A Canadian model for providing high-quality, timely and relevant evidence to meet health system decision-maker needs: the SPOR Evidence Alliance
Abstract
Canada has made great progress in synthesizing, disseminating, and integrating research findings into health systems and clinical decision-making; yet gaps exist in the research-to-practice continuum. The Strategy for Patient-Oriented Research (SPOR) Evidence Alliance aims to help close gaps by providing decision-makers with evidence that is timely, context sensitive, and demand driven to better inform patient-oriented practices and policies in health systems. In this article, we introduce a model established in Canada to support decision-maker needs for high-quality evidence that is patient oriented to enhance health systems performance. We provide an overview of how this model was implemented, who is involved, who it serves, as well as its organizational structure and remit. We discuss key milestones achieved to date and the impact this initiative has made within the health research community. The strength of the SPOR Evidence Alliance lies in its unique ability to simultaneously: (i) serve as a national platform for researchers to stay connected and collaborate to minimize duplication of efforts and (ii) facilitate access to research knowledge for patient partners and decision-makers. In doing so, the SPOR Evidence Alliance is supporting health policy and practice decisions that support and strengthen Canada’s dynamic health systems.
Background
Canada has a strong health research landscape with internationally respected expertise in basic biomedical research, large administrative and clinical databases, and world-leading expertise in knowledge synthesis (Canadian Institutes of Health Research 2011b). Yet, notable gaps exist in the capacity to synthesize, disseminate, and integrate the research knowledge base to inform health policies and practices (Canadian Institutes of Health Research 2011b). Furthermore, the traditional role of patients, caregivers, and health consumers as research participants, as opposed to partners in research, has resulted in a significant disconnect between what is important to quality of care and what researchers want to study. For example, a review on kidney dialysis revealed that only 20% of the clinical trials addressed an issue that a patient considered to be in their top-10 priorities (Jun et al. 2015). Therefore, not engaging patients as active partners in research may risk wasting public funds on findings that are not tailored to patient needs for improved health outcomes (Patrick et al. 2018).
To enhance the research-to-practice continuum and improve patient experiences with the health care system, the Canadian Institutes of Health Research (CIHR) formed the Strategy for Patient-Oriented Research (SPOR) enterprise in 2011 (Canadian Institutes of Health Research n.d.-d; (Canadian Institutes of Health Research 2016). A set of specialized patient-oriented research networks and research service centres has been established since, including the creation of the SPOR Evidence Alliance in 2017 through a competitive process. The objective of the SPOR Evidence Alliance is to establish a concerted, well-resourced, and collective approach to building a patient-oriented, rapid learning health system in Canada (Canadian Institutes of Health Research 2011a). The SPOR Evidence Alliance provides decision-makers with research that is context sensitive (i.e., contextualized to a specific setting) and demand driven (i.e., focused on a priority identified by a decision-maker) to inform health system practices, services, and (or) policies in a timely manner.
The SPOR Evidence Alliance specializes in supporting decision-makers’ needs for evidence using knowledge synthesis, guidelines, and knowledge translation. CIHR defines knowledge synthesis as a systematic approach to summarizing all available evidence on a particular topic using comprehensive literature searches and advanced qualitative and quantitative synthesis methods (Canadian Institutes of Health Research n.d.-b). Guidelines often start with knowledge syntheses to develop recommendations that guide decisions of practitioners, patients, and policy makers about appropriate health care for specific clinical or public health problems (Field and Lohr 1990; Shekelle et al. 1999; Canadian Institutes of Health Research n.d.-e). Knowledge translation is a dynamic and iterative process that includes knowledge synthesis, dissemination, exchange, and ethically sound application of scientific knowledge to improve health system performance (Canadian Institutes of Health Research n.d.-c; Straus et al. 2009).
Knowledge synthesis and knowledge translation products help decision-makers stay abreast of the rapidly growing volume of scientific literature published daily. If a decision-maker makes important health decisions based on findings of a single study and ignores all available studies on the topic, a potentially misleading and harmful decision can be made (Antman et al. 1992; Ioannidis 2005a; 2005b). Conversely, use of knowledge synthesis in the development of practice guidelines helps improve quality of care and health outcomes (Grimshaw et al. 2004; The Lancet 2014).
This paper is the third core paper in a series of four papers that focus on the SPOR Evidence Alliance’s collaborative model to support rapid knowledge translation. The series includes an introductory paper (Tricco et al. 2022), a second paper that describes the governance structure of the SPOR Evidence Alliance (Lunny et al. 2022), and a final paper describing patient engagement in the SPOR Evidence Alliance (Li et al. 2022). The purpose of this paper is to describe the SPOR Evidence Alliance’s research query services model for generating and supporting decision-maker needs for research evidence across Canada and beyond.
How does the SPOR Evidence Alliance respond to decision-maker needs through the research query services?
The SPOR Evidence Alliance accepts research queries using two targeted online forms through which decision-makers can submit their research requests. For policy makers, health system managers, guideline developers, and health care providers seeking evidence to inform a health policy or practice decision, a detailed form is available specifically for this type of request (SPOR Evidence Alliance n.d.-c). The SPOR Evidence Alliance’s central coordinating office reviews these requests as they come in, and coordinates research teams to respond to the requests using well-established and methodologically rigorous approaches in knowledge translation (Graham et al. 2006; Esmail et al. 2020), knowledge synthesis (Joanna Briggs Institute 2019; Higgins et al. 2022), or guideline development (Brouwers et al. 2020). For patients, caregivers, and general health care consumers who would like to suggest health topics that are important to them, a separate form is available online (SPOR Evidence Alliance n.d.-a). Specific to the patients, caregivers, and consumers, these requests are reviewed and prioritized on an annual basis using a modified James Lind Alliance Priority Setting Partnerships approach to identify three priority research topics each year (James Lind Alliance 2021). The top three identified priority topics are selected for further study, with the work being conducted in partnership with patient partners and fully funded by the SPOR Evidence Alliance.
