This is the first comprehensive study investigating how the soil properties associated with a natural carbonatite deposit influence the plant and soil microbial communities in a Great Lakes–St. Lawrence forest ecosystem. As with most of this part of North America, the deposit is covered by a layer of overburden from past glaciation; however, despite this it was found that some areas above the deposit had an identifiable soil chemical signature indicative of SRC. The variable soil chemistry resulted in differences in plant community structure. This was demonstrated in areas of moderate SRC influence that had different plant species than those areas of low/negligible influence. However, the influence of SRC did not appear to be a dominant factor in shaping soil microbial communities. Nevertheless, several bacterial and fungal OTUs were found to be differentially abundant between areas of high and low/negligible SRC influence.
SRC, soils, and the influence of the deposit on the overlying ecosystem
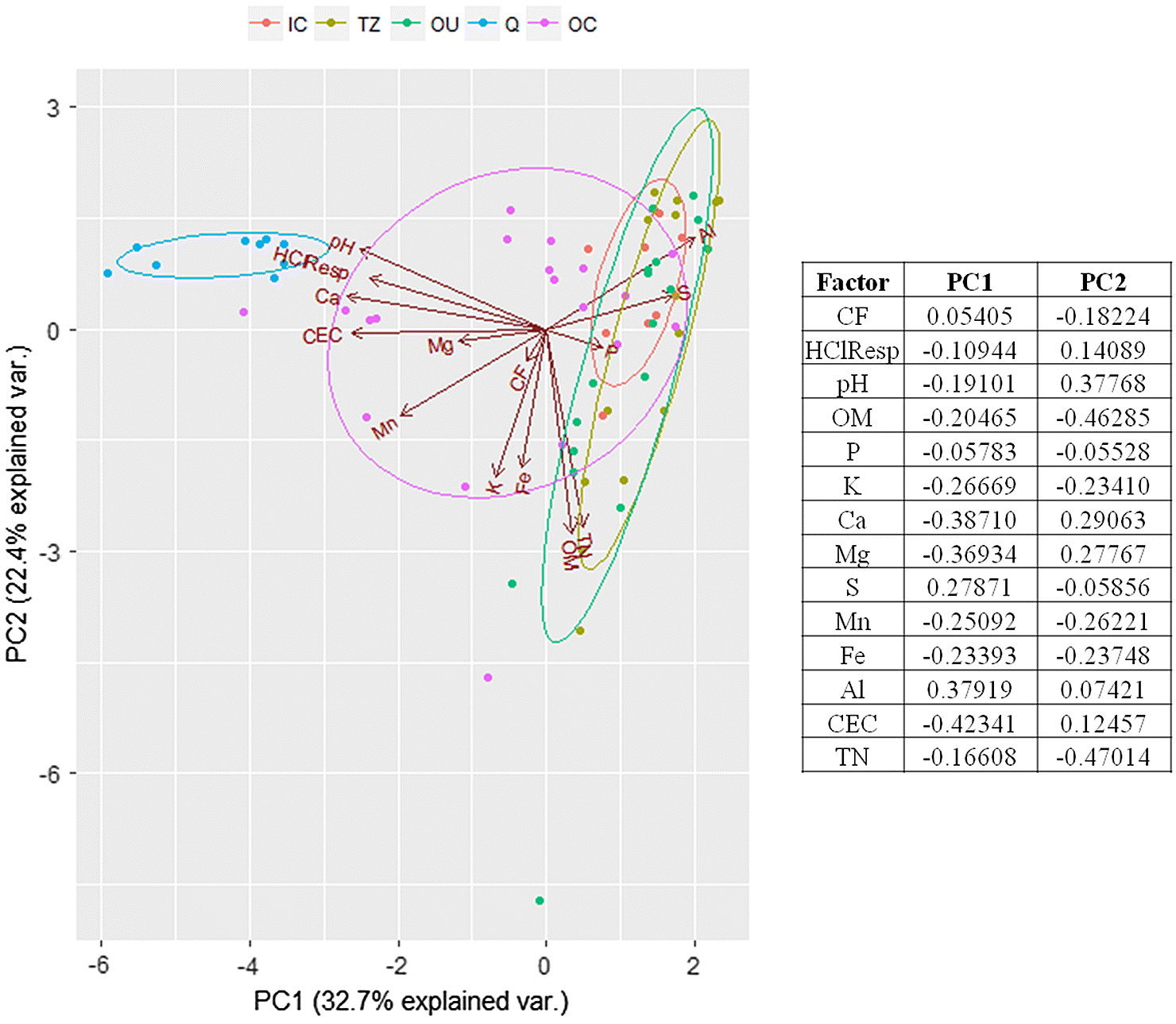
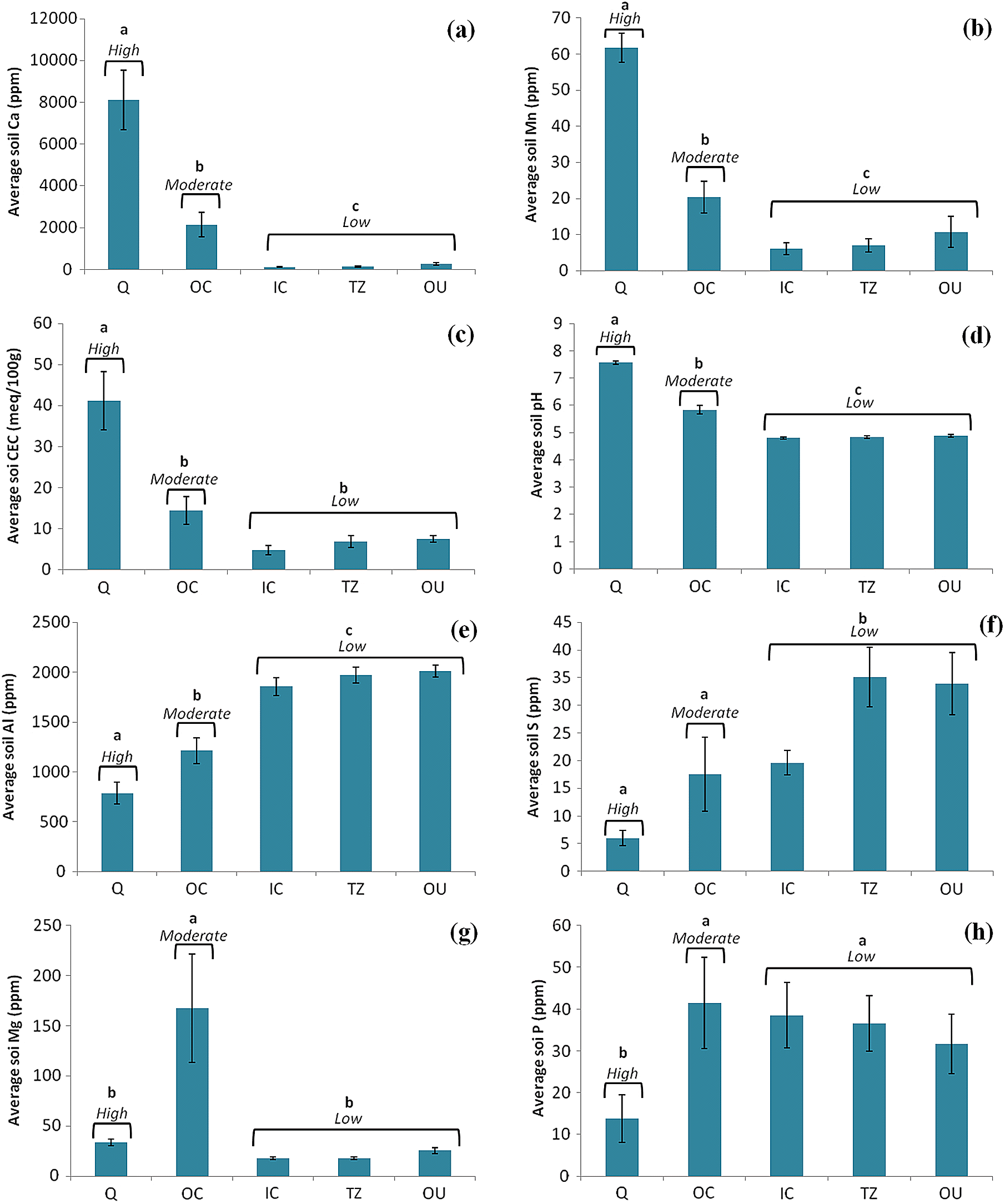
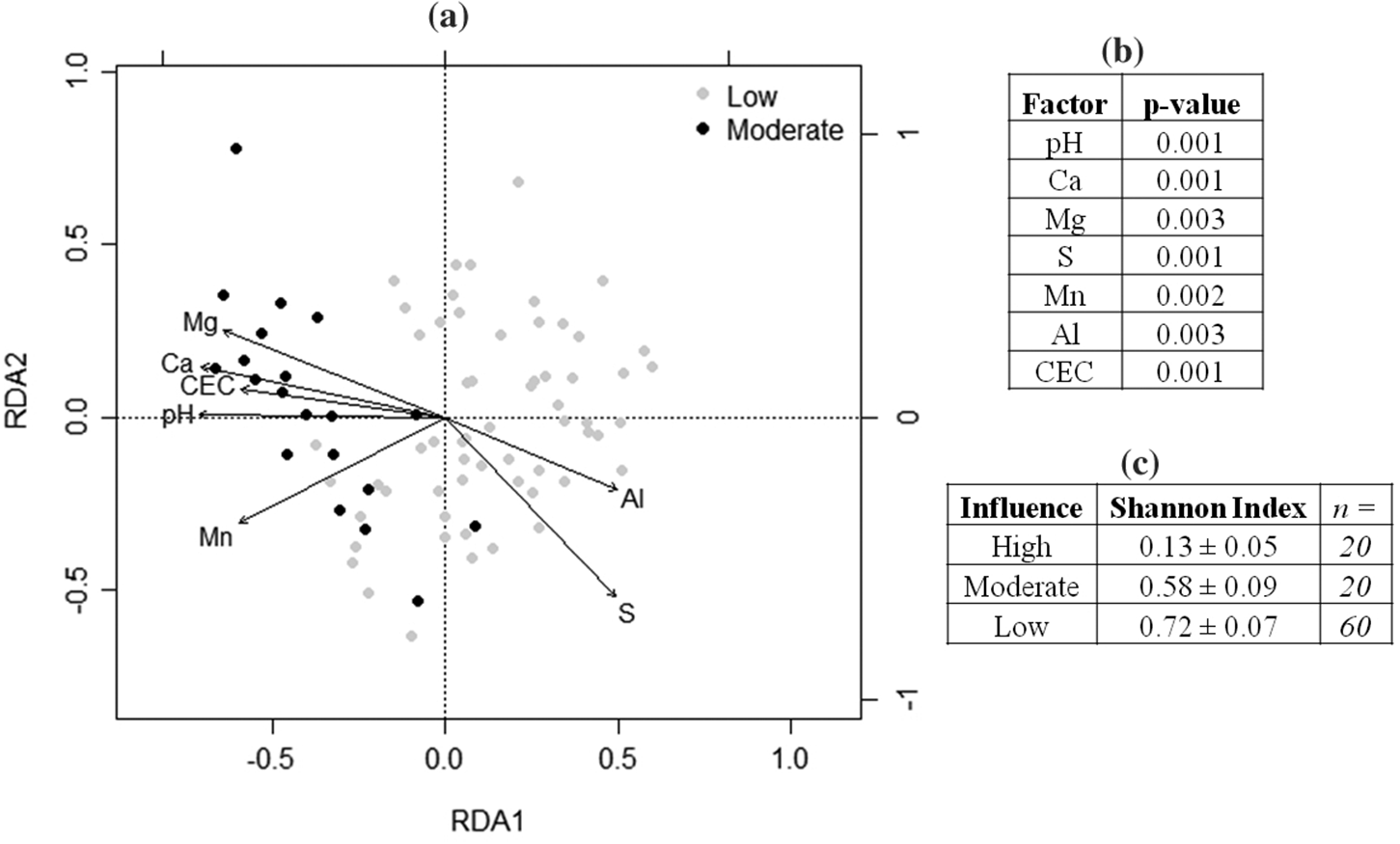
Several factors indicated an influence of the SRC deposit on soil chemistry, as revealed by a comparison between zones. Because CEC, Ca, Al, S, Mn, and pH values of the OC soils (moderate influence) were often more similar to those of the exposed SRC comprising the Q samples (high influence) than to those of other zones (low/negligible influence), the increases in these parameters were considered to be a signature of SRC. The primary mineral of SRC is calcite, and like many other carbonatite deposits it is accompanied by a variety of nutrient-bearing accessory minerals such as biotite and apatite (
