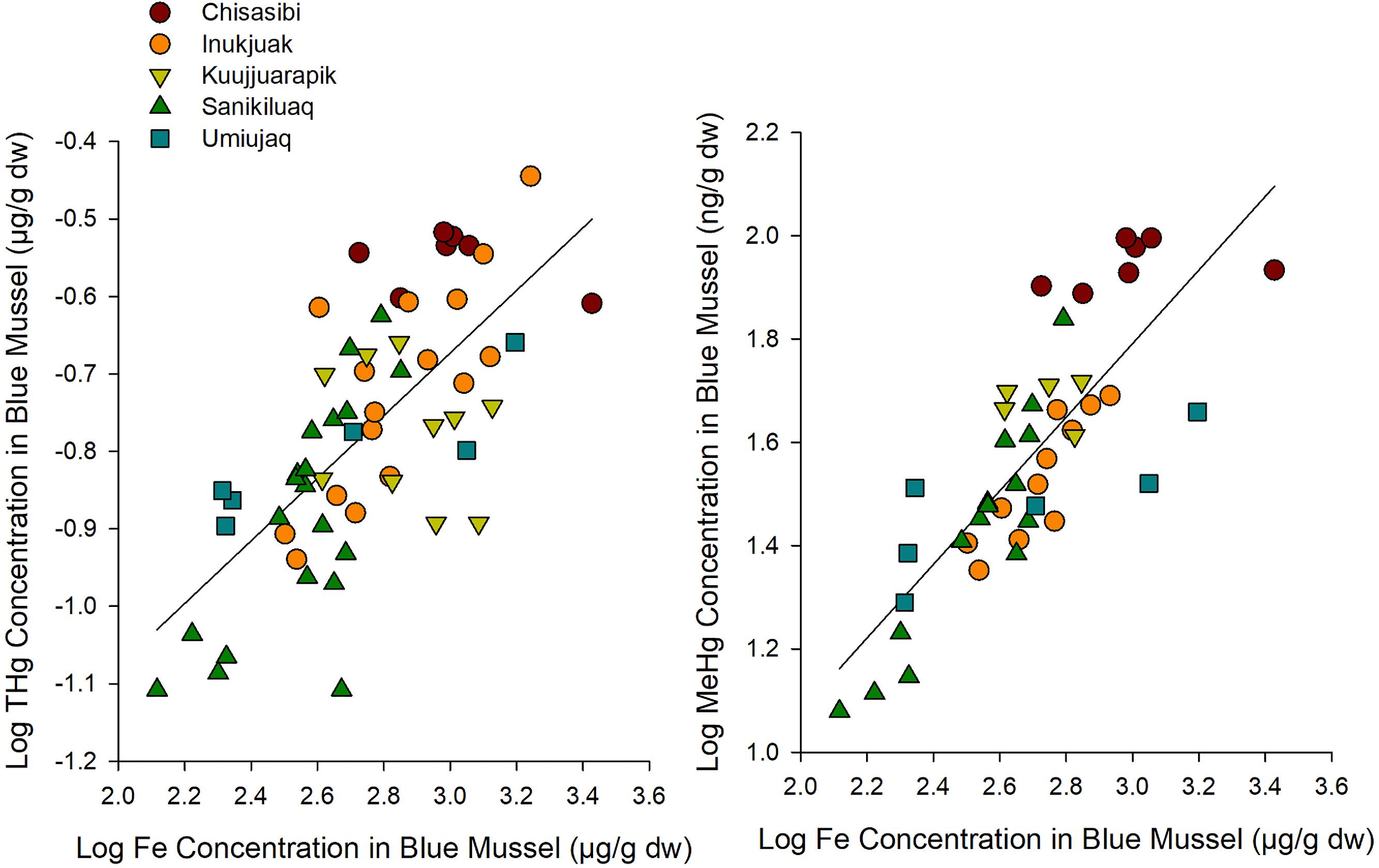
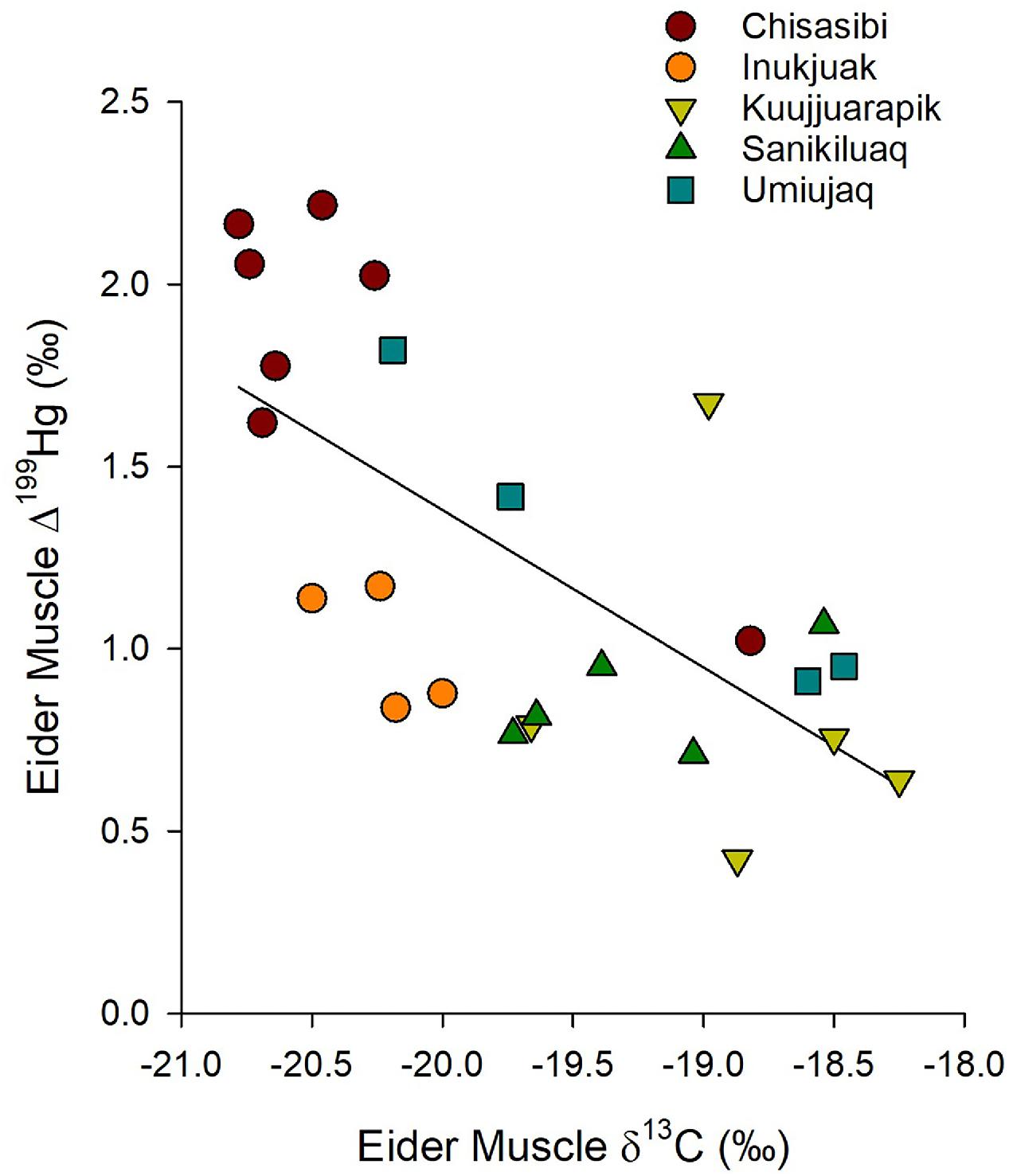
Spatial patterns of mercury bioaccumulation were investigated using two bioindicator species found throughout the study area: blue mussel (
Mytilus edulis) and common eider (
Somateria mollissima). Blue mussel is a sedentary filter-feeding invertebrate that feeds predominately on pelagic rather than sediment carbon (
Rosa et al. 2018), and it is widely studied around the world to monitor pollution (
Beyer et al. 2017). Common eider is a diving sea duck that feeds on molluscs (including blue mussel), crustaceans, and echinoderms in benthic habitats (
Ouellet et al. 2013). Common eiders in east Hudson Bay and east James Bay are the
sedentaria subspecies, which are non-migratory and remain year-round by overwintering in open water of polynyas and leads of ice particularly around the Belcher Islands (
Mallory et al. 2004;
Goudie et al. 2020). In spring, the eiders disperse to breeding grounds on islands and in coastal areas of James Bay and Hudson Bay, where they remain for much of the open-water season to breed, rear their young, and molt (
Goudie et al. 2020).