For a research query to be eligible, they must fulfill a set of criteria (Box 1).
Box 1. Query Eligibility for the SPOR Evidence Alliance
1.
Query must relate to a health topic according to the World Health Organization definition (World Health Organization 2002).
2.
Query concerns an existing or planned health policy or practice decision.
3.
Query request must be made by or on behalf of a decision-maker.
4.
Query request can be addressed with a knowledge synthesis, guideline, or knowledge translation approach.
If the query satisfies the eligibility criteria, an intake call with the query submitter is held to clarify the research request within five business days. An experienced librarian then performs preliminary literature searches using bibliographic databases and study registries (e.g., Open Science Framework, PROSPERO, Joanna Briggs Institute) to ensure that similar work is not already available or in progress elsewhere. This is also conducted within five business days. The SPOR Evidence Alliance’s nominated principal investigator, the Executive Committee (consisting of researchers, trainees, patient partners, and decision-makers from across Canada), and the central coordinating office (the administrative hub of the SPOR Evidence Alliance, housed within St. Michael’s Hospital, Unity Health Toronto) are responsible for reviewing the research topics and project work plans and budgets to advise on potential research duplication, feasibility of methods, relevance of the research question, and opportunities for patient and other stakeholder engagement. For questions related to drugs and health technologies, the SPOR Evidence Alliance checks with the Canadian Agency for Drugs and Technologies in Health (CADTH) (Canadian Agency for Drugs and Technologies in Health n.d.-b) and the CIHR Drug Safety and Effectiveness Network (DSEN) (Canadian Institutes of Health Research n.d.-a) to ensure that research efforts are not being duplicated.
After confirming that the current or any forthcoming scientific evidence does not adequately address the knowledge gap identified, the research project is assigned to a SPOR Evidence Alliance research team with relevant methodological (and contextual, as applicable) expertise. Standard research requests take up to 12 months to complete. For urgent, time-sensitive requests (e.g., urgent research priorities), the SPOR Evidence Alliance can address these requests within 3 months, and more recently for COVID-19 related queries in as little as five business days. The SPOR Evidence Alliance has 12 principal investigators and 17 research teams located across Canada and one international research team with subject matter expertise in a range of health topics and conditions as well as methodological expertise in knowledge translation, knowledge synthesis, guidelines development and patient-oriented research. Figure 1 provides an overview of the query response process.
