Open drug discovery of anti-virals critical for Canada’s pandemic strategy
Abstract
In the event of the current COVID-19 pandemic and in preparation for future pandemics, open science can support mission-oriented research and development, as well as commercialization. Open science shares skills and resources across sectors; avoids duplication and provides the basis for rapid and effective validation due to full transparency. It is a strategy that can adjust quickly to reflect changing incentives and priorities, because it does not rely on any one actor or sector. While eschewing patents, it can ensure high-quality drugs, low pricing, and access through existing regulatory mechanisms. Open science practices and partnerships decrease transaction costs, increase diversity of actors, reduce overall costs, open new, higher-risk/higher-impact approaches to research, and provide entrepreneurs freedom to operate and freedom to innovate. We argue that it is time to re-open science, not only in its now restricted arena of fundamental research, but throughout clinical translation. Our model and attendant recommendations map onto a strategy to accelerate discovery of novel broad-spectrum anti-viral drugs and clinical trials of those drugs, from first-in-human safety-focused trials to late stage trials for efficacy. The goal is to ensure low-cost and rapid access, globally, and to ensure that Canadians do not pay a premium for drugs developed from Canadian science.
Introduction
In times of crisis, we reflexively turn to open science as a solution (OECD 2020). Canada’s Innovation, Science and Industry Minister, Navdeep Bains, lauded Ontario researchers for their rapid ability to isolate the SARS-CoV-2 virus that causes COVID-19 and join the global scientific effort to combat the pandemic. He tweeted: “Open Science is key to global efforts to treat and prevent the spread of COVID-19.”
Scientific discovery has historically been an open enterprise, and open science has a proven track record in accelerating science. Open science comprises a set of institutional policies and practices that foster collaborative relationships and infrastructure with minimal reliance on restrictive intellectual and other property rights (Ali-Khan, et al., 2018). Open science has been instrumental in mission-driven, international, coordinated efforts for the public good, such as the Human Genome Project. However, the increasing emphasis on commercialization of public science—university patenting, spin-off company creation, and technology licensing for revenue—has reduced the scope of open science (David 2008, 2014; Bubela and Caulfield 2010; Popp Berman 2012). The current emphasis on proprietary principles for foundational technologies and at every further step along the drug discovery continuum diminishes our capacity for collective action to solve complex health problems (Sampat 2020). At this time, we confront just such a complex health problem: the COVID-19 pandemic, with the next emergent pandemic potentially on the horizon, the “G4” swine flu virus (Centers for Disease Control and Prevention 2020b).
Canada needs a proactive innovation strategy to address current needs and to anticipate future pandemics. Canada and governments around the world made a tactical error in failing to build public capacity to develop novel anti-virals, preferring instead to hand off drug discovery and development to the private sector. The flaw in this logic for emerging pathogens is that it assumes that the private sector has an incentive to invest in drugs for emerging threats that may never occur. The private sector does not, which results in market failure. Advance preparation through development of novel drugs against viruses with pandemic potential is possible, but we need a shared strategy, leadership and coordination among industry, academia, government and philanthropy, which, we argue an open drug discovery model can provide. Canada has invested in open science and can be a global leader in supporting an open science innovation strategy for pandemic preparedness.
Here we discuss how an open science model for drug discovery can support mission-oriented research and development (R&D), as well as commercialization. Open science shares skills and resources across sectors, reducing both individual and collective risk of failure. It avoids duplication because everyone knows what everyone else is doing, while it provides the basis for rapid and effective validation due to full transparency. Not relying on any one company or sector, it is a strategy that can adjust quickly to reflect changing incentives and priorities. While eschewing patents, it can ensure high quality drugs, low pricing, and access through existing regulatory mechanisms. Open science practices and partnerships decrease transaction costs, increase diversity of actors, reduce overall costs, open new, higher-risk/higher-impact approaches to research, and provide entrepreneurs freedom to operate and freedom to innovate.
We argue that it is time to re-open science, not only in its now restricted arena of fundamental research, but throughout the drug discovery pathway. Our model maps onto a strategy to accelerate discovery of novel broad-spectrum anti-viral drugs and clinical trials of those drugs, from first-in-human safety-focused trials to late-stage trials for efficacy. The goal is to ensure low-cost and rapid access, globally, and to ensure that Canadians do not pay a premium for drugs developed from Canadian science. The utility of open science is clear in a pandemic. However, it equally applies to other areas of market and collective action failure—neglected and ultra-rare diseases—and beyond.
We presage our argument with a discussion on why Canada and the world needs a proactive, targeted anti-viral strategy (the mission), followed by a discussion of role of the State in supporting mission-oriented research and development, especially where there is market failure. Such market failure exists when there is a lack of incentives for for-profit companies and a lack of private-sector financing models. We then make the case why open science at each stage along the drug research and discovery continuum might accelerate the mission towards pandemic preparedness and conclude with recommendations for how such a model might be implemented.