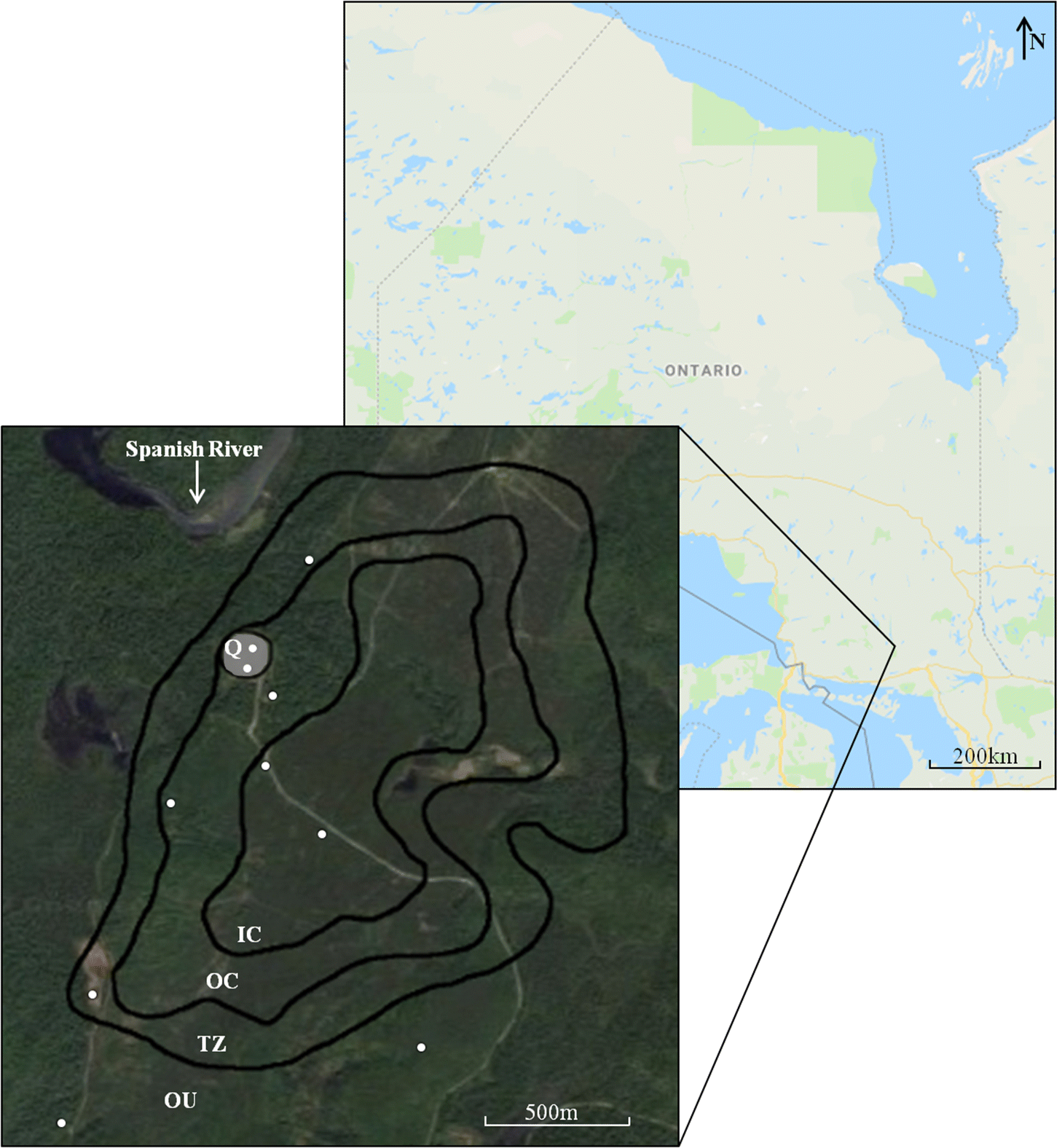
Sage 1987). Despite there being an overburden layer of variable depth (0 m at the quarry to at least 56 m elsewhere) covering the deposit, it appears that these minerals are affecting some of the sampled soils.
Kunzendorf and Secher (1987) made a similar suggestion for the Qaqarssuk carbonatite deposit in Greenland, which is covered by soils formed from alluvial deposits that are enriched in Nb and P originating from the underlying carbonatite. Thus, it can be inferred that in some areas the depth of the overburden is shallow enough that weathered SRC exerts an effect on the soils. This needs to be validated as there is not a reliable overburden map for the whole deposit. It is assumed