Elucidating metabolic pathways and function
GABA was first identified in potato tuber (
Solanum tuberosum L.) in 1949 (
Steward et al. 1949); however, most research on GABA over the next 50 years focused on its role as an inhibitory neurotransmitter in mammals (
Ngo and Vo 2019). Our initial interest in GABA metabolism was triggered by difficulty in tagging the biosynthetic pathway for arginine, an important storage protein amino acid in developing soybean cotyledons, using radiolabelled glutamate (Glu); surprisingly, Glu was rapidly and predominantly metabolized to CO
2 and GABA (
Micallef and Shelp 1989a,
1989b). Consequently, the focus shifted, and several key findings were reported shortly thereafter: Glu- and pyridoxal phosphate-dependent Glu decarboxylase (GAD) is the primary enzyme generating the GABA (
Tuin and Shelp 1994,
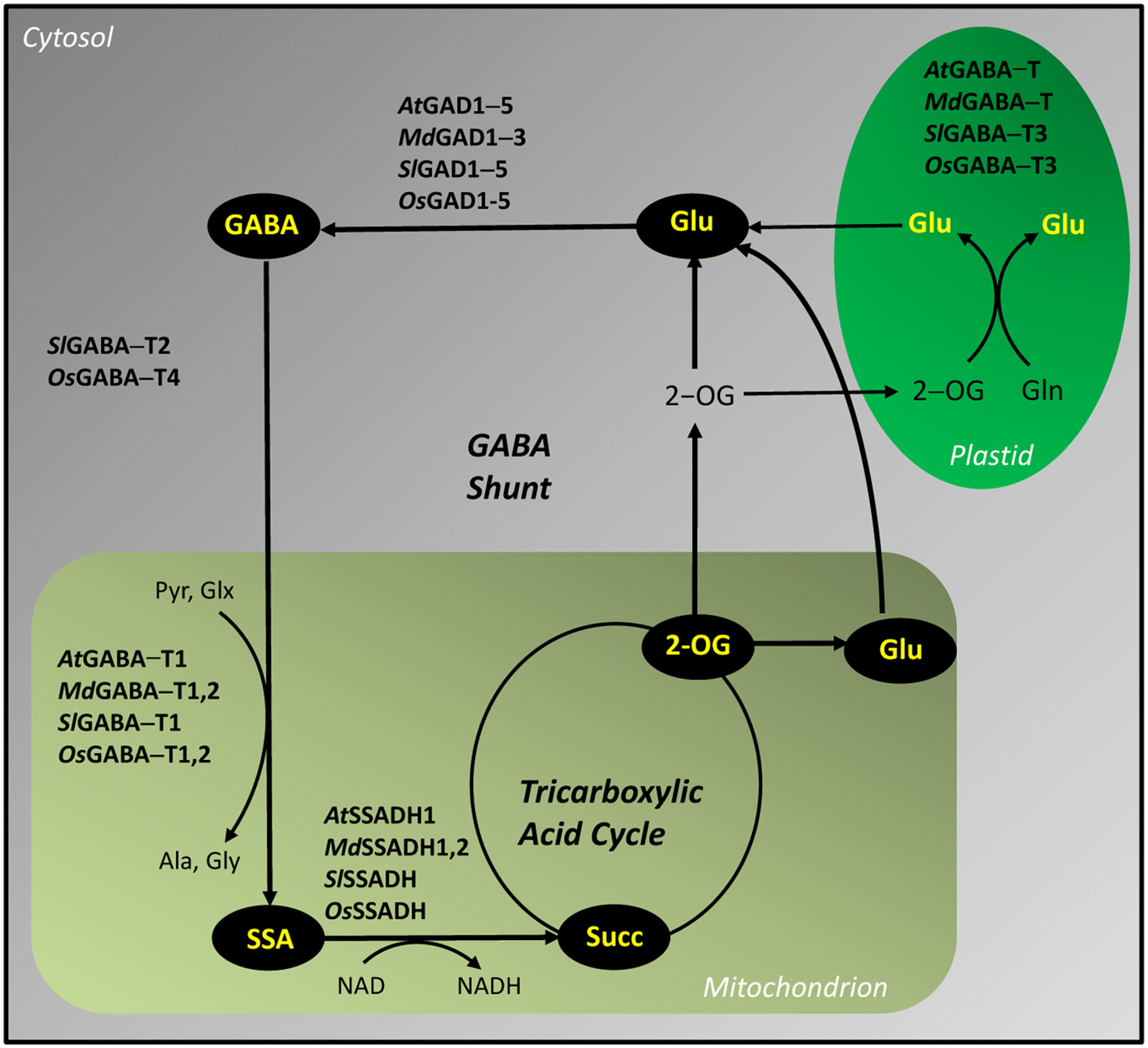
1996), and GAD is cytosolic, whereas the second and third enzymes of the GABA shunt (GABA transaminase or GABA-T, succinic semialdehyde dehydrogenase or SSADH) are mitochondrial (
Breitkreuz and Shelp 1995;
Busch and Fromm 1999;
Clark et al. 2009a) (
Fig. 1). Perhaps most significant was the finding that plant GAD, unlike bacterial and mammalian GADs, binds calcium-calmodulin (Ca
2+-CaM) to its C-terminal autoinhibitory domain, resulting in activation of the enzyme at neutral pH, but not at its acidic pH optimum (
Baum et al. 1993;
Ling et al. 1994;
Arazi et al. 1995;
Snedden et al. 1995). At that time, CaM was receiving attention for its role in stress signaling (
Zelinski 1998).