Why a proactive, targeted anti-viral strategy?
Reliance on any one strategy to combat and control SARS-CoV-2 and other viruses with pandemic potential leaves Canada and the world exposed to a long-term, high risk of health, social, and economic problems. The current COVID-19 pandemic offers a stark example. While we hope for an effective vaccine, the immunology of many viruses with pandemic potential teaches us that there is no guarantee that any vaccine will confer long-term immunity for the majority of the population. The average time from pre-clinical development through to market authorization for a vaccine is >10 years, and only 6% make it through to market entry (Pronker et al. 2013). As illustrations, massive international investment and efforts have still not resulted in an effective HIV vaccine, and it took 43 years from discovery of the Ebola virus to vaccine (Mullard 2020). While some COVID-19 vaccine candidates have demonstrated potential, others are falling victim to the proprietary model under which they were developed, with concerns mounting as to cost, national priority, and global access.
Similarly, while repurposing old drugs to identify anti-viral activity appears as an attractive quick win, it is a high-risk strategy with a limited history of success. There are currently hundreds of treatments under consideration as anti-virals for COVID-19, the majority of these are repurposing efforts, including over 20 high-throughput preclinical screening programs of antiviral drug candidates underway globally by a mix of public and private sector actors (Milken Institute 2020). The three most notable repurposing efforts to identify anti-viral treatments for COVID-19 have been hydroxy chloroquine (HCQ), a repurposed anti-malarial drug; Gilead’s Remdesivir, an RNA polymerase inhibitor that failed in Ebola, based on demonstrated potent antiviral activity in vitro and efficacy in animal models of COVID-19; and the HIV therapeutic combination of lopinavir-ritonavir, which did not demonstrate clinical benefit in hospitalized COVID-19 patients (RECOVERY: Randomised Evaluation of COVID-19 Therapy 2020a, World Health Organization 2020b).
The pendulum of enthusiasm for HCQ has been well documented, starting with social media posts by Elon Musk, French researchers, and President Trump characterizing is as a “game changer” in March 2020 (Sattui et al. 2020). Early but poorly designed studies suggested its efficacy (Funck-Brentano and Salem 2020), and the US Food and Drug Administration (FDA) issued an Experimental Use Authorization (EUA). A now retracted study highlighted adverse effects of the drug (Lenzer 2020; Offord 2020), which resulted in the World Health Organization (WHO) suspending the HCQ arm of its Solidarity clinical trial (World Health Organization 2020b); other trials followed suit. A large observational US-based study found no benefit from the drug (Geleris et al. 2020), and the final nail in the coffin of HCQ clinical trials around the world came from the well-designed, pragmatic UK-based RECOVERY trial, which demonstrated a lack of efficacy in early June (RECOVERY: Randomised Evaluation of COVID-19 Therapy 2020b). HCQ also did not prevent COVID-19 when taken prophylactically, as recommended by President Trump (Boulware et al. 2020). By 15 June 2020, the FDA had revoked its EUA. Distressingly, there were nearly 200 trials of HCQ, including many registered with Health Canada, of which more than a third planned to enroll fewer than 100 people—too few to make conclusions and preventing the patients from participating in trials for other drugs.
Remdesivir is similarly not a wonder drug (Kmietowicz 2020; Mahase 2020a). A randomized, placebo controlled, double-blinded clinical trial in China demonstrated no statistically significant clinical benefit to patients (Wang et al. 2020). Preliminary results from a randomized, controlled UK clinical trial found a statistically significant reduction from 15 days to 11 days hospitalization for patients with a respiratory tract infection, but there was no overall reduction in mortality (Beigel et al. 2020). Ten versus five days of dosing did not improve patient outcomes (Goldman et al. 2020). The current pricing in developed countries has been set at US$2340 for a five-day course, with US insurers, Medicare and Medicaid paying 33% more. Gilead is licensing to generic manufacturers to lower pricing in developing countries (Herper 2020). However, critics state that the value to health systems based on cost effectiveness could be as low as $310 if the drug does not lower mortality from the virus (Herper 2020). An international panel, including Canadians, recently reviewed the 23 randomised controlled trials, suggesting that the evidence base remained uncertain and that “the use of the drug may divert funds, time, attention, and workforce away from other potentially worthwhile treatments” (Mahase 2020b). Indeed, the RECOVERY trial has found that the low-cost anti-inflammatory steroid, dexamethasone maybe a better option, because it reduced death by up to one-third in hospitalized patients with severe respiratory complications of COVID-19 (Mahase 2020b, #18354; The RECOVERY Collaborative Group 2020).