Fig. 1.
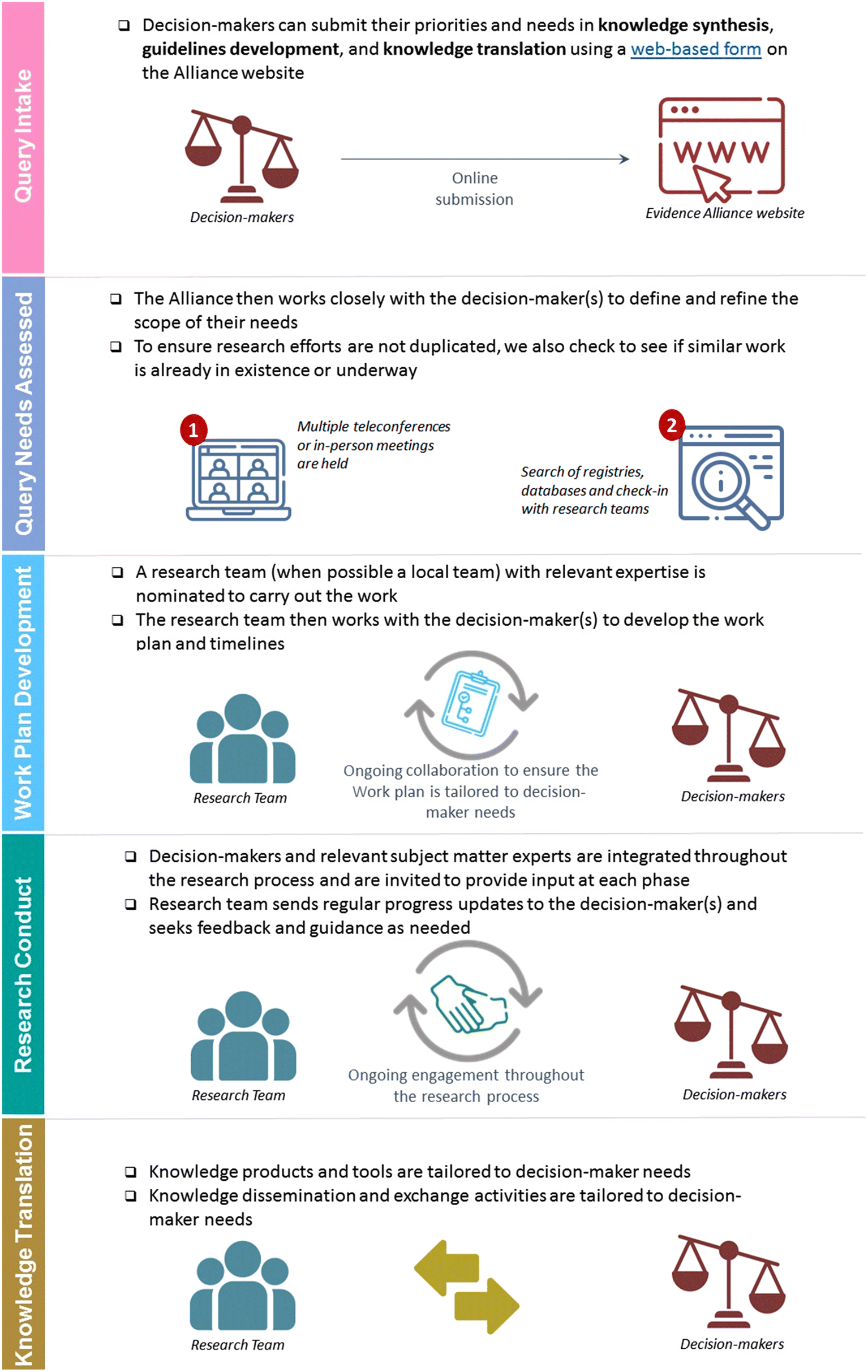
For knowledge translation, our philosophy is to use evidence-based theories, models, and frameworks, such as the Knowledge-to-Action cycle (Graham et al. 2006; Esmail et al. 2020). For knowledge synthesis, we match the decision-maker question to a specific method (e.g., systematic review, rapid review, scoping review, overview of reviews) using the “What Review is Right for You” tool (Knowledge Translation Program n.d.). Once the type of knowledge synthesis approach has been selected, our researchers use methodologically rigorous guidance developed by the Joanna Briggs Institute (Joanna Briggs Institute 2019) and Cochrane (Higgins et al. 2022) for synthesis of quantitative and qualitative studies, as well as appropriate reporting checklists such as the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and various extensions of the tool according to the specific research designs (Equator Network n.d.). For guideline development, adaptation, and implementation, we use transparent approaches based on rigorous methods proposed by groups such as the Canadian Task Force for Preventive Health Care (University of Alberta 2011), the US Task Force (U.S. Preventive Services Task Force n.d.), Guidelines International Network (Guidelines International Network n.d.), Grading of Recommendations Assessment, Development and Evaluation system (Guyatt et al. 2011) and the AGREE enterprise (AGREE n.d.; Brouwers et al. 2020).
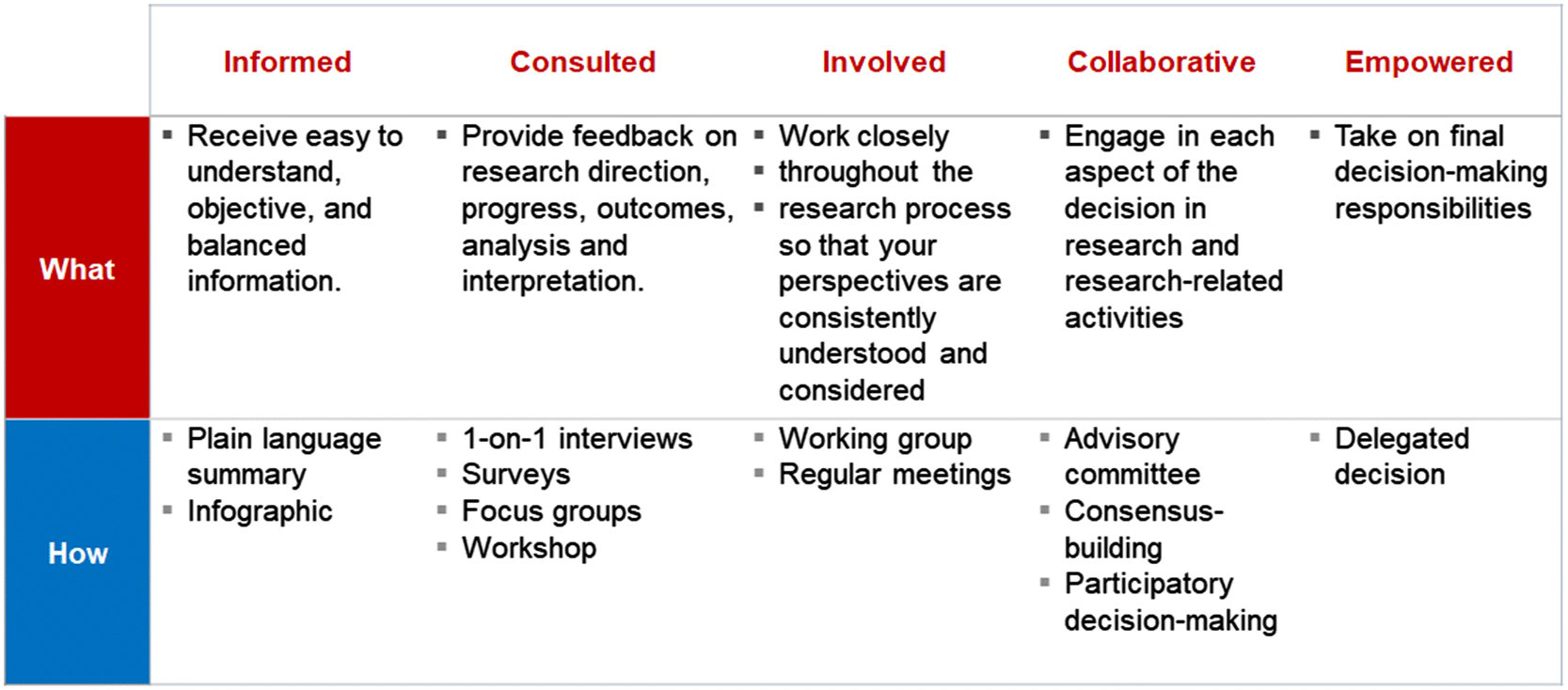
At the cornerstone of our query response is the use of an integrated knowledge translation approach to work collaboratively with the decision-makers who submitted the query or topic and maintain ongoing engagement from inception through to completion of the project (Tricco et al. 2018). The decision-makers provide feedback on the project work plan and timelines, results, and knowledge dissemination and exchange activities. The extent of engagement can range from informing (e.g., receiving regular project updates) to empowering (e.g., co-developing the research question), and is tailored to the decision-makers’ preference and availability (Fig. 2) (International Association for Public Participation n.d.). For example, a decision-maker may wish to be involved in the protocol development and final report writing stage, but may opt to only receive regular updates during the research conduct.
