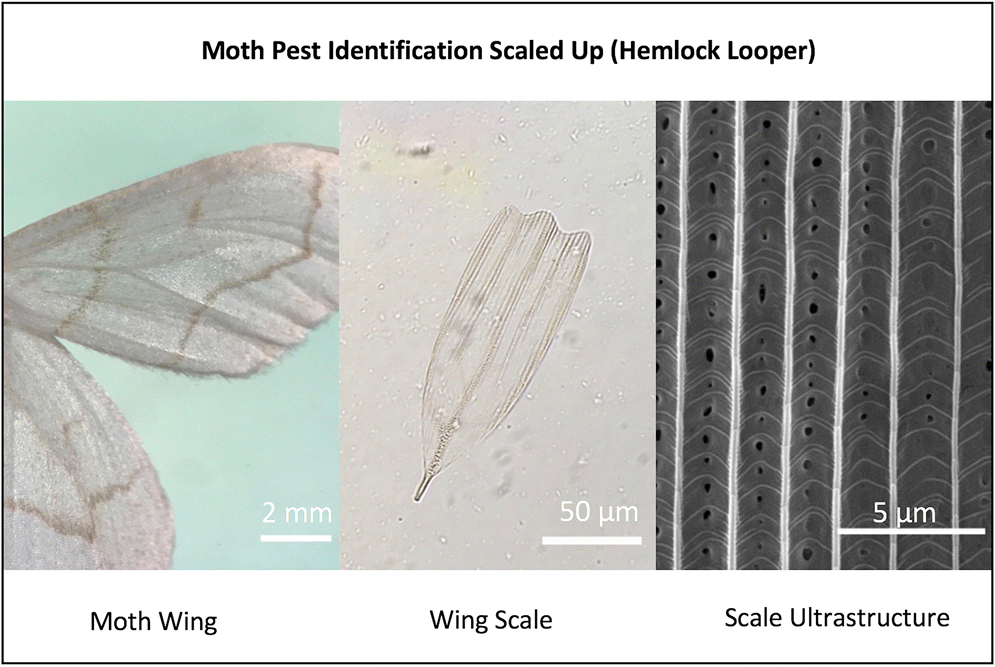
Introduction
Insect defoliators are a major source of ecological disturbance in boreal forests across eastern North America (
Volney 1996;
Zhang et al. 2014). Canadian forests are home to at least 106 species of insect defoliators, with the order Lepidoptera contributing the greatest number (70%) and some of the most destructive forest pests due to defoliation of their hosts (
Johns et al. 2016). Populations of these forest pests can increase to epidemic levels known as outbreaks. These outbreaks have generated a great deal of interest for economic and ecological reasons. Some species cause extensive damage to forests leading to severe reductions in the quality and abundance of harvestable timber resources. The economic impacts of these outbreaks can be significant. The eastern spruce budworm (
Choristoneura fumiferana Clemens; Tortricidae) outbreak of 1967–1993 affected 50 million ha of forest in eastern North America (
Sturtevant et al. 2015) with an estimated loss of 44 million m
3 of timber per year of the cycle. Economic models indicate potential losses of $10.8–15.3 billion in Atlantic Canada over the course of a cycle, depending on the severity of the outbreak (D.A. MacLean, unpublished data).
The population dynamics of spruce budworm have attracted special interest among ecologists because these populations cycle every 25–40 years over large areas. The factors that regulate and synchronize these cycles have been a long-standing interest among population ecologists (e.g.,
Swaine and Craighead 1924;
Morris 1963;
Blais 1983;
Johns et al. 2016;
Royama et al. 2017). The great length of spruce budworm cycles, however, means that relatively few cycles have been studied in detail and many of the interesting questions about the frequency of cycles—such as, how long have spruce budworm populations been cycling, what is the relationship between outbreak frequency and climate, has the geographical origin of outbreaks shifted through time—require long-term data necessitating a paleoecological approach (
Pureswaran et al. 2016).