In light of the above limitations, it is clear we cannot rely on repurposing to get us through this or the next pandemic. We need to prioritize the discovery and development of novel, targeted anti-virals as an essential component of the global arsenal to both prevent infection and treat COVID-19 and emerging pandemic viruses. Indeed, all leading experts know that even if our vaccine efforts are successful, anti-virals (and likely a cocktail of them) will be needed to treat a significant fraction of the infected population. Not all individuals can or will choose to be vaccinated (Schaffer DeRoo et al. 2020), vaccines may not offer complete immunity, especially in the elderly, and immunity may wane over time (Centers for Disease Control and Prevention 2020a).
The role of the entrepreneurial state and philanthropies in driving mission-oriented R&D
As global public goods, novel anti-virals need to be affordable, which implicates the form of R&D investment, the cost and scale-up of manufacture, uptake by health systems, and low-cost mode of administration to patients. However, because society treats most new medicines as private assets, pandemic drug research faces similar problems to areas where there are unmet medical needs, such as neglected and ultra-rare diseases, which suffer from a lack of market incentives due to low profit margins or small patient populations, respectively. Some economists consider these to be market failures, which occur when the distribution of goods or services by competitive markets is inefficient; incentives for individual decisions do not lead to rational outcomes for the collective (Stiglitz 1989). Collective action failures arise when two or more individuals cannot coordinate their actions to accomplish an outcome. This may be due to lack of appropriate incentives, lack of trust, informational asymmetries, or poor governance (Olson 1965; Ostrom 2005, 2015).
However, legitimizing state action in the context of market failure “concedes too much to market fundamentalists” (Kay 2007) because it is based on the premise that markets can be complete and efficient (Janeway 2012). In The Entrepreneurial State, Mazzucato (2011) argued that the historical role of the State in innovation is greater than stepping in to fix market failures. The State may take on risks over timelines that would be unacceptable to private capital; it may create new markets and networks of actors. These networks may harness the best of private and public sectors for national or global good in a manner that is symbiotic, not parasitic. Indeed, the innovations that have transformed societies, from transportation systems to space exploration, personal computers and the Internet all derived from massive State investment in science and innovation networks, where the investments were decoupled from pure considerations of economic return and “wasted” efforts that did not result in economic returns were considered the price of admission (Janeway 2012). While these State investments were generally coupled to national defence and security concerns, other initiatives, such as public health systems, also require the State to motivate collective action. These systems are similarly not market driven, but are based on different motivations, such as compassion, fairness, and solidarity (Kay 2007; Janeway 2012, p. 232).
In response to the COVID-19 pandemic, State funding for R&D for vaccines and drugs has been made available. For example, the United States Senate passed the Coronavirus Aid, Relief, and Economic Security (CARES) Act on 27 March 2020, which includes over US$6 billion dedicated to R&D. Specifically, the Act provides US$3.5 billion to the Biomedical Advanced Research and Development Authority (BARDA) for manufacturing, production, and purchase of vaccines, therapeutics, diagnostics, and related products, effectively creating a State-supported market for those products (Coronavirus Aid, Relief, and Economic Security (CARES) Act 2020). Canada and other countries have similarly invested heavily in R&D. By 20 July 2020, the Milkin Institute cited 197 vaccine candidates in development, globally, with 19 already in clinical trials (COVID-19 Vaccine Tracker 2020). Some leading candidates are being developed via public–private partnerships between BARDA or other US government agencies and Pharma/Biotech Firms. Demonstrating the power of the State in driving pandemic markets, the US government has committed US$2.1 billion to Sanofi and GlaxoSmithKline to supply 100 million doses of their jointly developed vaccine. The federal funding includes clinical trials and manufacturing (Thomas 2020). Nevertheless, concerns remain about the fair global distribution of a pandemic vaccine (Liu et al. 2020) and sufficient availability over time, as the vaccine may need to be given recurrently over a lifetime of risk.
This level of State intervention in vaccine and therapeutics R&D is commensurate with the estimated impact on the global economy. The World Bank, in its June 2020 Global Economic Prospects, projects a 5.2% contraction in global GDP in 2020 and the largest proportion of countries in recession in 150 years (worldbank.org/en/publication/global-economic-prospects). While the Bank calls for the strengthening of public health systems throughout the world in response to the current pandemic, history would suggest that the lessons of 2020 will rapidly fade from view.
In 2002, another coronavirus, the SARS-CoV virus, which causes severe acute respiratory syndrome, first infected humans in southern China. From there it spread to 26 countries, with 8000 reported cases and about 700 deaths, 44 in Canada (World Health Organization 2020a). The travel and tourism industry was severely impacted, but strict quarantine and contact tracing curtailed the spread of the virus and, by mid-2003 the World Health Organization declared the outbreak over. This episode should have been a global wakeup call to prepare for zoonotic viruses with pandemic potential. However, efforts at developing a vaccine stalled in preclinical studies as the outbreak ended (Jiang et al. 2005) and no anti-virals were developed.