Fig. 2.
Since the SPOR Evidence Alliance is a pan-Canadian research initiative, whenever possible, efforts are made to match decision-makers with local research teams. Not only does this approach facilitate collaboration among the decision-makers and researchers locally, but it also helps to build local research capacity, as well as creates and strengthens relationships. For example, if a decision-maker is seeking evidence to support a health policy decision in Québec, we connect them with our principal investigator Dr. Annie LeBlanc located in Québec to lead the query with her team of research staff, research trainees, and local subject matter experts. The SPOR Evidence Alliance uses a collaborative model to share its CIHR funding with the research teams who conduct the work.
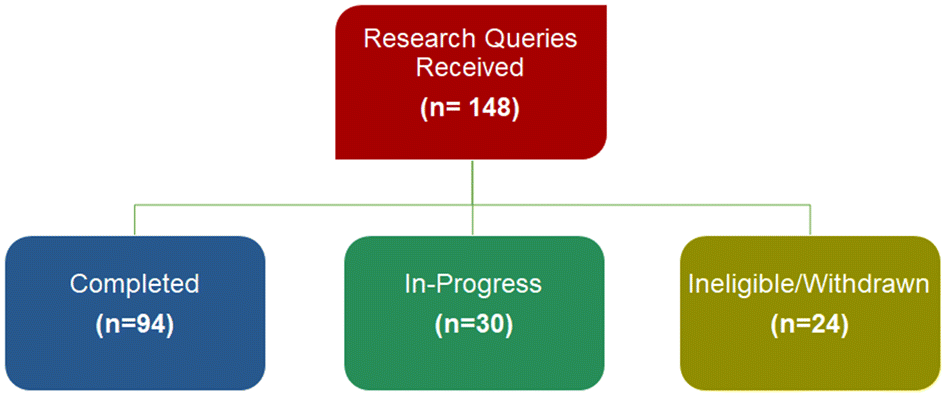
Between April 2018 and December 2021, the SPOR Evidence Alliance received 148 research queries and have addressed 94 of those, with 30 queries currently underway (Fig. 3).
Fig. 3.
More than 194 decision-makers, 180 patient and public partners, and 95 graduate students and research trainees were engaged across the 96 queries. Table 1 details the types of decision-makers that used our query services, the intended use of the research findings, and jurisdiction their decision impacts.
Table 1.
| Query characteristics | Addressed (n = 94), n (%) | In progress (n = 30), n (%) |
|---|---|---|
| Decision-maker Type | ||
| Policy maker | 61 (49.2) | 10 (8.1) |
| Health system manager | 17 (13.7) | 6 (4.8) |
| Other knowledge user | 8 (6.5) | 8 (4.0) |
| Guideline developer | 6 (4.8) | 0 (0.0) |
| Health care provider | 2 (1.6) | 6 (4.8) |
| Research application | ||
| Inform public health measures | 32 (25.8) | 5 (4.0) |
| Inform health system management | 26 (21.0) | 9 (7.3) |
| Inform guideline and clinical management | 24 (19.4) | 7 (5.6) |
| Inform economic and social responses | 6 (4.8) | 3 (2.4) |
| Inform knowledge translation | 5 (4.0) | 3 (2.4) |
| Inform patient engagement | 1 (0.8) | 3 (2.4) |
| Trainee involvement | ||
| Yes | 50 (40.3) | 13 (10.5) |
| No | 44 (35.5) | 17 (13.7) |
| Decision-maker reach | ||
| Provincial | 49 (39.5) | 19 (15.3) |
| National | 33 (26.6) | 9 (7.3) |
| International | 12 (9.7) | 2 (1.6) |
How does the SPOR Evidence Alliance engage patient partners in the research query services?
Patient involvement has been integral to the development of all activities of the SPOR Evidence Alliance. When developing the research query service model, patient partners provided input on the online query intake forms and supported the development of the query prioritization process for patient-submitted research topics. The SPOR Evidence Alliance began accepting health topic suggestions from patients, caregivers, and members of the public in November 2019.