During the late 1990s, the study of enzyme function transitioned from plant species such as tobacco (
Nicotiana tabacum L.), which provide abundant plant material for enzyme extraction and organelle fractionation, to
Arabidopsis thaliana, the first model plant for genomic and molecular research (e.g.,
Van Cauwenberghe and Shelp 1999,
Van Cauwenberghe et al. 2002). This facilitated identification of the genes encoding GAD (
AtGAD1-5), GABA-T (
AtGABA-T) and SSADH (
AtSSADH) and enabled their biochemical characterization as recombinant proteins generated in bacterial expression systems (e.g.,
Shelp et al. 2012a). Together, AtGABA-T and AtSSADH catalyze the conversion of GABA to SSA and then succinate. Notably, plant GABA-T and SSADH have different amino acceptor (pyruvate and glyoxylate versus 2-oxoglutarate) and co-factor (NAD
+ versus NADP
+) requirements than the bacterial and mammalian forms, respectively.
Complementary studies demonstrated that three of the five
Arabidopsis genes possess the CaM-binding domain (
Shelp et al. 1999,
2012a) (
Fig. 1).
AtGAD1-
5 also exhibit different expression profiles. For example,
AtGAD2 is strongly expressed and widely distributed throughout the shoot and roots (
Shelp et al. 2012b); the GABA level in the shoot of the
atgad2 mutant is less than 25% of that in the wild type (WT), but the level in the roots is unaffected (
Xu et al. 2021). In contrast,
AtGAD1 is strongly and widely distributed in roots (
Shelp et al. 2012b), and the GABA level in roots of the
atgad1 mutant is only 15% of the WT (
Bouché et al. 2004).