that weathering of SRC fragments and their incorporation into the soils is responsible for the SRC signature identified in the OC sites surveyed here. This assumption is justified based on the similar soil chemistry between OC and Q sites, and by previous works demonstrating carbonatite influence on soil chemistries (e.g.,
Kunzendorf and Secher 1987;
Vestin et al. 2006;
Ahn et al. 2014). However, this study has not verified the presence of carbonatite minerals in the soil outside of the quarry (Q). Although the soil chemistry of the OC sites was similar to that of the Q, many values were significantly lower in the OC and it is likely that the overburden present in the OC dilutes the SRC influence. Additionally, topography, hydrology, and vegetation presumably play important roles in the transfer of carbonatite elements into the glacial till soil. For instance, where the deposit is near the surface at higher elevations, a signature of SRC might be found both in soils at these locations and in nearby topographical lows where downward water movement through the soils would have transported some minerals or elements. Nutrient translocation by the action of plants could also have occurred and a mechanism for this is provided by the nutrient-uplifting hypothesis of
Jobbágy and Jackson (2004), which posits that trees (and other vegetation) are active in transporting lithosphere-derived nutrients to the surface of soils. This translocation occurs when lithosphere nutrients are incorporated into above- and below-ground plant biomass, and subsequently released when the plant organic matter decomposes close to the soil surface.