A number of direct and indirect paleoecological methods have been attempted for reconstructing the history of past outbreaks for a variety of forest insect pests. Dendrochronological techniques pioneered by
Swaine and Craighead (1924) and
Blais (1954) have been the most widely applied and most successful so far. The most popular method is based on a comparison of ring-widths of host and nonhost tree species of the target insect from a given site. The method, however, is limited by the age of the oldest trees available, which in the case of balsam fir, the preferred host of spruce budworm, and white pine, a commonly used nonhost species, is 300–400 years. Furthermore, the method is indirect and cannot distinguish other possible agents of ring-growth suppression, such as pathogens.
The most direct approach has been to recover fossil head capsules of spruce budworm larvae from sediments, although
Davis et al. (1980) found that the capsules occurred too sporadically to provide reliable estimates of outbreak periodicity.
Bhiry and Filion (1996) recovered head capsules of spruce budworm and hemlock looper (
Lambdina fiscellaria Guenée; Geometridae) in association with insect-chewed hemlock needles dating from the mid-Holocene Hemlock Decline, but again, these head capsules were neither abundant nor consistently present from sample to sample. It is perhaps unsurprising that the direct study of head capsules in sediments has proven to be unrewarding because, unlike the abundant head capsules of chironomids whose larvae are aquatic, the terrestrial larvae of lepidopterans are unlikely to fall into lakes and become incorporated into sediments as fossils.
Simard et al. (2002,
2006) devised the technique of using species-identifiable frass to determine past outbreaks of spruce budworm; however, the frass did not always preserve in peats, resulting in fragmentary records. Thus, whereas some of the existing methods can produce useful century-scale records, no method has been able to produce detailed records of outbreaks on a millennial scale.
Recently,
Navarro et al. (2018) introduced the novel use of moth wing scales to reconstruct past outbreaks of spruce budworm. Because wing scales are made of chitin, which is known to preserve well in lake sediments and peats, the technique offers the potential of producing detailed records of outbreaks over millennial time scales.
Navarro et al. (2018) used linear discriminant analysis based on shape and other characteristics of wing scales as viewed by light microscopy to identify scales from spruce budworm, hemlock looper, and forest tent caterpillar moths (
Malacosoma disstria Hübner; Lasiocampidae). Their analysis was able to correctly discriminate 68% of spruce budworm scales.
We have been independently developing the use of moth scales to reconstruct past outbreaks of forest moth pests. In this paper we examine the potential of using moth-scale ultrastructure to discriminate between the main species of moth pests of eastern North America. The taxonomic potential of wing-scale ultrastructure has been long recognized (
Downey and Allyn 1975;
Ghiradella 1984,
1991). Scanning electron microscopy (SEM) of moth-scale ultrastructure has been used to discriminate between families (
Simonsen 2001;
Simonsen and Kristensen 2001) and between species (
Yang and Zhang 2011) of lepidopterans. Navarro et al.’s (2018) analysis included three species: spruce budworm, hemlock looper, and forest tent caterpillar. Our analysis builds upon those three species by including an additional two species: eastern blackheaded budworm (
Acleris variana Fernie; Torticidae) and jack pine budworm (
Choristoneura pinus Freeman; Torticidae). Additionally, we conducted analyses to determine if we can differentiate between male and female moth scales for each of the five species.