In addition to States, philanthropies have also historically funded mission-oriented research. In the current context, an example is the Drugs for Neglected Diseases initiative (DNDi), which is an international not-for-profit R&D organization, funded initially by the 1999 Nobel prize money awarded to Médicins Sans Frontiers (MSF) to develop affordable treatments for neglected diseases (DNDi: Drugs for Neglected Diseases Initiative 2020a). In addition to MSF, its founding partners are the Institut Pasteur, Fundação Oswaldo Crux, the WHO’s Special Programme for Research and Training in Tropical Diseases, the Malaysian Ministry of Health and the Indian Council of Medical Research. DNDi initiates and coordinates global collaborations, bringing together the strengths of public, private, academic, nonprofit, and philanthropic sectors. It has over 180 partners in over 40 countries.
The above factors lead to the conclusion that States and philanthropies have a role to play not only in controlling the current pandemic but also in mission-oriented science to prepare for future pandemics. Several viral families that cross species boundaries and therefore have pandemic potential in a naïve human population are already known. While public health measures include improved surveillance systems and rapid deployment of measures to reduce spread, we have the opportunity to harness R&D strengths in anti-viral drug discovery, and coordinate efforts in both the private and public sectors. Pandemic preparedness cannot be left to the market. Promising drugs may be developed that will never be used because the pandemic never materializes or materializes many years beyond the period of monopoly protection. Given the average success rate of drugs through clinical development (<10%) (Hay et al. 2014), some degree of “wasted” effort towards drugs that will not work or never be used must be accepted. The analogy is to fire extinguishers, the majority of which are never used. The alternative may be many lives lost unnecessarily, and global economic disaster. In the next section we suggest that the most efficient strategy for developing pandemic anti-virals is State/philanthropy-supported open science.
Why open science?
Open science is a key to both advancing antiviral R&D and ensuring sustainability of R&D in preparation for future pandemics. Open science comprises a set of practices that avoid restrictive intellectual property rights. These practices relate to publications, data, research reagents, methods, software and other tools, and infrastructure. Open science aims is to reduce costs associated with negotiations over intellectual property rights, thereby lowering the transactional barriers to collaboration; promote data re-use and recontribution; distribute project risk; increase rigour and trust through transparency and reproducibility of results (Jasny et al. 2017); reduce redundant research, for example, by enabling access to data that disproves a hypothesis or mechanism of action; and enable the more rapid generation of new hypotheses (Gold et al. 2019). In the context of clinical trials, expanding open science further along the drug discovery continuum provides a mechanism to share the results from projects and trials that do not meet their goals, avoiding multiple trials of compounds based on the same disproven hypotheses, which has the added benefit of not exposing clinical trial participants to the risks of duplicative and ineffective interventions (Guizzaro 2018). Open science even redefines the notion of failure as “waste” as shared negative or equivocal results will contribute to learning—the more various nontoxic interventions are tested in people, the more we will learn about human biology and physiology.
Ironically, the COVID-19 hydroxychloroquine story illustrates the dangers of proprietary science and the need for openness and transparency in clinical research (Offord 2020). Both The Lancet and the New England Journal of Medicine were forced to retract high profile studies (Mehra et al. 2020a, 2020c) that purported to demonstrate the harmful effects of the drug, including cardiac complications (Mehra et al. 2020b; The Lancet Editors 2020). Those studies relied on a proprietary database of patient data from US health systems held by Surgisphere Corporation and were neither independently audited nor accessible to the academic and clinician authors of the papers. Similarly, the development of the Moderna vaccine and Gilead Sciences stories highlight the risk of reliance on proprietary and venture-backed interests of biotech companies (Sampat 2020). The latter is unlikely to price Remdesivir at a price that will enable global access and the former is now mired in a patent dispute. A recent decision by the US Patent and Trademark Office Patent Trial and Appeal Board (2020) threatens US biotech Moderna’s lead vaccine candidate. Moderna had attempted to invalidate a patent which covers part of its RNA technology held by Arbutus Biopharma (while Arbutus has an office in Vancouver, all of its R&D and other assets are in Pennsylvania). The decision leads to uncertainty and delay, as licensing negotiations may need to take place, depending on whether the Arbutus patent covers both drug discovery and vaccine development (Garde 2020).
In the context of pandemic preparedness, open science needs to apply further along the drug discovery continuum than is currently the norm (Fig. 1). Open science provides a platform through which to simplify the creation and administration of public–private–philanthropy partnerships to address target discovery and drug discovery by eliminating reliance on intellectual property. Open science also has the potential to diversify project teams by making it easier, faster, and less expensive for small firms, communities and others to participate in projects (Gold 2016). Finally, open science efforts can leverage mechanisms—such as contracting or regulatory mechanisms—to reduce final drug costs.
Fig. 1.