The soil chemistry differences above the deposit indicate that in some areas SRC has had a strong influence on the soils. Above-ground differences in plant community composition are therefore likely linked to changes in soil chemistry. Several plant species unique to the moderate influence sites (i.e., the OC) are typically those colonizing areas post-disturbance or that are tolerant to alkaline soil pH. For instance,
C. peregrina has been considered a weed, and post-fire disturbance conditions are favourable for its growth (
Hall et al. 1976). As none of the outer core sites displayed signs of recent disturbance (e.g., being dominated by younger trees or displaying signs of fire damage on older wood), it is likely that the abundance of ruderal species in the OC is not due to a disturbance event but to the presence of SRC. With the stark differences in soil pH between areas of moderate and of low/negligible SRC influence, it appears that one of the main effects of the deposit has been the creation of ecological microhabitats (“islands”) of more basic soil pH in what would otherwise be a large surrounding area (“sea”) of more acidic soils. The more basic the soils, the greater likelihood they will be colonized by species tolerant of soil pH changes, i.e., ruderal species. Based on the Shannon diversity indices, where areas of moderate influence were seen to have lower indices than those areas without SRC influence, we hypothesize that the altered soil chemistry caused by SRC constrains the abundance and diversity of the plant species present in these areas. The data collected regarding tree species show the same trend whereby in moderate influence sites there were fewer tree species and fewer individuals than in low/negligible sites (
Table S3). This might be due to the soil chemistry restricting the types and number of plant species and (or) the spatial separation from areas where more alkaline-adapted species are present and could serve as seed sources. These species could also be less tolerant of the higher, potentially toxic Al concentrations found in the more acidic soil conditions. A similar situation whereby the bedrock and soil chemistry controlled the distribution of plant species was found in a broad survey of rock habitat vegetation performed by
Tyler (1996). To verify this hypothesis, the soil pH across the deposit area could be assessed, and the areas of higher pH (and thus presumably under carbonatite influence) identified and surveyed for vegetation community composition. Such a study would also assist in determining the magnitude of overburden depth and hydrology/topography in expressing the effects of the carbonatite on the deposit area soils.
Although it was expected that the soil microbial communities would be significantly shaped by differences in pH or by the abundance of elements provided by the weathering of SRC’s nutrient-bearing minerals, the data did not support this hypothesis. For instance, many species of bacteria that have been associated with the weathering of carbonatite minerals (e.g.,
Burkholderia (
Uroz et al. 2011) or
Pseudomonas (
Colin et al. 2017)) were not more abundant in the moderate- or high-influence sites than in the low/negligible sites. The only bacterial OTU consistently in higher abundance in SRC-affected areas was tentatively identified as a member of the genus
Arthrobacter belonging to the family Micrococcaceae. Members of this genus have been shown to utilize a diverse set of carbon/nitrogen sources (
Hagedorn and Holt 1975). Furthermore, their ability to metabolize aliphatic amino acids and aromatic hydrocarbons (
Hagedorn and Holt 1975), as well as their nitrification activity, were found to be higher at more neutral than acidic pHs (
Brierly and Wood 2001). The ability to utilize a variety of nutritional substrates and to nitrify under the more basic soil conditions in SRC-affected areas would be beneficial to this microbial group as SRC does not contain appreciable amounts of N, and these characteristics could explain the higher presence of
Arthrobacter in areas with the SRC signature. This would be especially important in the Q, where the soils almost exclusively consist of SRC particles. In terms of the fungal OTUs, most were found to decrease in abundance with increasing SRC influence. This is in agreement with the observations made by
Rousk et al. (2010) that fungi prefer acidic soils whereas bacteria prefer neutral/alkaline soils.
The most abundant bacterial families detected in our soil samples were either saprotrophic and (or) associated with the rhizosphere of plants (
Table S4). Similarly, aside from the Pseudeurotiaceae, most fungal families were saprotrophic and (or) ectomycorrhizal (
Table S4). The identity of tree species dominant in a given area can be a strong determinant of fungal community composition (
Urbanová et al. 2015). As such, differences in leaf litter composition and (or) mycorrhizal hosts may provide an explanation for the differences seen in fungal abundances between low/negligible and the high or moderate SRC influence soils. Additionally, increasing the pH to values similar to those observed in the moderately-influenced sites has previously been reported to negatively affect the survival of ectomycorrhizae on
P. abies roots (
Lehto 1994). This suggests that the increased soil pH resulting from the presence of SRC may not be conducive to ectomycorrhizal colonization of the tree species at the deposit. Given our focus in this study on the assessment of the colonization of herbaceous plants by arbuscular mycorrhizal fungi, the colonization of trees by ectomycorrhizal fungi needs to be investigated in future studies.