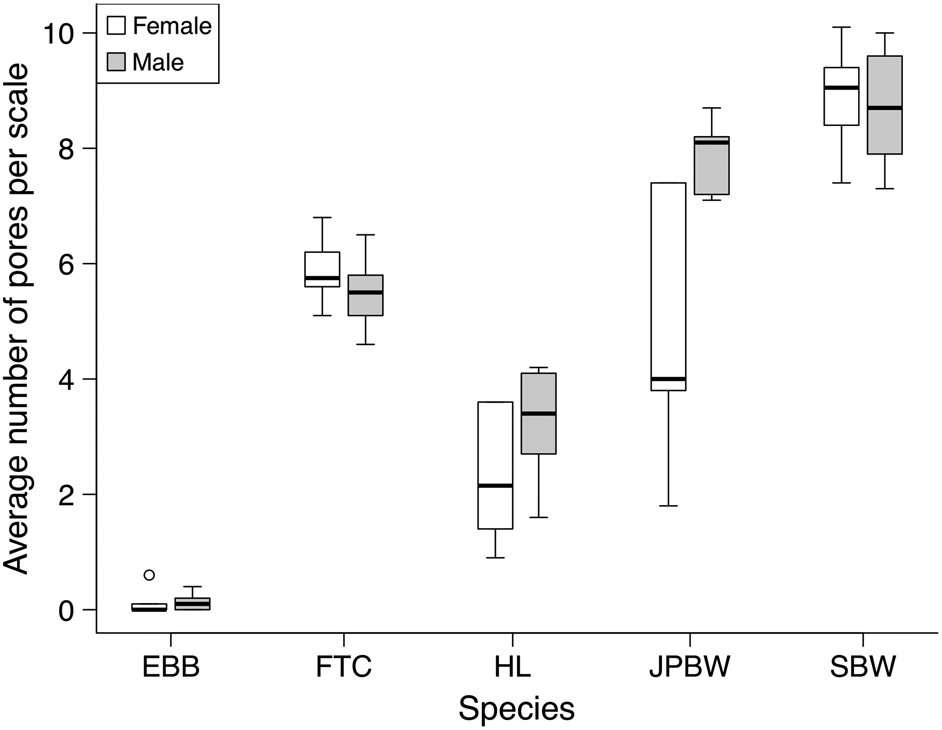
Rhainds and Heard (2015) found that swarms formed during SBW outbreaks are female dominated. Thus, identification of predominately female scales in the fossil record could indicate the presence of swarms, typical during periods of high population densities. Our approach is to use SEM images of scale ultrastructure for qualitative morphological and quantitative morphometric comparisons between the five outbreak species.
Materials and methods
Specimens
We examined the wing-scale ultrastructure of the five most common moth pest species in eastern North America: eastern spruce budworm, hemlock looper, forest tent caterpillar, jack pine budworm, and eastern blackheaded budworm. Six males and six females of each species were examined.
Male spruce budworm moths were obtained from pheromone traps in Dalhousie, New Brunswick. Female spruce budworm, jack pine budworm, and blackheaded budworm moths were provided by the Great Lakes Forestry Centre, Sault Ste Marie, Ontario. Male jack pine budworm and blackheaded budworm specimens were caught in pheromone traps located in New Brunswick and Cape Breton, Nova Scotia, respectively. Hemlock looper specimens, both male and female were obtained from the Laurentian Forestry Centre, Quebec. The Forest Management branch of the Department of Energy and Resource Development, New Brunswick, donated forest tent caterpillar moths.
Preparation and examination of wing scales
We used forceps to scrape scales onto carbon-taped stubs separately from the dorsal side of a forewing and corresponding hindwing for each individual. The stub-mounted scales were then sputter-coated with gold for conductivity using an Edwards S150A coater. We collected secondary electron images of scales at a magnification of 5000× at the University of New Brunswick Microscopy and Microanalysis Facility with a JEOL JSM-6400 Scanning Electron Microscope using an accelerating voltage of 15 kV. Images were acquired using a Digiscan II controlled by Gatan Digital Micrograph software.