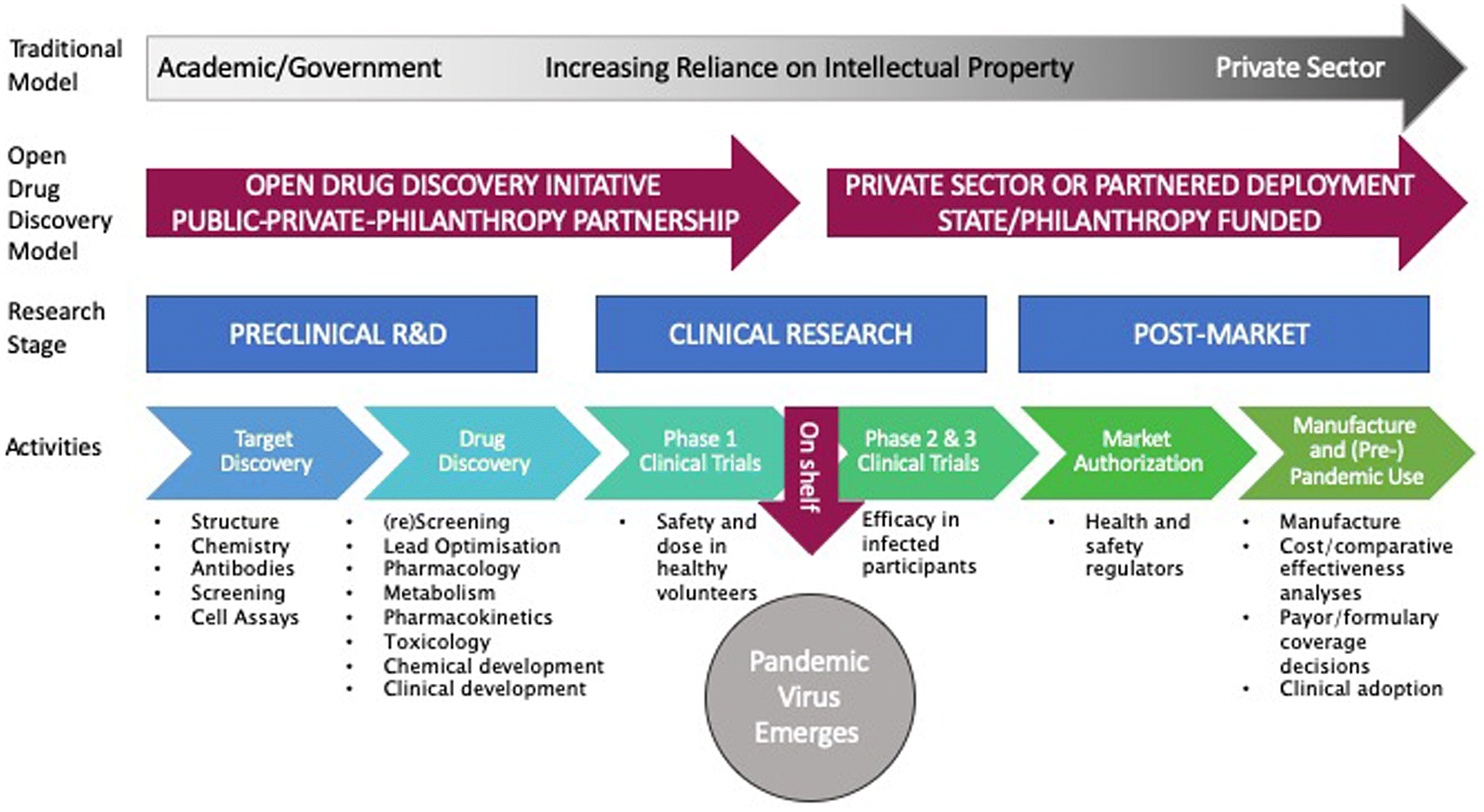
We recommend a strategy of coordinating the rapid development of novel anti-viral drug candidates and taking the most promising candidates through Phase 1 studies for safety and dosing, usually in healthy volunteers. Those derisked candidates, which have demonstrated safety in humans, may then be “left on the shelf,” poised to be tested for efficacy when a new virus with pandemic potential emerges. The drug candidate can be advanced into patients in Phase 2 or 3 clinical trials by any actor anywhere in the world, recognizing that these trials would be financed by public or philanthropic sources, or potentially by private sources that commit to pricing and distribution terms that privilege access. Those sources would further create the market for manufacturing, distribution, and use. Here we discuss the benefits and challenges for open science along the drug discovery continuum.
Mission-oriented research
Open science can accelerate mission-oriented research. For example, the sequencing of the human genome constituted one of the greatest scientific achievements in history. In under 15 years, the project went from conception to completion. Its success is not solely due to technological advances, however. The Human Genome Project would never had succeeded as quickly, or provided its immense social and economic good, had the scientific community not carried out its science under open science principles. This transformational position allowed scientists, institutions and nations to set aside proprietary interests, which had been delaying progress, and instead focus on coordinated collective action, on reducing duplication of effort, and on providing freedom for innovators to use the information to advance both public and private-sector R&D. In sum, open science underpinned the project’s success. Other sequencing initiatives have followed suit, including sequencing efforts for other model organisms and pathogens. Researchers continue to add value to public genomics databases, such as GenBank, by contributing improved or new data, or by adding to new knowledge through the annotation of gene function. The rapid sharing of the SARS-CoV-2 genome by researchers in China initiated R&D of diagnostics, therapeutics and vaccines around the world.