AtGAD4 expression is negligible in both shoot and roots, but is strongly induced by various stresses, including hypoxia (
Miyashita and Good 2008). The
atgaba-t mutant is phenotypically normal, except for lower seed production, and the leaf GABA level increases up to 16-fold. The
atssadh mutant overaccumulates GABA and H
2O
2 by two- and four-fold, respectively (
Bouché et al. 2003;
Fait et al. 2005). The product of the SSADH reaction, succinate, contributes to the production of carbon skeletons and NADH via the tricarboxylic acid cycle and to the generation of ATP via the mitochondrial electron transport chain, which prevents the accumulation of reactive O
2 species (
Tuin and Shelp 1994;
Bouché et al. 2003)
Baum et al. (1996) demonstrated that transgenic tobacco plants constitutively overexpressing a mutant petunia (
Petunia x hybrida Vilm.) GAD lacking the CaM-binding domain (
GADΔC) have elevated levels of GABA and are stunted and infertile. Yet,
McLean et al. (2003) were able to select transgenic tobacco plants overexpressing
NtGAD or
NtGADΔC and exhibiting a normal phenotype; the GABA levels were up to 3-fold higher in the shoot, and up to 28-fold higher in the root than in the WT. The high-GABA plants exhibit resistance to the root-knot nematode and the oblique-banded leafroller (
MacGregor et al. 2003;
McLean et al. 2003). Overexpression of the petunia
GAD∆C under control of a developmentally regulated seed promoter increases the GABA level in mature
Arabidopsis seeds by approximately 50-fold compared to the WT (
Fait et al. 2011).
In summary, fundamental inquiry into the role of GABA in plants led to the elucidation of its metabolic route and how it might be manipulated in important economic crops. In the late 1990s there was considerable interest protecting intellectual property related to gene discovery and utility. Consequently, we began to prepare a patent application that included GABA-T and high-GABA plants, but the university, ever aware of the costs associated with the patenting process, looked for an industry partner to share the burden. In the end, the potential partner initiated its own application and neither party completed the process. Furthermore, at the time the safety of consuming elevated levels of GABA in plant-derived foodstuffs was unknown. Nonetheless, the pace of plant-GABA research continued to increase over time (251 publications prior to 1997 versus 3342 papers published from 1997 to 2016, an 883% increase over a 20-year period) and changes in GABA levels were realized by cultural management and genetic engineering strategies. Below, several case studies are discussed of GABA enrichment in economic crops (i.e., tomato (Solanum lycopersicum L.) fruit, rice (Oryza sativa L.) grain, sprouting grains and seeds, controlled atmosphere-stored apple (Malus x domestica Borkh.) fruit, with emphasis on research from the main scientists contributing to these advances.
Managing the hyperaccumulation of GABA in sprouts of cereal grains and legume seeds
Sprouting/germination is a useful processing step for improving the nutritional and potential disease-prevention qualities of cereal grains and legume seeds (
Gani et al. 2012;
Gu et al. 2017;
Idowu et al. 2020;
Munarko et al. 2022). For example, it reduces the level of phytic acid, which interferes with the absorption of minerals by the body, and the level of gluten, a protein of concern to celiacs (
Kim et al. 2012). Furthermore, sprouting increases the GABA level in grains and seeds of various crops, including brown rice, buckwheat, waxy wheat, oat, adzuki bean, kidney bean, lentil, lupin, sesame, soybean, and pea (
Cho and Lim. 2016;
Gan et al. 2017).
The accumulation of GABA in germinated brown rice, quinoa (
Chenopodium quinoa Willd.) and adzuki bean (
Vigna angularis (Willd.) Ohwi & H. Ohashi) is also associated with elevated GAD activity and expression of the various GAD genes (
Liu et al. 2005;
Zhao et al. 2017;
Hussain et al. 2020;
Jiang et al. 2021;
Zhang et al. 2021). Elucidation of the precise role(s) of the various GADs, as well as GABA-Ts and SSADHs, requires additional study using RNAi or gene editing technologies. Nonetheless, germinated cereal grains and legume seeds are now being commercially marketed as a rich source of GABA. For example, germinated brown rice (GBR) has 15 times more GABA than standard brown rice, and 10 times the level in germinated white rice (
Coconuts Bangkok 2019). GBR was developed for marketing in Japan in 1995. GBR products were developed and marketed first by Domer Co. (Ueda City, Nagano Pref.) and the city government, Mino-cho of Kagawa Pref., was one of the earliest organizations engaged in the production of GBR (
Patil and Khan 2011).