Wing scale comparisons
Using terminology from
Downey and Allyn (1975) and
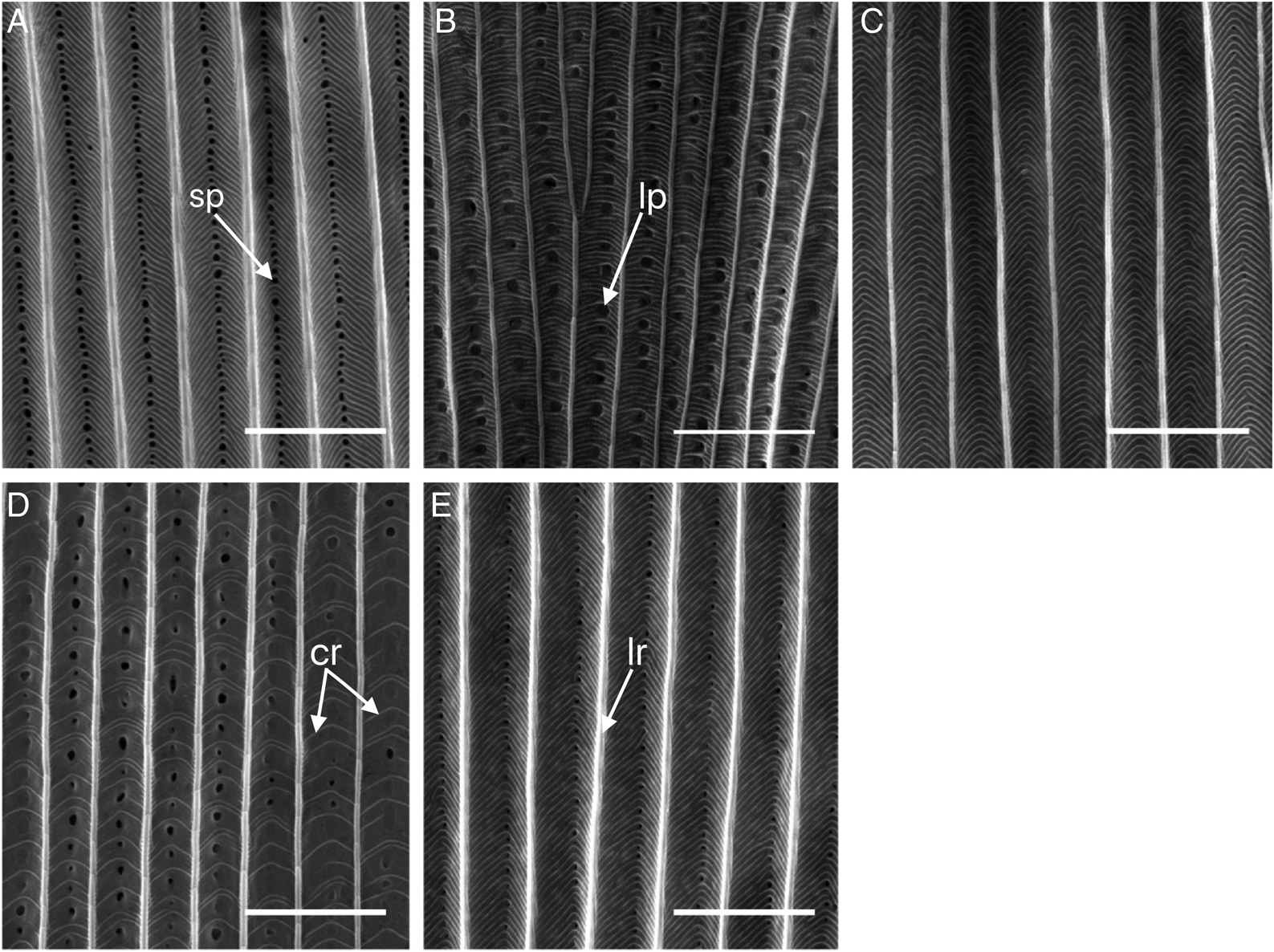
Ghiradella (1991), we first examined scales for differences in qualitative ultrastructure traits, including spacing of longitudinal ridges and cross ribs and occurrence of pores between longitudinal ridges (
Fig. 1). We measured several ultrastructural traits for quantitative analysis. To determine the uniformity of cross-rib spacing, we counted the number of cross ribs within a selected 5 μm central section of the scale surface and measured the distance between them. Pores are a prominent feature of many scales. We counted the number of pores in a selected 5 μm section and measured five pores from that section for pore height (diameter parallel to longitudinal ridges), pore width (diameter perpendicular to longitudinal ridges), and calculated the area of individual pores using the formula:
A =
πab, where
a is the radius along the horizontal axis and
b is the radius along the vertical axis of the pore. If pores were absent in the 5 μm section, but present elsewhere on the SEM image, measurements were taken for up to five randomly selected pores. We also measured the width of longitudinal ridges and distance between ridges. Measurements were made using imaging software Fiji version 1.0 (
Schindelin et al. 2012).