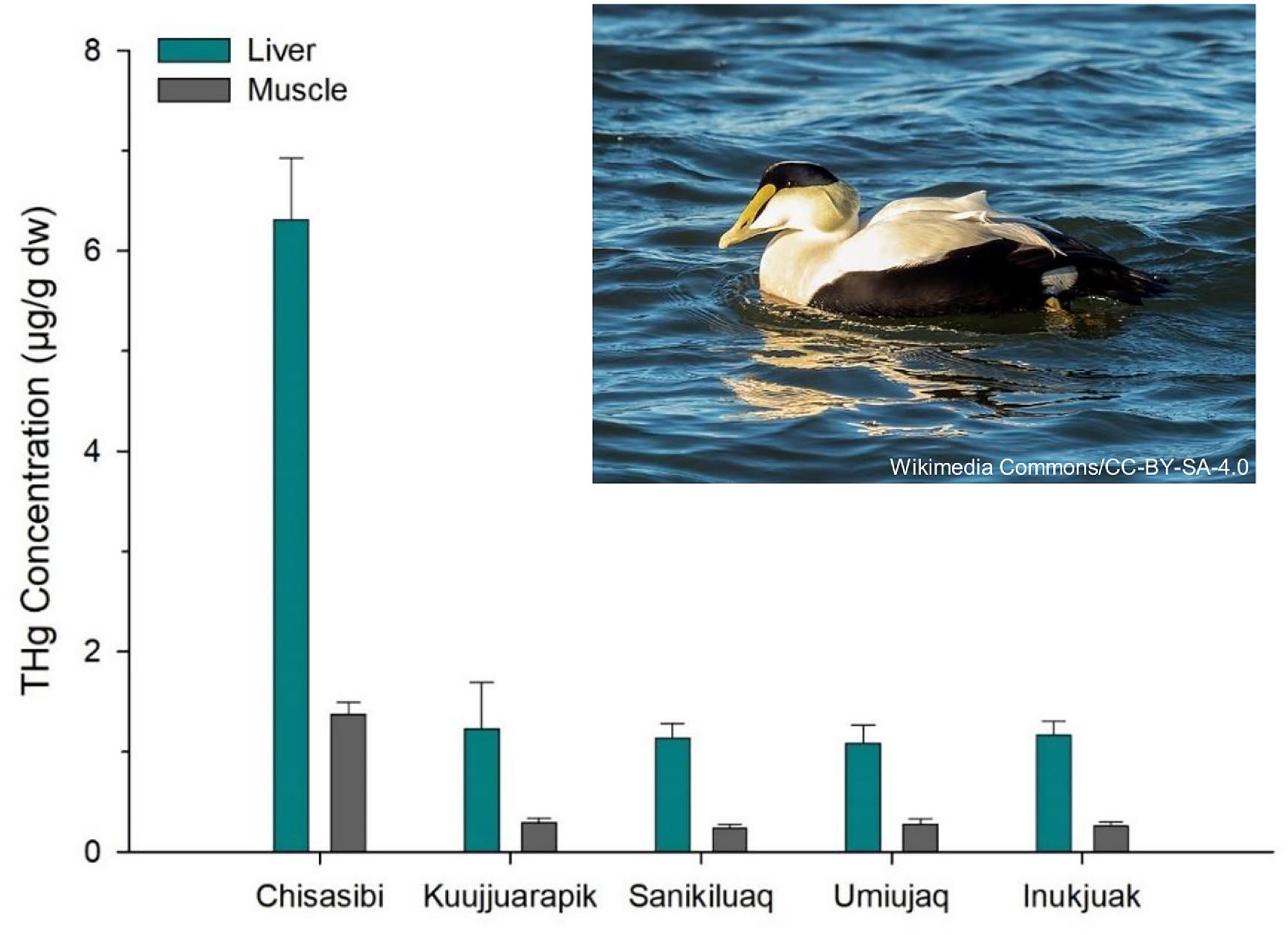
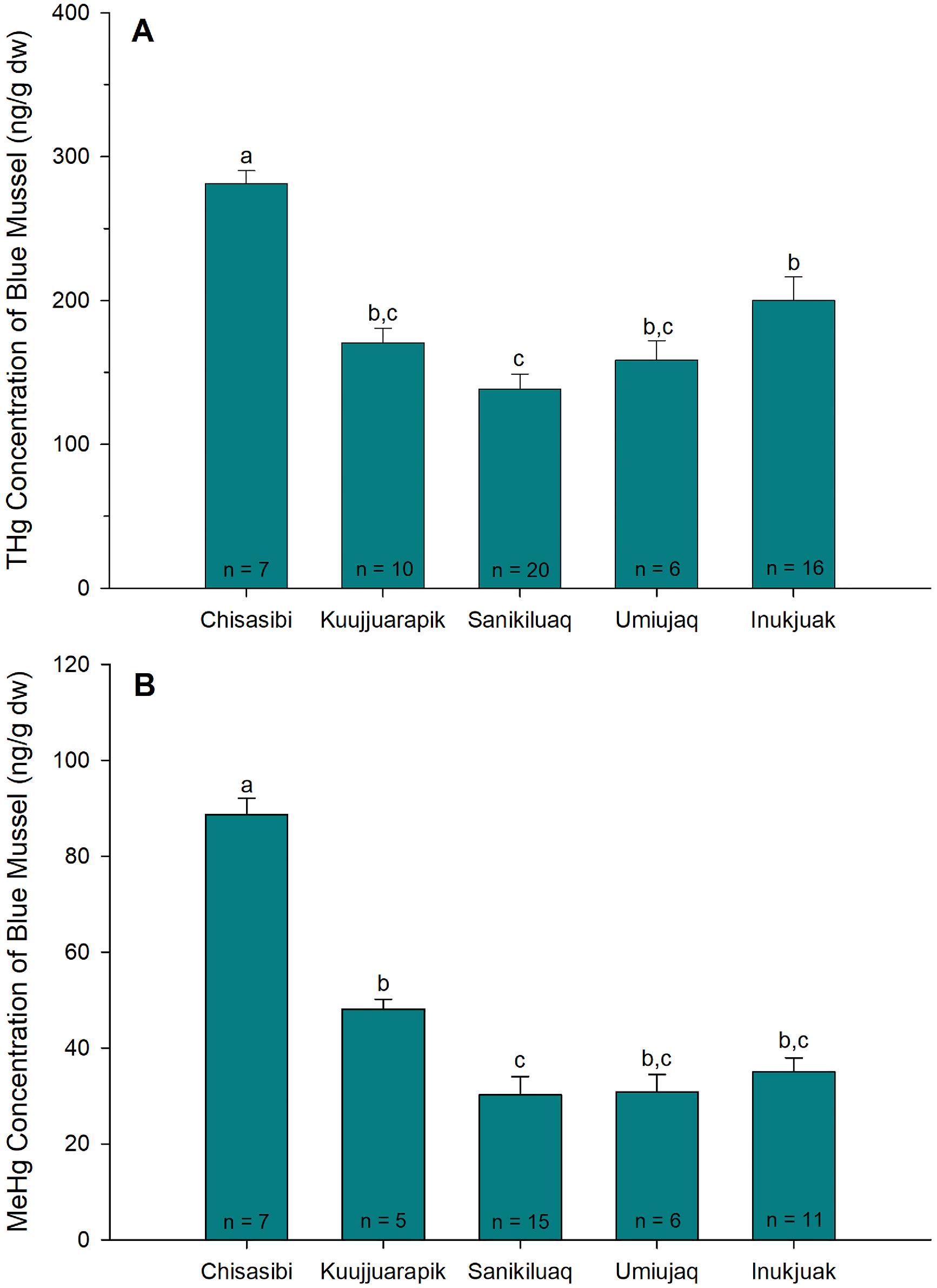
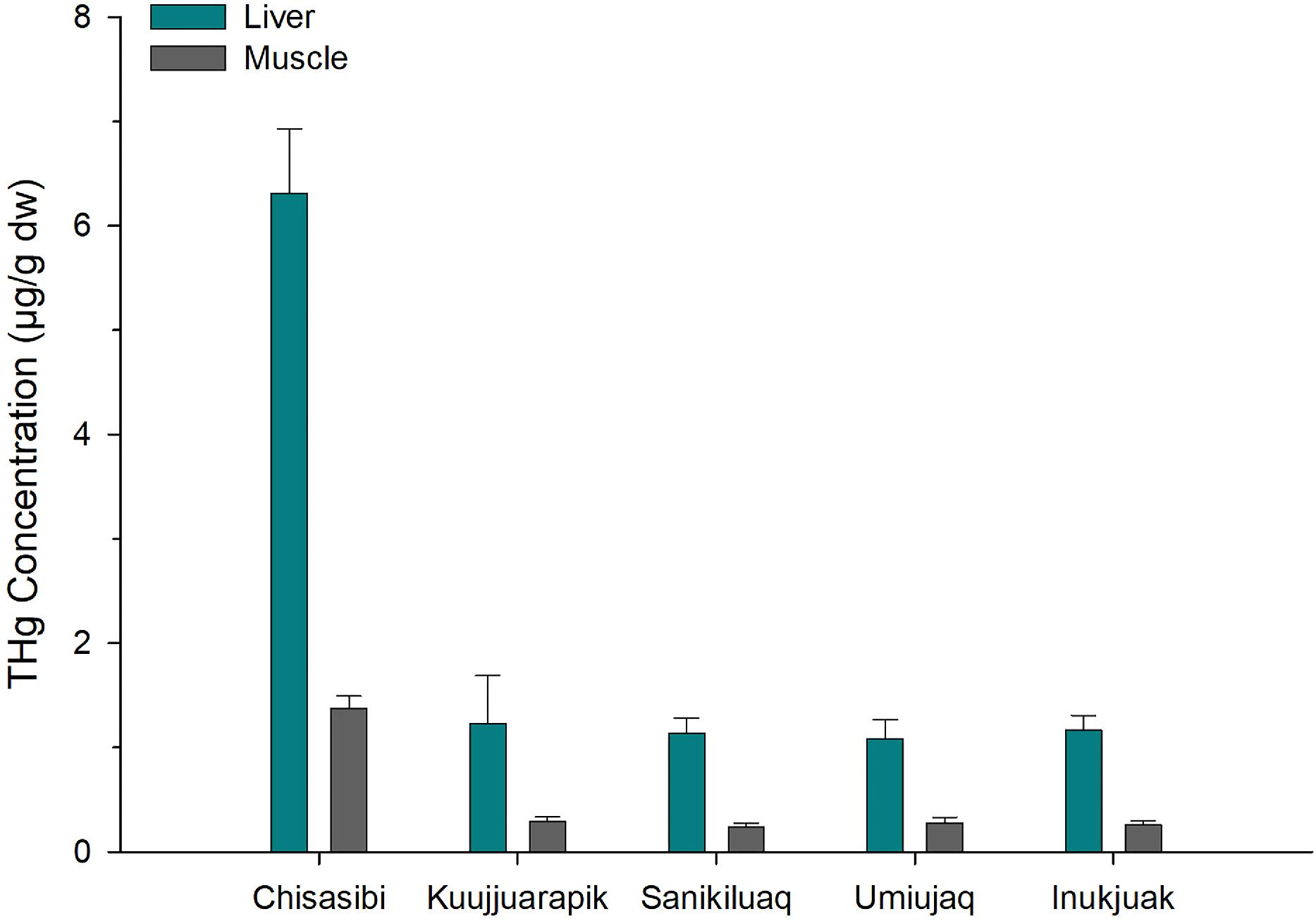
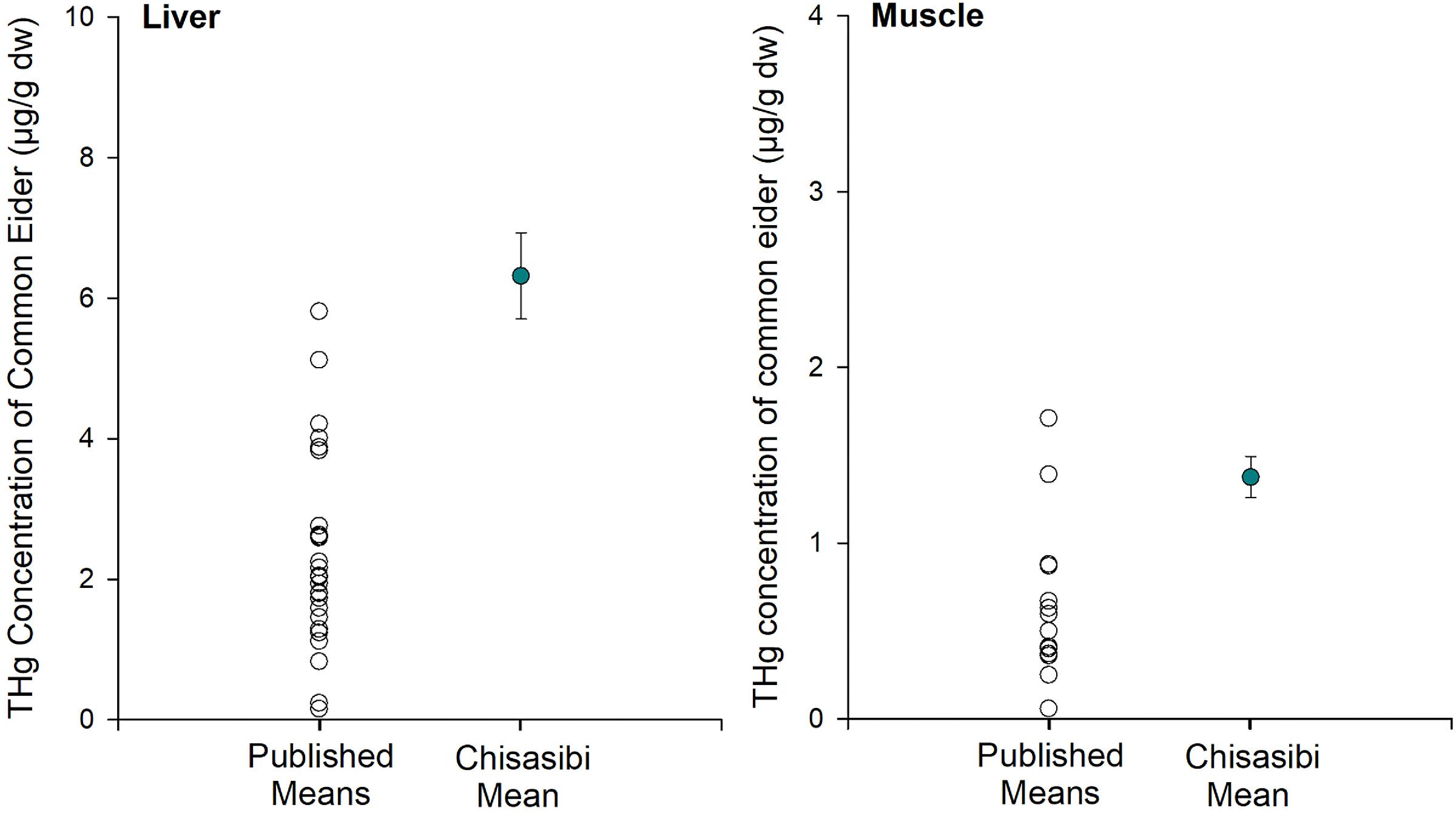
Blue mussels and common eiders were collected by local hunters near each of their communities. The collections were completed during the open water season (June to early December) between 2014 and 2017, though additional common eiders were also collected at Chisasibi in 2021 to increase the sample size. Blue mussels were collected by hand or trawl net from several near-shore sites per community. Ten individual mussels were collected per site to form a composite sample, and the number of composite blue mussel samples varied from 6 to 20 per community, representing sampling of multiple sites over a 2- or 3-year period. A total of 59 composite blue mussel samples were obtained for the study. Blue mussels were frozen whole in polyethylene zip bags and shipped to the National Wildlife Research Centre (NWRC, Ottawa, Canada) for laboratory processing. Common eiders were captured with a firearm