Breeding and genetic engineering to hyperaccumulate GABA in tomato fruits and rice grains
Breeding and genetic engineering of GABA-enriched tomato fruit was spearheaded by Professor Hiroshi Ezura and co-workers at the University of Tsukuba in Japan (
Gramazio et al. 2020). Early research indicated that fruit of
Solanum pennellii Correll, a wild tomato relative, contains higher GABA levels than fruit of the domesticated species (
Table 1). Typically, GABA increasingly accumulates in fruits of modern tomato cultivars such as Micro-Tom from flowering to the mature green stage, then decreasing during ripening (
Rolin et al. 2000;
Akihiro et al. 2008). In contrast, the GABA-enriched cultivar DC03-9 does not show a rapid decline in GABA after the breaker stage (
Table 1). Three cytosolic CaM-dependent SlGADs are present in the fruit, though
SlGAD2 and
SlGAD1 are expressed during the first two-thirds of fruit development, and
SlGAD1 is expressed during the second half (
Akihiro et al. 2008). GABA levels in red fruit are not reduced in RNAinterference (RNAi)
-SlGAD1 lines, whereas they are reduced by 28%−77%, 72%−95%, and 64%−93%, respectively, in
RNAi—SlGAD2,
RNAi—SlGAD3, and
RNAi--SlGADall lines, with little effect on overall plant growth, indicating that the expression of
SlGAD2,3 is correlated with GABA accumulation in red fruit (
Takayama et al. 2015).
Three pyruvate/glyoxylate-dependent SlGABA-Ts are present in tomato fruit, but only SlGABA-T1 is mitochondrial. SlGABA-T2 and SlGABA-T3 are cytosolic and plastidial, respectively (
Akihiro et al. 2008;
Clark et al. 2009b) (
Fig. 1). The expression of all three
GABA-Ts is similar during the first half of fruit development, but the expression of
GABA-T2,3 declines thereafter (
Akihiro et al 2008). Loss-of-function analyses confirmed that pyruvate and glyoxylate-dependent GABA-T activity is essential for GABA catabolism in tomato fruit and showed that
SlGABA-T1 is the major gene responsible (
Koike et al. 2013;
Li et al. 2018). RNAi plants with suppressed
SlGABA-T expression are dwarf and infertile. Notably, in DC03-9 the expression of GA
BA-T1,2 remains steady during the beaker and red stages of development, but GABA-T activity increases (
Saito et al. 2008). A single mitochondrial NAD
+-dependent
SlSSADH is present in the fruit and its expression is steady throughout development (
Akihiro et al. 2008). Thus, the enriched GABA level in DC03-9 fruit is likely due to elevated GABA synthesis rather than catabolism.
Fruit of transgenic tomato plants constitutively overexpressing
SlGAD3 exhibit a normal phenotype and possess up to five-fold higher GABA levels than the WT (
Takayama et al. 2015) (
Table 1). Fruit-ripening-specific overexpression of
SlGAD3ΔC increases the fruit GABA levels even more, but the fruit never turn red (
Takayama et al. 2017). Thus, sufficient GABA could be accumulated for practical use, but strict regulations for genetically modified organisms (GMOs) at the time limited their subsequent utilization.
CRISPR/Cas9 technology-mediated deletion of the C-terminal autoinhibitory domain in
SlGAD2 and
SlGAD3 also increases fruit GABA levels (
Table 1), but plant growth, flowering, and fruit yield are reduced in the former case, suggesting that
SlGAD3 is a suitable CRISPR/Cas9 target for increasing GABA levels in tomato fruit (
Nonaka et al. 2017). This was followed by the production of F
1 hybrid lines with elevated GABA levels by crossing the MicroTom
SlGAD3 mutant created with CRISPR/Cas9 to a WT cultivar (
Lee et al. 2018). Subsequently,
Li et al. (2018) exploited a multiplex CRISPR/Cas9 system to demonstrate that suppression of
SlGABA-TP1 and
SlSSADH increases leaf and fruit GABA levels and reduces plant height and fruit set. Interestingly, leaf GABA accumulation is associated with the inhibition of GAD activity and leaf necrosis in the SSADH mutant with the accumulation of reactive O
2 species. More recently, the Sanatech Seed Company of Japan released a commercial tomato cultivar with elevated GABA levels; this was created by expressing a mutant GAD gene lacking the CaM-binding domain (presumably
SlGAD3∆C) using CRISPR/Cas9 (
Waltz 2021) (
Table 1). Notably, CRISPR/Cas9-engineered plant materials are being considered genome edited, rather than genetically modified, thereby reducing some opposition to their commercialization.