Statistical analysis
Nonmetric multidimensional scaling
Clustering of scales and individual moths by wing scale ultrastructure was visualized by nonmetric multidimensional scaling (nMDS) using the metaMDS function from the vegan package (version 2.4-6;
Oksanen et al. 2018). Dissimilarity matrices for the nMDS were based on Bray–Curtis distance measures.
Unique features for species identification
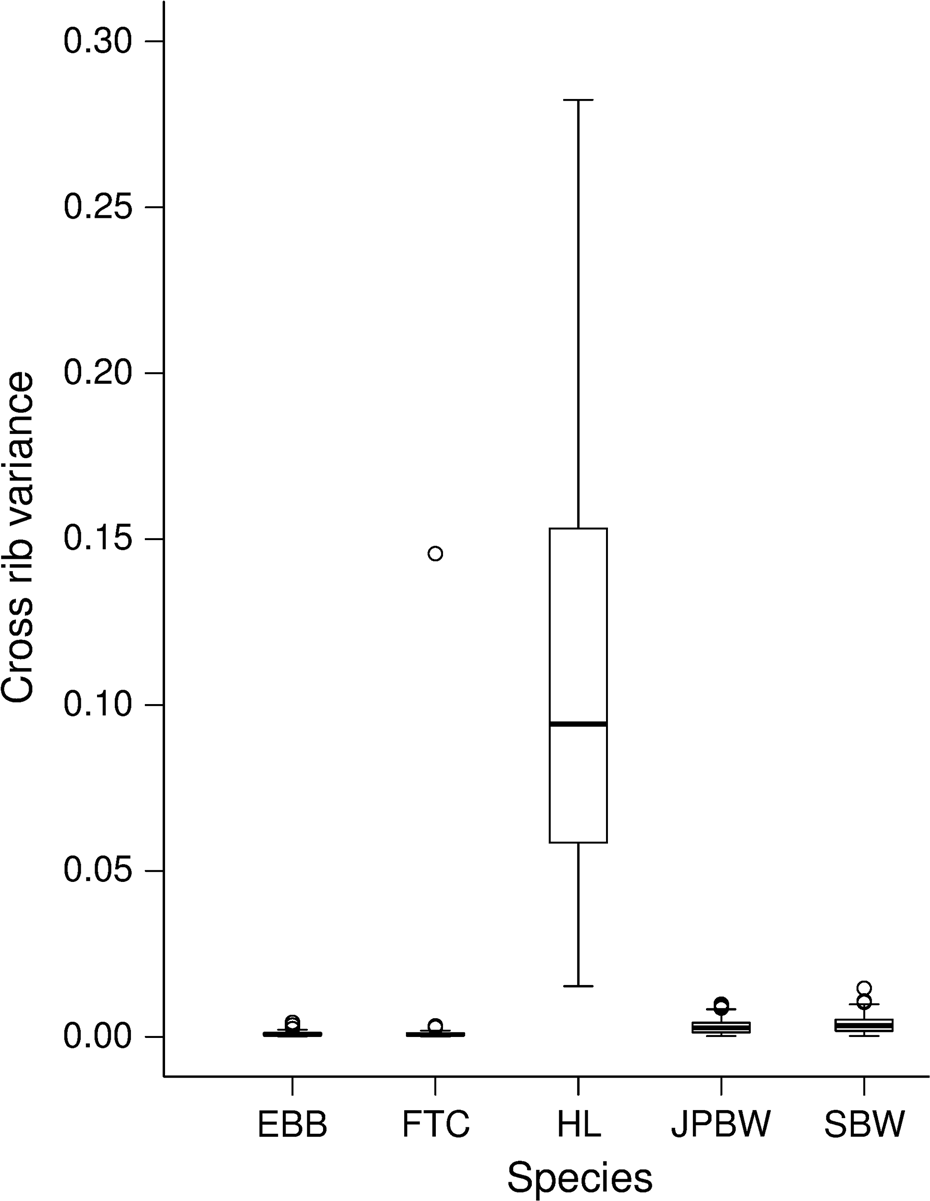
Two species (hemlock looper and blackheaded budworm) displayed unique scale features. Hemlock looper scales clearly displayed nonuniform spacing between cross ribs (
Fig. 1D) and blackheaded budworm scales had few (if any) pores (
Fig. 1C). To test statistically if nonuniform spacing between cross ribs could be used to distinguishing hemlock looper scales from the four other species, variance in the spacing between cross ribs per scale was analyzed using a linear mixed-effects model (LMM). Model parameters included fixed effects of species, sex, a species by sex interaction, and a random effect of moth (to account for repeated measures). Hemlock looper was used as the reference species for the conditional model of the fixed effects (i.e., treatment contrasts) and females were used as the reference sex. Conditional models allowed for comparisons in the variance in the distance between cross ribs of each individual species to hemlock looper. The “lmer” function from the “lme4” package (version 1.1-17;