Central to our query services is to carefully consider opportunities for meaningful patient partner engagement on projects. Although not all projects are appropriate for patient involvement (e.g., subject matter not relevant for patients, such as a review exploring organizational absorptive capacity of new research knowledge), whenever possible, we encourage research teams to identify and support patient partner involvement in activities that would help support the incorporation of the patient lens into the research project so that the research findings are patient oriented and contribute to patient-centred health policy and practice decisions. An example of how research teams have meaningfully included patient voices in their work is seen in the work led by our principal investigator (Dr. Pertice Moffitt) from the Northwest Territories (NWT). In 2018, the Government of NWT reached out to the SPOR Evidence Alliance seeking scientific evidence for effective strategies to address family violence, which is seven times higher in the region compared with the national average (Burczycka et al. 2018). The SPOR Evidence Alliance considered this a high priority topic to explore and embarked on a shared funding partnership to support this work. The query submitter worked with Dr. Moffitt and her local team of research trainees, research staff, and subject matter experts, with oversight from a multi-stakeholder group that included Indigenous community partners from the region. A scoping review with sharing circles was conducted. Sharing circles are a type of community-based research approach that follows a conversational and relational process that is more consistent with an Indigenous worldview than the more western method of focus group interviews (Lavallée 2009; Rothe et al. 2009; Kovach 2010). The Government of NWT has used the research findings to develop a mitigation strategy that is culturally appropriate to foster trust and respect in the community.
The SPOR Evidence Alliance continues to explore ways to enhance patient partner engagement in the query services. Several consultations with research teams and patient partners revealed that formal training sessions would be helpful in facilitating meaningful engagements. The SPOR Evidence Alliance partnered with two patient partners with education backgrounds to co-develop and co-deliver a 3-week training program on rapid reviews tailored for patient and public partners in research. Twenty-four students successfully completed the pilot program in May 2021 (Table 2).
Table 2.
| Participant Characteristics | n (%) |
|---|---|
| Gender | |
| Female | 21 (88) |
| Male | 3 (13) |
| Location | |
| Alberta | 2 (8) |
| British Columbia | 2 (8) |
| Manitoba | 1 (4) |
| New Brunswick | 2 (8) |
| Newfoundland and Labrador | 1 (4) |
| Nova Scotia | 2 (8) |
| Ontario | 9 (38) |
| Quebec | 4 (17) |
| Saskatchewan | 1 (4) |
| Age Group | |
| 18–35 years | 2 (8) |
| 36–55 years | 7 (29) |
| 56–64 years | 3 (13) |
| 65–80 years | 3 (13) |
| Not reported | 9 (38) |
| Perspective | |
| Family member or caregiver (an unpaid individual who attends to the needs of a child or dependent adult) | 3 (13) |
| Interested member of the public | 1 (4) |
| Patient or former patient | 1 (4) |
| Patient or former patient, family member or caregiver (an unpaid individual who attends to the needs of a child or dependent adult) | 2 (8) |
| Patient or former patient, family member or caregiver (an unpaid individual who attends to the needs of a child or dependent adult), interested member of the public | 7 (29) |
| Not reported | 10 (42) |
How does the SPOR Evidence Alliance build researcher capacity?
To build researcher capacity in knowledge synthesis and knowledge translation within a patient-oriented research environment, many of our research queries include early career researchers or research fellows/trainees either as a co-lead (alongside a senior researcher) or as a team member. This allows early career researchers (i.e., <5 years after an initial academic appointment) and trainees to build a diverse research portfolio by gaining exposure to unfamiliar research areas or methods and learn to collaborate with decision-makers from diverse knowledge and experience. Across the 124 queries, 20 were led by an early career researcher and 63 queries included graduate students and trainees (n = 113) as part of the research team.
For example, the British Columbia Ministry of Health requested a systematic review in 2019 to identify key indicators of successful patient and family caregiver engagement within their provincial health care system (Hamilton et al. 2019). A post-doctoral research fellow led this work with oversight and guidance from a senior researcher and in collaboration with a family caregiver as co-researcher. The investigators of this query were co-authors of the Patient Engagement in Research Framework (Hamilton et al. 2018), which they are using to ensure that the patient co-researcher has a meaningful research experience.
How does the SPOR Evidence Alliance disseminate and promote uptake of its research?
Our research query services have produced a number of knowledge products such as peer-reviewed journal publications, reports, and other publications (e.g., protocols, research briefs, plain language summaries) (Fig. 4). To date, this work has resulted in 34 peer-reviewed publications/submissions, with 104 reports delivered, and 109 other knowledge products such as power point presentations, 1-page briefs, blog posts, op-eds, and infographics.
Fig. 4.

When applicable, at the conclusion of the research project, results are submitted to an open access peer-reviewed journal to make findings available to broader audiences. The SPOR Evidence Alliance also encourages dissemination of findings at scientific meetings and, in some instances, provides funding to allow trainees and early career researchers to attend scientific meetings and conferences for the purpose of disseminating study findings. A one-page executive summary in plain language is also co-created with patient partners for each completed project and posted on the SPOR Evidence Alliance website (SPOR Evidence Alliance n.d.-d).
How does the SPOR Evidence Alliance work with other query service providers in Canada?
Knowledge users in Canada can benefit from other similar existing research services, such as those offered by the national-level CIHR DSEN (Canadian Institutes of Health Research n.d.-a) and CADTH (Canadian Agency for Drugs and Technologies in Health n.d.-b). The scope of DSEN queries is limited to the safety and (or) effectiveness of prescribed drugs available in the Canadian market and can only be accessed by federal regulators; federal, provincial, and territorial drug plans; and organizations mandated to support government decision-making with respect to drugs (e.g., Public Health Agency of Canada) (Canadian Institutes of Health Research 2012). In contrast, CADTH query programs involve health technologies and medical devices, and these queries are only accepted from Health Canada, provincial ministries, regional/local health authorities, hospitals, or federally or regionally administered health care programs in a contributing jurisdiction (Canadian Agency for Drugs and Technologies in Health n.d.-a). Whenever the SPOR Evidence Alliance receives queries that may fall within the scope of DSEN or CADTH research services, we check with these groups first to see if the request is better directed to them, and whether there are any redundancies or duplication. If similar work is underway, we inform the query submitter and arrange to have the findings shared with them. In some instances, an update of the existing knowledge base to capture recently published research may be considered.