Professor Kazuhito Akama at Shimane University in Japan led efforts to genetically engineer rice grain with elevated GABA levels. Five
GAD genes are present in maturing rice seed. Four of these possess the C-terminal CaM binding domain; the C-terminus of OsGAD2 does not bind CaM, but is still autoinhibitory (
Akama et al. 2001;
Akama and Takaiwa 2007).
OsGAD1 shows the highest expression, followed by
OsGAD5,
OsGAD4 and
OsGAD2,3 (
Zhao et al. 2017). The expression of all five
OsGADs tends to increase with seed maturation. Four GABA-Ts are present in maturing rice seed, with GABA-T1,2 located in mitochondria, OsGABA-T3 in plastids, and OsGABA-T4 in the cytosol (
Shimajiri et al. 2013a) (
Fig. 1). The three organelle-located GABA-Ts utilize pyruvate and glyoxylate, but not 2-oxoglutarate, as amino acceptors. The activities of recombinant OsGABA-T3 and OsGABA-T1, respectively, are approximately 200- and 50-fold that for recombinant OsGABA-T2. The expression of
OsGABA-T1 is higher than that for
GABA-T2,3,4 in maturing seeds;
OsGABA-T1,2,4 expression increases throughout seed maturation, whereas
OsGABA-T3 expression declines (
Shimijiri et al. 2013b;
Zhao et al. 2017).
RNA interference of
OsGABA-T1 suppresses the expression of
OsGABA-T1, as well as
OsGABA-T2, and increases GABA levels in brown and white rice by approximately 20 and 10 times and 10 and five times, respectively, after 10 days and 4 months of storage (
Zhou et al. 2015) (
Table 1). Seed-specific overexpression of
OsGAD2ΔC and RNAi suppression of GABA-T leads to sustained and high GABA levels in brown rice (
Akama et al. 2009;
Shimajiri et al. 2013b,
Kowaka et al. 2015). CRISPR/Cas9 technology-mediated deletion of the C-terminal autoinhibitory domain in Os
GAD3 increases the seed GABA level by seven-fold and this is accompanied by higher grain weight and protein content than WT brown rice (
Akama et al. 2020)
Using exogenous GABA to mitigate postharvest deterioration and crop stress
During postharvest storage, horticultural commodities are often exposed to multiple abiotic stresses (i.e., low temperature, low O
2, elevated CO
2) to reduce ethylene production and respiration, so that ripening/senescence is delayed and nutritional and sensory quality is preserved (
Lum et al. 2016;
Aghdam et al. 2022). Overall, these studies indicated that improvements in the marketability of horticultural products are generally associated with the promotion of GABA biosynthesis and GABA shunt activity, with or without the accumulation of GABA. During prolonged storage, the onset of senescence-related injury may also be associated with the accumulation of GABA.
Our research, conducted in collaboration with Dr. Gale Bozzo at the University of Guelph and Dr. Jennifer DeEll at OMAFRA, focused on apple fruits, since they are stored, more than any other botanical fruit, under controlled atmosphere conditions. Furthermore, gene sequences for the key steps in GABA metabolism in apple fruit were identified by comparison to the
Arabidopsis genome (
Brikis et al. 2018;
Aghdam et al. 2022), allowing elucidation of the biochemical properties and subcellular location of multiple isoforms of the encoded proteins (
Fig. 1), as well as their transcript abundance. Exogenous GABA was never considered to be an effective protection against the multiple abiotic stresses imposed during postharvest storage because its penetration through the apple skin was presumed to be low. However, many other researchers demonstrated that exogenous GABA significantly alleviates chilling injury, bacterial/fungal decay, and loss of quality in postharvest fruits, vegetables and cut flowers during storage (see references in
Aghdam et al. 2022).