Bates et al. 2015) was used to conduct the LMM; uniformity of cross ribs was log transformed prior to analysis to achieve normality. Most blackheaded budworm scales had no pores, leading to an excessive number of zeros in the data. As such, a zero-inflated generalized linear mixed-effect model (ziGLMM) was used to determine if number of pores could be used to differentiate blackheaded budworm from the other species. The same fixed and random effects from the LMM were used as model parameters for the ziGLMM but treatment contrasts were done using blackheaded budworm as the reference species. Our ziGLMM was conducted using the “glmmTMB” function (family = gaussian) from the “glmmTMB” package (version 0.2.2.0;
Brooks et al. 2017). The “drop1” function (package: “stats” version 3.4.0;
R Core Team 2017) was used to evaluate the contribution of fixed effects in both univariate analyses. First the species by sex interaction was evaluated, when it was not significant we removed the interaction term and compared reduced models with one of the fixed effects removed to the full model with both effects present (following
Zuur et al. 2009). Where the interaction was significant, we did not evaluate the main effects independently.
Discriminant analysis
No unique features of scale ultrastructure were identified to differentiate among forest tent caterpillar, jack pine budworm, and spruce budworm. Therefore, we applied a discriminant analysis (DA) to determine whether ultrastructure could be used to differentiate wing scales of these three species. DA requires independent observations (