Foundational research
Current open science initiatives for COVID-19 mirror longstanding, global initiatives to render research publications, reagents, and data more open and accessible. Access to knowledge, data and reagents facilitates transparency, provides opportunity for replication of results—an essential component of the scientific method—avoids duplication of effort, and leads to novel hypotheses and avenues for research. Without restrictions imposed by intellectual property rights, such as patents, follow-on research is unencumbered. In other words, follow-on-researchers are assured of their “freedom-to-operate”, i.e., to use, improve on knowledge, data and reagents, and potentially to commercialize resultant innovations.
In the context of COVID-19, publishers of scientific journals and research funders around the world have committed to making preprints of submitted but not yet peer-reviewed articles and peer-reviewed research articles freely available (OASPA 2020). The publishers have further committed to ensuring that COVID-19 submissions include a data availability statement, which conforms with the FAIR principles of the Research Data Alliance; these apply to data (or any digital object), metadata (data about the digital object), and infrastructure. FAIR data are Findable via metadata and identifiers in a registry or searchable resource; Accessible, including openly or via authentication or authorization; Interoperable with other data, applications or workflows; and Re-useable, meaning the metadata and data are well-enough described so that they can be replicated or combined in different settings (Wilkinson et al. 2016). Building on open research datasets, other initiatives are providing automated tools for analysis. Others have pledged to make their “intellectual property available free of charge for use in ending the COVID-19 pandemic and minimizing the impact of the disease” through licensing (Open COVID Pledge 2020). Similarly, in Canada and globally, genomics initiatives, such as CanCOGEN (genomecanada.ca/en/cancogen) engaged in COVID-19 related research deploy open science principles; however, a discussion of these initiatives is beyond the scope of this article.
Drug discovery research
Canada hosts world-leading open science initiatives in support of precompetitive biomedical R&D. For example, the Structural Genomics Consortium (SGC) is a global public–private open science partnership with a Toronto campus that carries out early-stage drug discovery research and makes all its output freely available. Its funding and drug discovery partners include national and provincial funding agencies, global Pharma and biotech companies, patient organizations, and disease foundations. Its work in ascertaining the three-dimensional structure of medically relevant proteins accounts for 15% of global output of new human protein structures (thesgc.org). Leaders of the SGC in 2011 noted that 75% of research on human proteins still focused on 10% of proteins, many of which were known prior to the mapping of the human genome in 2001 (Edwards et al. 2011). They noted, however, that the unrestricted availability of high-quality tools, in this case chemical probes for identified druggable targets on the proteins, led to diversification of research. In response to this observation, the SGC began to make available high-quality chemical probes to support drug discovery (Edwards et al. 2009). The chemical probes against novel protein targets are made available to the scientific community without IP restrictions, enabling scientists around the world to interrogate the biological function, disease relevance, and druggability of these proteins. The open accessibility of these SGC chemical probes (more than 25 000 samples have been distributed to scientists around the world) has spurred downstream innovation in clinical development against novel disease targets, including here in Canada (Arshad et al. 2016). The first chemical probe (inhibitor) against the WDR5 protein, part of a very important protein family mutated in cancer, developed through an open science collaboration between the SGC and the Ontario Institute for Cancer Research (OICR), formed the basis of a promising novel leukemia drug by Toronto-based start-up Propellon Therapeutics (Gold and Morgan 2019).
The SGC shares all of its data and materials without restrictive intellectual property (IP). Within weeks of the release of the SARS-CoV-2 viral sequence, the SGC had purified half of the ∼25 different SARS-CoV-2 proteins and had begun to distribute them to Canadian scientists in all sectors. These proteins are key reagents to develop medicines, serve as potential vaccine candidates, and facilitate the development of serology diagnostics. Similarly, the Toronto-based Centre for Phenogenomics (TCP) is part of an international network of data and biorepositories for rodent models for biomedical R&D. Biorepositories, such as TCP and its US equivalent, the Jackson Laboratory (JAX). This networks simplifies the exchange of rodent reagents (e.g., sperm, embryos, mice, rats) and associated data as freely as possible via simplified conditions of use, which reduce transaction costs otherwise associated with more complicated material and data transfer agreements (MTAs and DTAs) (Bubela et al. 2017). TCP is in the final stages of validating its COVID-19 mouse model, essential for pre-clinical research, for distribution to the Canadian research community.