Others within the SPOR enterprise also offer query services in knowledge synthesis, so we also check for redundancy and duplication with those entities. When appropriate, the SPOR Evidence Alliance research team collaborates with other SPOR entities to respond to a query. For example, the Ministère de la Santé et des Services sociaux (Québec Ministry of Health and Social Services) reached out to the SPOR Evidence Alliance in April 2018 seeking evidence on the scope of family physician practice to inform a position statement on the nature of family practice in Quebec (Zomahoun et al. 2021). To respond to this query, the SPOR Evidence Alliance partnered with the knowledge synthesis platform of the Québec SUPPORT Unit, which is the provincial research service centre funded by the SPOR enterprise.
During the COVID-19 pandemic, the SPOR Evidence Alliance collaborated and coordinated with the McMaster Health Forum’s COVID-END platform (McMaster Forum n.d.), CAN-COVID (CanCOVID n.d.), and the Ontario Ministry of Health and Long-Term Care in prioritizing and addressing an overwhelming demand for rapid scientific evidence related to the pandemic. Since the pandemic, the SPOR Evidence Alliance responded to 37 requests for knowledge synthesis by decision-makers at the provincial, national, and international levels (SPOR Evidence Alliance n.d.-b). To ensure ease of access to research findings, we post information about all completed or in-progress COVID-19-related projects on the SPOR Evidence Alliance website (SPOR Evidence Alliance n.d.-d). When applicable, we also support research teams to register the research topic with the National Collaborating Centre for Methods and Tools (National Collaborating Centre for Methods and Tools n.d.), to publish their findings on medRχiv (pre-print server for health sciences) (medRxiv n.d.), and prepare submissions to open access peer-reviewed journals.
Discussion and conclusions
The SPOR Evidence Alliance was founded in August of 2017 with a 5-year nonrenewal grant from the CIHR and with the support of 41 cash and in-kind sponsors from public and not-for-profit sectors. Over the past 3 years of operation, the SPOR Evidence Alliance has established an inclusive and collaborative model to accept and respond to decision-maker and stakeholder requests for high-quality evidence in a timely manner.
Our network of research teams across Canada have conducted 94 unique purpose-driven research projects to address a knowledge gap and decision-maker evidence needs. During the COVID-19 pandemic, the SPOR Evidence Alliance’s collaborative approach has helped to coordinate with research groups nationally and internationally in addressing an outpour of demand for urgent scientific evidence by decision-makers and stakeholders globally. The strength of the SPOR Evidence Alliance lies in the meaningful engagement of decision-makers (including patient partners) in all research activities. An inclusive and collaborative model is used to gather input from decision-makers who are requesting the query service and other stakeholders who might be impacted by the research results in shaping the health research landscape in a meaningful way. The SPOR Evidence Alliance continues to explore ways to enhance meaningful patient partner engagement in query responses with rapid timelines.
Our model of collaboration and purpose-driven research ensures that efforts to address health research needs of decision-makers are appropriately informed, thereby reducing inefficiencies and waste. As the 5-year funding period of the SPOR Evidence Alliance nears, it is important to strategically build sustainable partnerships with sponsors and decision-makers nationally and internationally, so that we can continue to build a rapid learning health system across Canada and beyond.
Funding
This research was conducted through the SPOR Evidence Alliance which is supported by the Canadian Institutes of Health Research (CIHR) under Canada’s Strategy for Patient-Oriented Research (SPOR) initiative, and the generosity of partners from 41 public and not-for-profit sectors across Canada. SES is funded by a Tier 1 Canada Research Chair in Knowledge Translation. ACT is funded by a Tier 2 Canada Research Chair in Knowledge Synthesis. ACT leads the Drug Safety and Effectiveness Network (DSEN) Methods and Applications Group for Indirect Comparisons (MAGIC) funded by the Canadian Institutes of Health Research (DMC-166263). LCL holds a Tier 2 Canada Research Chair in Patient-Oriented Knowledge Translation.
Acknowledgments
We gratefully acknowledge Sinit Michael for referencing and providing logistical support in the preparation of this manuscript.
References
AGREE. n.d. Advancing the science of practice guidelines [online]: Available from agreetrust.org/.
Antman EM, Lau J, Kupelnick B, Mosteller F, and Chalmers TC. 1992. A comparison of results of meta-analyses of randomized control trials and recommendations of clinical experts. Journal of the American Medical Association, 268(2): 240–248.
Brouwers MC, Spithoff K, Lavis J, Kho ME, Makarski J, and Florez ID. 2020. What to do with all the AGREEs? The AGREE portfolio of tools to support the guideline enterprise. Journal of Clinical Epidemiology, 125: 191–197.
Burczycka M, Conroy S, and Savage L. 2018. Family violence in Canada: a statistical profile, 2017 [online]: Available from www150.statcan.gc.ca/n1/pub/85-002-x/2018001/article/54978-eng.pdf.