Zuur et al. 2007). Because we had repeated measures (multiple scales per moth), which violate assumptions of independence, we pooled values and reported each trait as the mean value per individual moth (
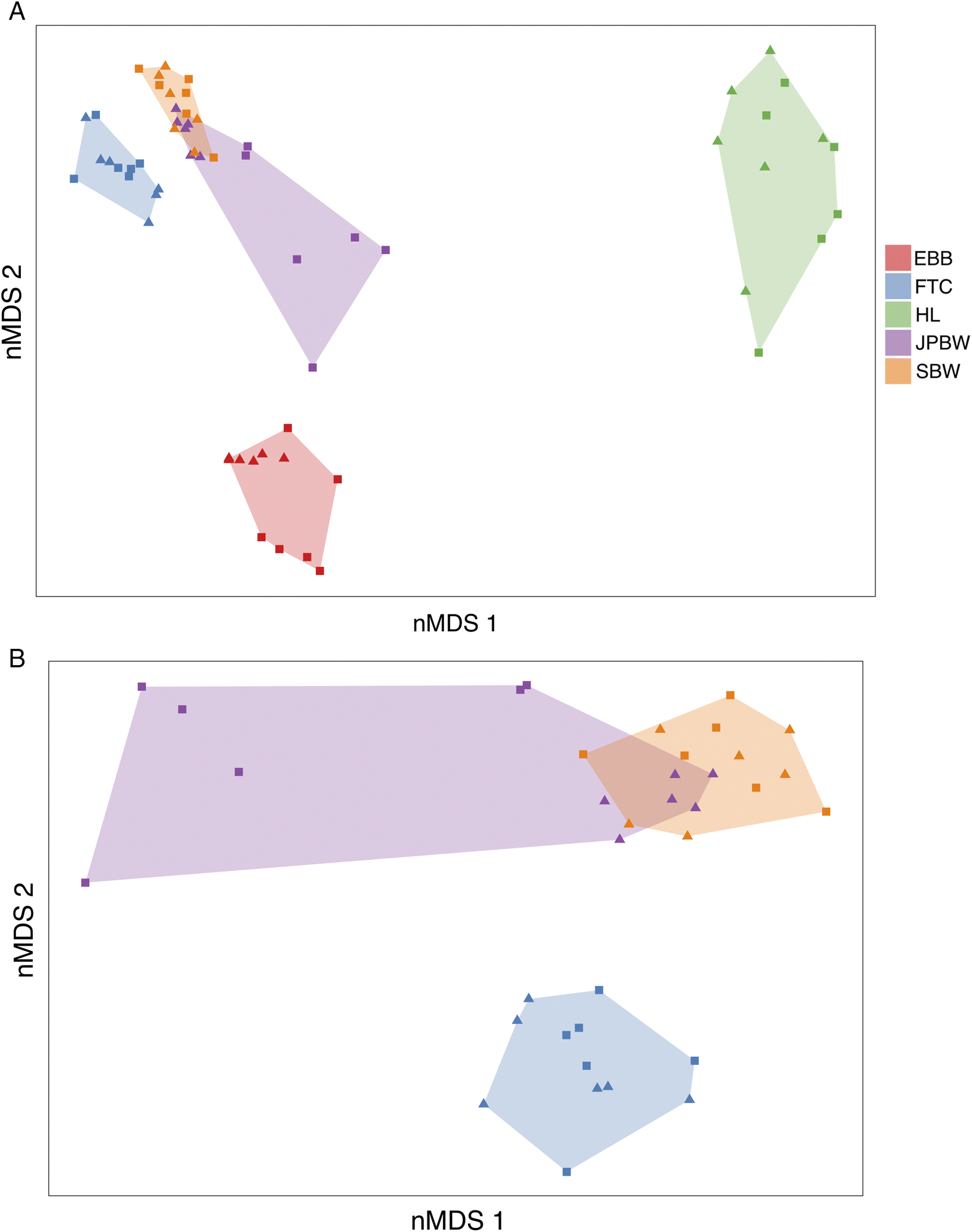
Supplementary Material 1). Given our small sample size (six of each sex per species) and high degree of overlap for female and male forest tent caterpillar and spruce budworm (
Fig. 2) we did not include sex as a grouping factor in our DA (i.e., we used the DA to group individuals by species only).
An underlying assumption of DA is that the variables used to discriminate among groups follow a multivariate normal distribution. Although normality of the individual traits does not ensure a multivariate normal distribution, it can be used as a reasonable first step in achieving multivariate normality (
Looney 1995). As such, each trait was visually assessed to determine if it generally followed a normal distribution. Traits deviating from normality (e.g., skewed or bimodal distributions) even after normalizing transformations were applied (i.e., log, arcsine square root transforms) were not included in the DA. Four candidate traits were identified: number of cross ribs, distance between longitudinal ridges, width of longitudinal ridges, and the number of pores. With these four candidate traits we formally tested all the underlying assumptions of DA. The only assumption not met was homogeneity of variance. We therefore used a quadratic discriminant analysis (QDA) over a linear discriminant analysis as QDA does not have an underlying assumption of homogeneity (
Zuur et al. 2007).