from sites near each community, with sample sizes ranging from 14 to 24 birds over a 2- or 3-year period, except for Inukjuak where only 4 birds were collected in 1 year. Breast muscle and the liver were removed from each bird after collection, frozen in polyethylene zip bags, and shipped to NWRC for laboratory processing. A total of 77 common eiders were obtained for the study. The repeat sampling of blue mussel and common eider at each community incorporated both local spatial variation and inter-annual variation.
Laboratory methods
In the laboratory, biotic samples were homogenized and freeze-dried prior to chemical analysis. Blue mussels were de-shelled, and the whole-body soft tissue of 10 individuals from a site was homogenized with an Omni Mixer with wide window shafts to form a composite sample. Individual common eider livers were also mechanically homogenized while eider muscle samples were manually homogenized in their containers after drying. All samples were freeze-dried for a minimum of 48 h.
Total mercury (THg) was measured in blue mussel, eider liver, and eider muscle with a Direct Mercury Analyzer (Milestone Inc., Shelton, Connecticut, USA) at NWRC. For a small subset of samples (liver and muscle of 14 eiders collected in 2021), THg was measured following wet digestion by atomic absorption spectrometry at RPC laboratories (Fredericton, New Brunswick, Canada) due to lab closure at NWRC. Analytical duplicates were measured every 10 samples with a relative percent difference (RPD) of <10% (
n = 14). During the project, six types of certified reference materials (CRMs) were analyzed showing mean recoveries of 86%–102% (
n = 108; see
Rohonczy et al. 2024, for details). An inter-laboratory comparison of eider mercury results from RPC with those from NWRC showed high precision (mean RPD = 10%,
n = 10).
Methylmercury was also measured on a subset of blue mussel samples (n = 44) with a cold-vapour atomic fluorescence spectrometer at NWRC or Flett Research Ltd (Winnipeg, Manitoba, Canada). Samples were digested prior to analytical detection with nitric acid at NWRC and with KOH-methanol at Flett Research Ltd. Duplicate analytical measurements had a mean RPD = 4% (n = 7). Recoveries of CRMs averaged 90% (range of 85%–97%) for both DORM-3 fish protein (n = 4; National Research Council of Canada) and NIST 2976 mussel tissue (n = 6; National Institute of Science and Technology).
A suite of 24 elements was measured in blue mussel samples (n = 59) with an inductively coupled plasma mass spectrometer (ICP-MS) at NWRC or the Alberta Institute of Technology Futures (Vegreville, Alberta, Canada). Only elements that were above analytical detection (Ag, As, Ba, Cd, Co, Cu, Fe, Li, Mn, Mo, Ni, Pb, Se, Sr, U, V, and Zn) are reported here. Analytical duplicates were measured every 10 samples with deviations generally <10%. Mean recoveries of individual elements ranged from 92% to 105% for measurement of 2–8 types of CRMs, with the exception of Pb, which had a lower mean recovery (77%). Details on the quality assurance and quality control of measurements for individual elements are provided in the supplementary materials (Table S1).