With the current rate of global warming, the temperature is expected to rise by 1.5–2.4 °C by 2050, field-grown crops will be subjected to more extreme weather events and concurrent and multiple climate changes (e.g., drought, waterlogging/O
2 deprivation, salinity), which reduce crop yields (
Shelp et al. 2021a). Because of the positive effects of endogenous and exogenous GABA on the levels of reactive O
2 species, membrane stability, non-enzymatic and enzymatic antioxidants, and crosstalk among phytohormones, exogenous application of GABA was recently posited to be an effective and sustainable strategy against multiple stress under both open and closed environments. For example, GABA binding to receptors can interfere directly with ion transport in stomata and root epidermal cells, and with the activity of phytopathogens, thereby enhancing tolerance to drought, waterlogging (hypoxia), and disease (
Shelp et al. 2021a). And promoting the activity of the GABA pathway can restore or partially restore stress-induced losses in respiration and energy generation.
Novel elite corn lines with enriched carotenoid levels
Carotenoids are acquired in the diet and associated with protection from many chronic diseases, including an eye disease known as age-related macular degeneration (ARMD). ARMD is associated with loss of lutein and zeaxanthin from the macular region of the eye (see references in
Burt et al. 2013). Currently, it is the leading cause of vision loss in people over 50, affecting approximately 2.5 million Canadians (
Fighting Blindness Canada 2020).
It was known that egg carotenoids are readily absorbed into the human body, and that the dietary intake of lutein by humans could be enhanced by supplementing the diet of laying hens with marigold (
Tagetes erecta L.) petal extracts (
Delgardo-Vargas et al. 1998). Professor Elizabeth Lee and co-workers at the University of Guelph posited and confirmed that the carotenoid levels, as well as the zeaxanthin:lutein balance, in field corn could be improved by allele mining of the Orange Flint race using traditional breeding techniques (
Burt et al. 2010,
2011a,
2011b). Subsequently, the feasibility of using high-carotenoid corn to generate high-lutein and (or) high-zeaxanthin eggs, and the utility of using the high-carotenoid lines as donors to develop elite high-carotenoid inbred lines with high carotenoids were demonstrated (
Burt et al. 2013). Accordingly, the future to apply high-carotenoid corn seemed bright. Furthermore, a leading agriculture company was interested in introgressing the high carotenoid content into sweet corn for human consumption. In the meantime, however, a global ingredient manufacturer in the eye health space generated marigold lines with elevated levels of both lutein and zeaxanthin, which reduced the interest of corn producers in commercializing the high-carotenoid corn.
Eco-friendly control of Botrytis infection
Botrytis cinerea, the causal agent of the gray mold disease, is the most economically important necrotrophic fungal plant pathogen in the world (see refs in
Seifi et al. 2019). While conventional fungicides against the gray mold disease are available, there are situations where they do not offer complete control, and negative health and environmental consequences have been ascribed to some of them. Therefore, the development of novel resistance-enhancing and eco-friendly controls for such an impactful disease seemed important for production of horticultural and field crops, as well as organically grown crops.
Many natural compounds are known to activate defense responses against a certain type of biotic or abiotic stress. Recently, we demonstrated that exogenous application of spermine (Spm), unlike other polyamines, induces strong resistance against
B. cinerea in tomato, common bean, and
Arabidopsis plants (
Seifi et al. 2019). Furthermore, co-application of Spm with salicylic acid, a priming agent against a broad spectrum of phytopathogens, suppressed disease in a tomato-
B. cinerea pathosystem in a synergistic interaction. Since there are many other important pathogens with a similar nectrophic mode of infection (e.g.,
Sclerotinia sclerotiorum and
Alternaria solani), this finding appeared to be a significant step towards establishing novel eco-friendly methods to control a wide range of pathogens (
Seifi and Shelp 2019). A patent application was submitted, but our industry partner was unable to replicate the synergistic interaction in greenhouse and field trials with various crop species, so the application was abandoned.