Training and testing the QDA
Seventy percent of our data was randomly selected and used to train our model (
n = 25) and the remaining 30% was used to test its accuracy (
n = 11). Since our goal was to identify species from single scales, we also randomly selected one-third (
n = 120) of the individual scales (i.e., not pooled values per moth) to test our model’s predictive ability at the scale level. The process of randomly selecting training data, moth-level test data, and scale-level test data was bootstrapped 100 times. For each bootstrap we reported the accuracy (percent correct identifications) for the training and moth-level test data. For the scale-level test data, we reported the percent of correct identifications per species and the percent of scales misidentified as either of the two additional species (e.g., percent of spruce budworm scales identified as forest tent caterpillar, percent spruce budworm identified as jack pine budworm, etc.). Our QDA was conducted using the “qda” function from the “MASS” package (version 7.3-49;
Venables and Ripley 2002). All statistical analyses were conducted using R software (version 4.4.0;
R Core Team 2017).
Discussion
The purpose of the methods developed in this paper and that of
Navarro et al. (2018) is to identify fossil moth scales to species in an effort to reconstruct the outbreak histories of specific forest moth pests. Fossil Lepidoptera wing scales provide direct evidence of the past occurrence of moth species and are a promising new paleoecological proxy to reconstruct past insect disturbance (
Montoro Girona et al. 2018;
Navarro et al. 2018). The use of scale ultrastructure to identify fossil scales is an important contribution to this growing field.
We showed that blackheaded budworm and hemlock looper can be categorically separated from forest tent caterpillar, jack pine budworm, and spruce budworm based on unique qualitative morphological traits of scale ultrastructure. This result suggests that distinctive morphological traits could be used to identify unknown fossil moth scales of hemlock looper and blackheaded budworm. Blackheaded budworm was included in our analysis because it shares the same preferred host species as spruce budworm and, historically, outbreaks of the two species have overlapped both spatially and temporally, as during their outbreaks in the 1940s in eastern Canada (
Miller 1966). It is therefore important to be able to separate these two taxa. Also, although Navarro et al.’s (
2018) analysis correctly classified a high proportion of hemlock looper scales (79%), we determined that hemlock looper can be categorically separated from the other four major moth outbreak species based on scale ultrastructure, without the need for DA.
QDA showed that forest tent caterpillar was well separated from the budworm species (jack pine budworm and spruce budworm), with only 24% of forest tent caterpillar scales incorrectly assigned to either jack pine budworm or spruce budworm. We found that most misidentifications were between spruce budworm and jack pine budworm (39% of spruce budworm incorrectly assigned as jack pine budworm, and 23% jack pine budworm incorrectly assigned as spruce budworm), indicating a high degree of similarity in scale ultrastructure between these two species. Spruce budworm and jack pine budworm are closely related species both belonging to the genus
Choristoneura (
Freeman 1967), whose scales we correctly distinguished from forest tent caterpillar 76% of the time. The hosts and outbreak characteristics of spruce budworm and jack pine budworm, however, differ. Jack pine budworms have a strong preference for jack pine (
Pinus banksiana Lambert) (
Volney 1988) but, larvae will occasionally feed on other pine species (
Kulman and Hodson 1961) as opposed to spruce budworm, whose hosts are chiefly balsam fir (
Abies balsamea (L.) Miller), white spruce (
Picea glauca (Moench) Voss), and black spruce (
Picea mariana Miller). Jack pine stands are found primarily on dry, sandy soils. Given the strong preference jack pine budworm has for its host and its host for particular edaphic conditions, study sites could be selected to minimize the past local occurrence of jack pine trees and therefore be able to presume that any
Choristoneura scale found is likely from spruce budworm.
Fossils, such as wing scales, are commonly found in suboptimal condition. The method developed by
Navarro et al. (2018) differs from ours in that scale morphometrics can only be measured on entire scales, excluding any scales that were broken or folded. A potential advantage of our method when applied as a paleoecological tool is that scale ultrastructure can be observed as long as a central fragment of a scale is present. If fossils are damaged or folded, our method provides the advantage of being able to identify a greater proportion of fossil scales towards establishing a reasonable count; a significant advantage if scales are not especially abundant at a site.