Canadian Agency for Drugs and Technologies in Health. n.d.-a. About the Rapid response service [online]: Available from cadth.ca/about-cadth/what-we-do/products-services/rapid-response-service.
Canadian Agency for Drugs and Technologies in Health. n.d.-b. CADTH [online]: Available from cadth.ca/.
Canadian Institutes of Health Research. 2011a. Two research-to-practice valleys: Canada’s strategy for patient-oriented research [online]: Available from cihr-irsc.gc.ca/e/44000.html#a2.2.
Canadian Institutes of Health Research. 2011b. Why is a strategy for patient-oriented research important now?: Canada’s strategy for patient-oriented research [online]: Available from cihr-irsc.gc.ca/e/44000.html#a1.2.
Canadian Institutes of Health Research. 2012. Framework for the management of DSEN queries [online]: Available from cihr-irsc.gc.ca/e/documents/framework-for-the_management-of_dsen_queries_e.pdf.
Canadian Institutes of Health Research. 2016. Evaluation of the strategy for patient-oriented research [online]: Available from cihr-irsc.gc.ca/e/49937.html.
Canadian Institutes of Health Research. n.d.-a. Drug safety and effectiveness network [online]: Available from cihr-irsc.gc.ca/e/40269.html.
Canadian Institutes of Health Research. n.d.-b. A guide to knowledge synthesis: a knowledge synthesis chapter [online]: Available from cihr-irsc.gc.ca/e/documents/knowledge_synthesis_chapter_e.pdf.
Canadian Institutes of Health Research. n.d.-c. Knowledge translation [online]: Available from cihr-irsc.gc.ca/e/29529.html.
Canadian Institutes of Health Research. n.d.-d. SPOR in action [online]: Available from cihr-irsc.gc.ca/e/51040.html.
Canadian Institutes of Health Research. n.d.-e. Synthesis resources [online]: Available from cihr-irsc.gc.ca/e/36331.html.
CanCOVID. n.d. CanCOVID [online]: Available from cancovid.ca/.
Equator Network. n.d. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement [online]: Available from equator-network.org/reporting-guidelines/prisma/.
Esmail R, Hanson HM, Holroyd-Leduc J, Brown S, Strifler L, Straus SE, et al. 2020. A scoping review of full-spectrum knowledge translation theories, models, and frameworks. Implementation Science, 15(1): 11.
Field MJ, and Lohr KN. 1990. Clinical practice guidelines: Directions for a new program. In Institute of Medicine Committee to Advise the Public Health Service on Clinical Practice Guidelines. National Academies Press, Washington DC.
Graham I, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, et al. 2006. Lost in knowledge translation: Time for a map? The Journal of Continuing Education in the Health Professions, 26(1): 13–24.
Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. 2004. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technology Assessment, 8(6): iii–iv, 1–72.
Guidelines International Network. n.d. [online]: Available from g-i-n.net/home.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. 2011. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology, 64(4): 383–394.
Hamilton C, Hoens AM, Backman CL, McKinnon AM, McQuitty S, English K, et al. 2018. An empirically based conceptual framework for fostering meaningful patient engagement in research. Health Expectations, 21(1): 396–406.
Hamilton C, Snow ME, Clark N, Gibson S, Dehnadi M, Lui M, et al. 2019. Quality of patient, family, caregiver and public engagement in decision-making in healthcare systems: A scoping review protocol. BMJ Open, 9(11): e032788.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, and Welch VA (editors). 2022. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. [online]: Available from training.cochrane.org/handbook.
International Association for Public Participation. n.d. IAP2 spectrum [online]: Available from iap2canada.ca/Resources/Documents/0702-Foundations-Spectrum-MW-rev2%20(1).pdf.
Ioannidis JP. 2005a. Contradicted and initially stronger effects in highly cited clinical research. Journal of the American Medical Association, 294(2): 218–28.
Ioannidis JP. 2005b. Why most published research findings are false. PLoS Medicine, 2(8): e124.
James Lind Alliance. 2021. The James Lind Alliance guidebook [online]: Available from jla.nihr.ac.uk/jla-guidebook/downloads/JLA-Guidebook-Version-10-March-2021.pdf.
Joanna Briggs Institute. 2019. JBI reviewer’s manual [online]: Available from wiki.joannabriggs.org/display/MANUAL.
Jun M, Manns B, Laupacis A, Manns L, Rehal B, Crowe S, et al. 2015. Assessing the extent to which current clinical research is consistent with patient priorities: A scoping review using a case study in patients on or nearing dialysis. Canadian Journal of Kidney Health and Disease, 2: 35.
Knowledge Translation Program. n.d. What review is right for you? [online]: Available from whatreviewisrightforyou.knowledgetranslation.net/.
Kovach M. 2010. Conversational method in Indigenous research. First Peoples Child & Family Review, 5(1): 40–48.
Lavallée L. 2009. Practical application of an indigenous research framework and two qualitative indigenous research methods: Sharing circles and Anishnaabe symbol-based reflection. International Journal of Qualitative Methods, 8(1): 21–40.
Li LC, Hoens AM, Wilhelm L, Bubber V, PausJenssen E, McKinnon A, et al. 2022. Patient engagement in the SPOR Evidence Alliance: Reflection and learnings. FACETS 7(1): 126–138.
Lunny C, Zarin W, Chaudhry S, Thomas SM, LeBlanc A, Desroches S, et al. 2022. An inclusive and diverse governance structure of the strategy for patient-oriented research (SPOR) evidence alliance. FACETS 7(1):
McMaster Forum. n.d. COVID-19 evidence network to support decision-making [online]: Available from mcmasterforum.org/networks/covid-end.
medRxiv. n.d. medRxiv [online]: Available from medrxiv.org/.