Clinical research
Drug discovery norms dictate that ideas from academia should be tested in clinical trials by the private sector, thereby shifting along the drug discovery continuum from precompetitive to competitive research, protected by patents and other forms of intellectual property. Indeed, many of the downstream efforts following on from SGC’s open science projects have followed this path. The OICR and its commercialization arm, FACIT, recently sold the Propellon Therapeutics leukemia program to Celgene in the largest deal for a preclinical drug in Canadian history (OICR NEWS 2019). This traditional approach has the advantage of attracting venture financing for further development of the drug, but in the absence of alternative models, the quid pro quo is that any knowledge gained remains proprietary, and private sector pressures influence the research agenda and potentially the accessibility and affordability of any drugs that receive market authorizations. The development of the Ebola vaccine out of the Public Health Agency of Canada’s Winnipeg laboratories and the subsequent public sector trialing of the vaccine following the 2014 Ebola outbreak, demonstrates the feasibility of a public sector-led drug development (Herder et al. 2020).
Given that the “first-in-man” test of an innovative idea or target is at its core a fundamental research exercise and a public good, open science is particularly apt, in the context of a pandemic, to carry out target and drug development through to end of Phase 1. Spreading risk across sectors and placing data in the public domain not only decrease duplication and increase coordination, but also ensures that another actor can accelerate its own development or pick up a project dropped by another. The SGC is pushing the boundaries of open science along the drug discovery continuum through the incorporation of a series of not-for-profit drug discovery companies (Edwards et al. 2017; Morgan et al. 2018). For example, the SGC spinoff, M4K Pharma Inc., is starting Investigational New Drug (IND)—enabling studies for a rare childhood brain cancer, diffuse intrinsic pontine glioma. M4K Pharma’s mission is to deliver safe, efficacious, and most importantly, affordable medicines that enable access to the patients that need them. M4K Pharma R&D is supported by multiple public, private and philanthropy funding sources, including the Ontario Institute for Cancer Research (OICR).
M4K Pharma follows the SGC model in rapidly sharing knowledge, results, data, and materials without patent restrictions (Morgan et al. 2018). These practices are inconsistent with a patent position that rewards inventions that are not already in the public domain. The patent bargain exchanges a 20-year right to exclude others from practicing the invention in exchange for the public disclosure of the invention in the patent document. Patents are published 18 months after the initial filing date of the patent, which should contain enough information that a skilled person could practice the invention. In reality, 18 months is not only too late during a pandemic but the information is rarely readily usable without the tacit know-how only shared through person–person collaboration or through licensing agreements. Costs for prosecuting the set of patents that would cover all aspects of drug discovery (e.g., the active ingredients, the formulation, the methods for manufacture, new uses, dosage forms) in multiple jurisdictions are in tens to hundreds of thousands of dollars and then enforcing those property rights against infringement, for example through litigation against would-be generic competitors, can run in the many millions of dollars.
How then, might an initiative or entity, such as M4K Pharma, retain control over its innovations to ensure it can deliver on its mission of affordable and accessible medicines? Patents are generally considered to be the most powerful tool to control an invention. Their purpose in drug discovery is to prevent generic or other competition, extending the patent term for as long as possible to command a monopoly price.
Instead of patents, we recommend using regulatory data protection and market exclusivities as mechanisms of control (Morgan et al. 2018). These regulatory mechanisms, described in detail in Morgan et al. (2018), arise automatically upon registration of a new compound as a drug, without additional cost, offering powerful protection, irrespective of patent protection or remaining length of the patent term. Data exclusivity is granted by drug regulatory agencies, such as the FDA, Health Canada, and the European Medicines Agency, over the information package on preclinical and clinical studies that support the case for market authorization for the intervention in question. Their effect is to prevent other companies from relying on these data for set periods of time (5–12 years), and often extend beyond the expiration of the 20-year patent period, most of which is lost during the 10–14 years it takes to bring a drug to market (Hay et al. 2014). Prior to expiry of data protection, to obtain marketing authorization, a would-be competitor would need to complete an entire preclinical and clinical development program sufficient to support its own independent product dossier, which in practice acts as a potent barrier to entry. Regulatory exclusivities provided to orphan disease products and novel antimicrobials offer even broader market protections (e.g., the 2012 US Generating Antibiotic Incentives Now (GAIN) Act) (Darrow and Kesselheim 2020).
Generic companies are able to produce off-patent drugs more cheaply because they can reference the information held by the regulators about safety and efficacy, without the added cost of repeating the studies. Regulatory product dossiers protected by data exclusivity provisions can therefore be licensed to enable manufacturing, impose price caps and to ensure global access. The benefit of relying on data exclusivity is that, unlike patents, it is not invalidated by prior public disclosure, making it possible to share scientific progress openly and quickly (Morgan et al. 2018). It also eliminates the additional costs of seeking patent protection and enforcing patents. Further, it does not preclude or require lengthy negotiations for follow-on innovation, as independent invention is not precluded by it.