Blue mussels and common eider muscle were analyzed for nitrogen, carbon, and sulfur isotope ratios on a DeltaPlus XP isotope ratio mass spectrometer interfaced to a Vario El III elemental analyzer via a Conflo II at the Ján Viezer Stable Isotope Laboratory at the University of Ottawa (Ottawa, Ontario, Canada). Isotope ratios are reported in delta notation as the per mil (‰) deviation from N2 (air) for nitrogen, Vienna Pee Dee Belemnite for carbon, and Vienna Canyon Diablo Troilite for sulfur. Analytical precision is typical <0.2‰ for carbon and nitrogen stable isotopes and <0.3‰ sulfur stable isotopes.
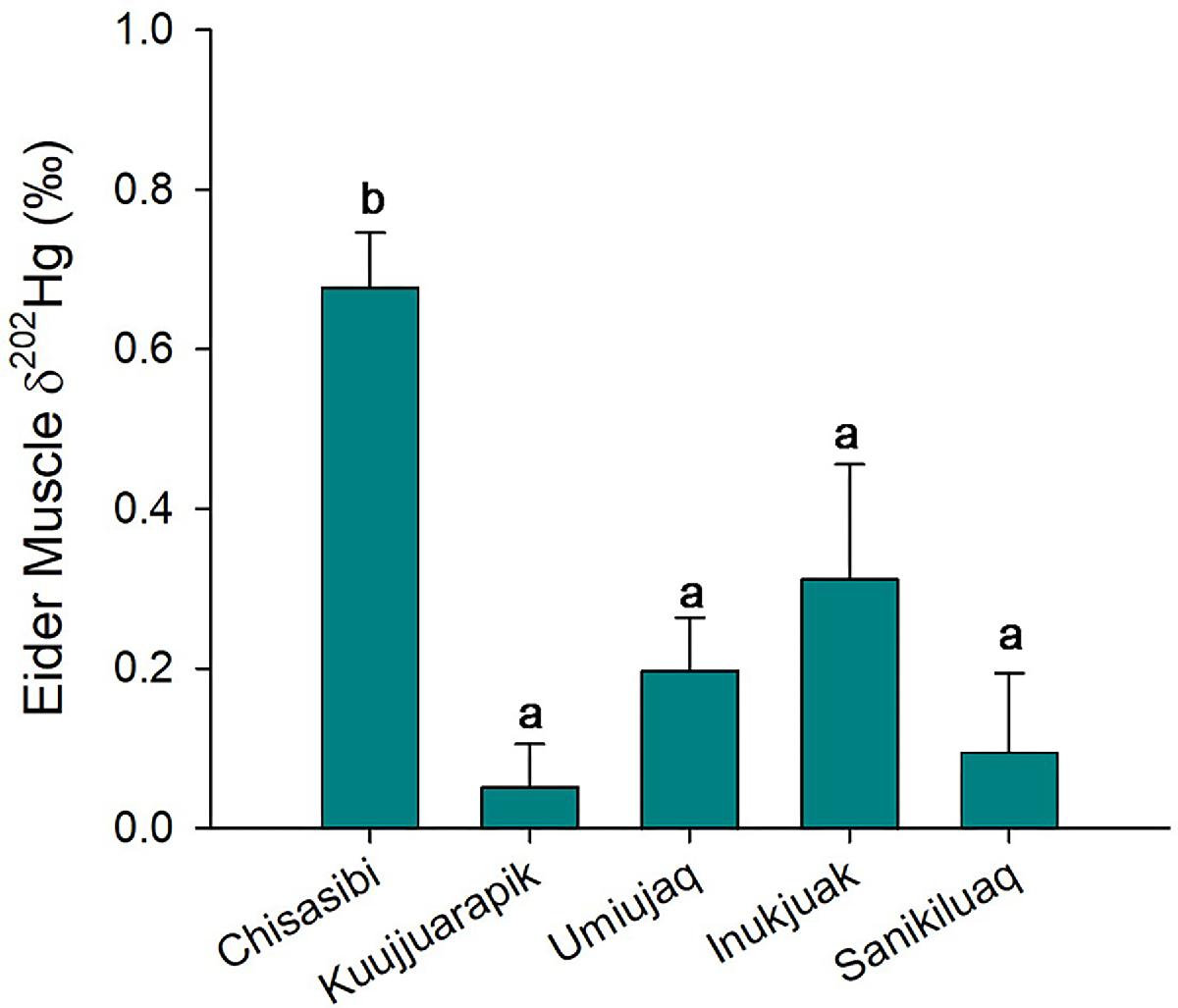
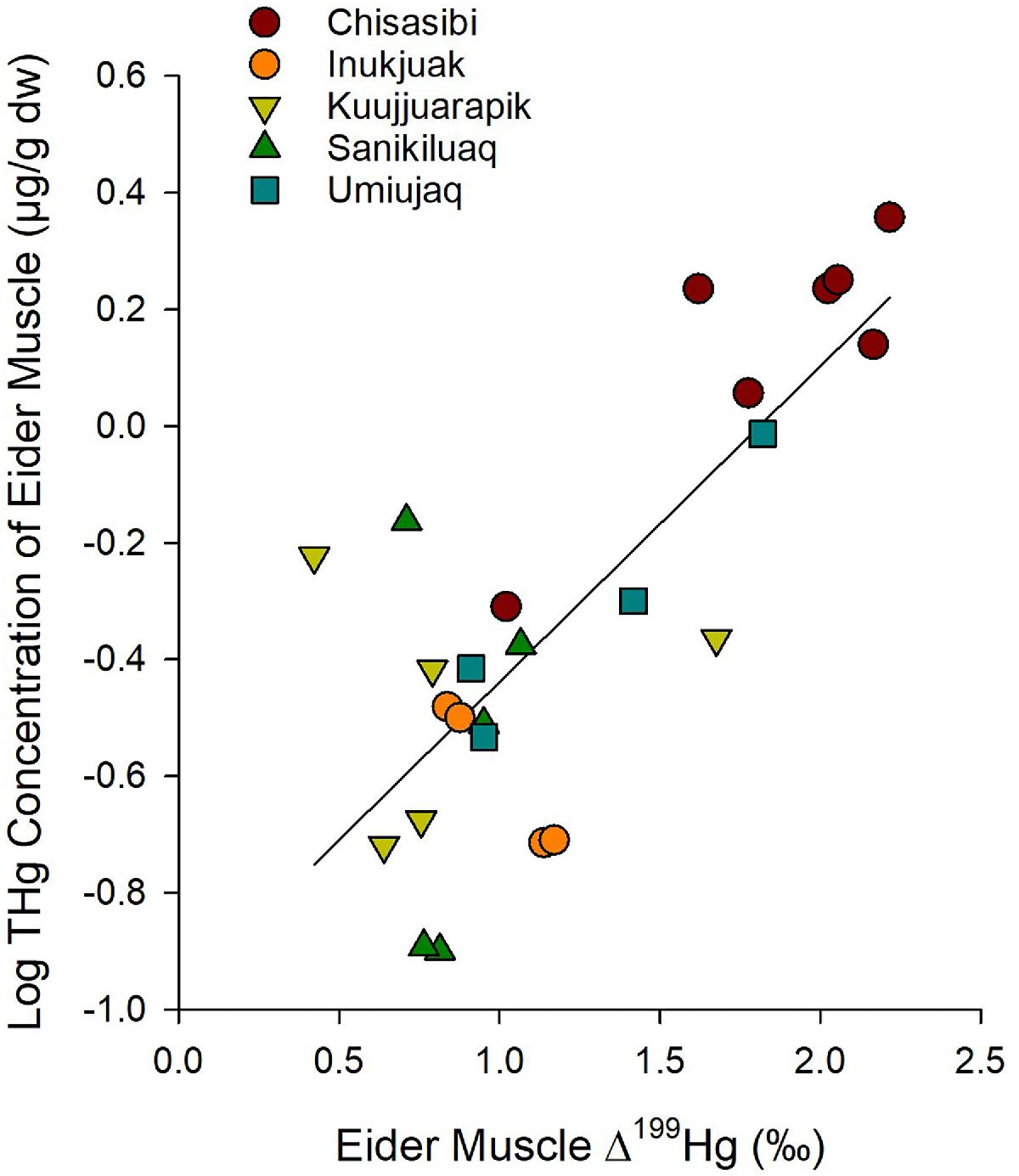
Mercury stable isotope ratios were measured on a subset of common eider muscle samples (n = 25) with a Nu Plasma II multi collector-ICP-MS instrument in the Water Quality Centre at Trent University (Peterborough, Ontario, Canada). Samples were digested using 3 mL of aqua regia in a 40 mL amber glass vessel by heating on a hot plate at 120 °C for 4 h. An ESI hydride ICP hydride generation system was used to generate a Hg0 vapor by the quantitative reduction of Hg(II) in solution using stannous chloride (3% w/v in 1 M HCl). Mercury isotope ratios are reported as the per mil deviation from the xxxHg/198 Hg ratio of the NIST SRM 3133 mercury standard. Results for replicate measurements of the RM-8610 cinnabar standard are provided in the supplementary materials (Table S2).