National Collaborating Centre for Methods and Tools. n.d. COVID-19 Rapid evidence reviews [online]: Available from nccmt.ca/covid-19/covid-19-evidence-reviews.
Patrick K, Kebbe M, and Aubin D. 2018. A home for patient-oriented research. Canadian Medical Association Journal, 190(20): E607.
Rothe JP, Ozegovic D, and Carroll LJ. 2009. Innovation in qualitative interviews: “Sharing Circles” in a First Nations community. Journal of the International Society for Child and Adolescent Injury Prevention, 15(5): 334–340.
Shekelle PG, Woolf SH, Eccles M, and Grimshaw J. 1999. Clinical guidelines: Developing guidelines. British Medical Journa, 318(7183): 593–596.
SPOR Evidence Alliance. n.d.-a. Asset map of Canadian clinical practice guideline developers [online]: Available from sporevidencealliance.ca/key-activities/cpg-asset-map/.
SPOR Evidence Alliance. n.d.-b. COVID-19 evidence synthesis [online]: Available from sporevidencealliance.ca/test-2/covid-19-evidence-synthesis/.
SPOR Evidence Alliance. n.d.-c. Policy-maker and healthcare provider research query [online]: Available from sporevidencealliance.ca/submit-a-request/make-a-query-suggestion-to-the-alliance/.
SPOR Evidence Alliance. n.d.-d. SPOR evidence alliance [online]: Available from sporevidencealliance.ca.
Straus S, Tetroe J, and Graham I. 2009. Defining knowledge translation. Canadian Medical Association Journal, 181(3–4): 165–168.
The Lancet. 2014. Research: Increasing value, reducing waste [online]: Available from thelancet.com/series/research.
Tricco A, Zarin W, Clement F, Abou-Setta A, Curran J, LeBlanc A, et al. 2022. Introducing the strategy for patient oriented research (SPOR) evidence alliance: A partnership between researchers, patients and health system decision-makers to support Rapid-learning and responsive health systems in Canada and beyond. FACETS 7(1).
Tricco A, Zarin W, Rios P, Nincic V, Khan PA, Ghassemi M, et al. 2018. Engaging policy-makers, health system managers, and policy analysts in the knowledge synthesis process: A scoping review. Implementation Science, 13(1): 31.
U.S. Preventive Services Task Force. n.d. Methods and processes [online]: Available from uspreventiveservicestaskforce.org/uspstf/methods-and-processes.
University of Alberta. 2011. Canadian task force on preventive health care [online]: Available from sites.ualberta.ca/∼mtonelli/manual.pdf.
World Health Organization. 2002. The conceptual basis for measuring and reporting on health. [online]: Available from who.int/healthinfo/paper45.pdf.
Zomahoun HTV, Samson I, Sawadogo J, Massougbodji J, Gogovor A, Diendéré E, et al. 2021. Effects of the scope of practice on family physicians: A systematic review. BMC Family Practice, 22(1): 12.
Information & Authors
Information
Published In

FACETS
Volume 7 • Number 1 • January 2022
Pages: 420 - 434
Editor: Debra Clendinneng
History
Received: 30 August 2021
Accepted: 26 October 2021
Version of record online: 17 March 2022
Notes
This paper is part of a collection titled “Strategy for Patient Oriented Research (SPOR) Evidence Alliance: A Canadian Model to Build Rapid-learning Health Systems”.
Copyright
© 2022 Authors Zarin, Lunny, Chaudhry, Thomas, Leblanc, Clement, Abou-Setta, Curran, Hutton, Florez, Li, Bonstein, Hamilton, Moffitt, Godfrey, Fahim, Straus, and Tricco, and The Crown. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Data Availability Statement
The data and materials used and (or) analysed during this study are available from the corresponding author on reasonable request.
Key Words
Sections
Subjects
Authors
Author Contributions
WZ helped manage the research initiative, helped develop the operational procedures, and wrote the manuscript.
CL developed the initial outline and wrote sections of the manuscript.
AL, FC, AMA-S, JAC, LCL, PM, CG, SES, and ACT obtained funding, conceptualized the research initiative, provided guidance on the operation and direction of the initiative, and reviewed the manuscript.
All contributed to the interpretation and narrative of the paper, reviewed and revised the content, approved the final version, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work.
Competing Interests
The other authors have nothing to declare.
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Wasifa Zarin, Carole Lunny, Sabrina Chaudhry, Sonia M. Thomas, Annie LeBlanc, Fiona Clement, Ahmed M. Abou-Setta, Janet A. Curran, Brian Hutton, Ivan D. Florez, Linda C. Li, Stephen Bornstein, Clayon B. Hamilton, Pertice Moffitt, Christina Godfrey, Louise Zitzelsberger, Leanne Gardiner, Christine Fahim, Sharon E. Straus, and Andrea C. Tricco. 2022. A Canadian model for providing high-quality, timely and relevant evidence to meet health system decision-maker needs: the SPOR Evidence Alliance. FACETS.
7(): 420-434. https://doi.org/10.1139/facets-2021-0132
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
Cited by
1. Comments regarding “A Canadian model for providing high-quality, timely and relevant evidence to meet health system decision-maker needs: the SPOR Evidence Alliance.”
2. An inclusive and diverse governance structure of the strategy for patient-oriented research (SPOR) Evidence Alliance