Open science anti-viral drug discovery initiatives
An anti-viral drug discovery program requires coordination of multiple, parallel, projects to feed a drug discovery pipeline and overcome failures in collective action. One model for such an approach is DNDi, the governance structure of which reduces roadblocks to collaboration while promoting follow-on R&D, manufacturing, and equitable and affordable access to its products (DNDi.org). Its Intellectual Property Policy specifies that wherever possible, the results of its work are placed in the public domain; patenting is the exception, and the need for any form of intellectual property is judged on a case by case basis (DNDi 2018). Patents are not utilized to seek rents or pose barriers to follow-on research but may be used to negotiate contracts that result in the greatest benefit for the neglected patients. DNDi may also acquire rights to technologies to enable further research and access to patients. To date, DNDi has developed a new all-oral curative drug, Fexinidazole, for sleeping sickness, caused by the parasite T.b. gambiense (DNDi: Drugs for Neglected Diseases Initiative 2020b), pediatric treatment for Chagas disease, a strawberry-flavoured pediatric treatment for HIV, and cheaper treatments for hepatitis C, and it has active drug discovery programs for visceral and cutaneous leishmaniasis, Chagas disease, filaria (river blindness), and clinical trials for mycetoma (DNDi: Drugs for Neglected Diseases Initiative 2020c).
Bringing together the coordinated governance structure of DNDi and the open science approach of the SGC and M4K Pharma, Canada and the global community have the opportunity to undertake international pandemic preparedness anti-viral drug discovery initiatives. Two such initiatives have been launched in the US (READDI.org) and Canada (VIMIpen.org). Working in concert, these initiatives will coordinate and evaluate anti-viral candidates from academic or other centres that agree to abide by its open science principles in the context of pandemic preparedness. Their coordinated activities cover target discovery, with a focus on novel targets on human proteins (not viral proteins), such as those that help the virus evade the host immune system or enable the virus to infect human cells; identification of promising compounds that hit those targets, development of early lead compounds; lead optimization and candidate selection; preclinical enabling studies of promising candidates; and Phase 1 studies for safety and dosing in health volunteers. Validated compounds are poised to deploy in Phase 2 and 3 studies if and when a new virus emerges. With guaranteed State and philanthropy funding for clinical trials, manufacture, and use, Pharma and generic firms will be ready to participate to stave off a pandemic and its economic and social consequences. These private-sector participants would be guaranteed a reasonable return on any late-stage investments, but price caps would acknowledge the upfront and risky State and philanthropy contributions to R&D.
Rapid placing of methods, reagents, data, and analyses into the public domain creates prior art that prevents others from patenting around the initiative’s products and methods. The initiatives retain rights over data packages submitted to regulators on preclinical and Phase 1 clinical studies as a powerful form of intellectual property that can be used to exert control. A consolidated governance structure enables the use of standard form agreements that support socially responsible licensing strategies for data and reagents (Roskams-Edris and Gold 2019). As evidenced by the DNDi and SGC, such initiatives attract partners in all sectors to work with leading researchers and institutions, enabling access to knowledge and research infrastructure. These partnerships especially benefit researchers in small and medium-sized enterprises (SMEs) who might otherwise not have access to cutting edge know-how and equipment. For example, partner artificial intelligence drug discovery SMEs would have the opportunity to develop, test, and improve their proprietary computational algorithms and models through access to the initiative’s experimental data (Gold et al. 2019).
Alternative models: too little, too late
Alternative models to overcome the challenges of drug discovery for neglected and pandemic diseases include patent pools and patent clearinghouses. A patent pool is an agreement between two or more patent owners to license their patents to one another or to third parties. Patent pools are often associated with complex technologies, with inter-depending technological requirements (Aoki and Schiff 2008). Examples in drug discovery include the United Nations supported Medicines Patent Pool, which has signed agreements with 10 patent holders for thirteen HIV antiretrovirals, one HIV technology platform, three hepatitis C direct-acting antivirals, and a tuberculosis treatment to enable generic manufacture of low-cost doses of drugs for use in low-resource settings (Medicines Patent Pool 2020). Two forms of clearinghouse exist: those that provide information about intellectual property, or those that facilitate the licensing of intellectual property. These are most prevalent in industries requiring firms to clear copyrights, for example in the music industry (Aoki and Schiff 2008).
Patent pools and clearinghouses have limited value during a pandemic. Both are ex ante solutions to the problem of restricting freedom to operate in follow-on R&D. They are analogous to closing the barn door after the horse has bolted, or in this case, after the delays caused in patenting and negotiations have been expended. While some firms have agreed to an Open COVID Pledge to render all COVID-related intellectual property accessible during the COVID-19 crisis (Open COVID Pledge 2020), this has engendered resistance from the pharmaceutical industry, thus causing further delays (Silverman 2020). Similarly, government mechanisms to force access to drugs, such as compulsory licensing, or to gain access to therapies developed using public research dollars, such as the U.S. National Institutes of Health march-in rights, have rarely been used and are again post-hoc remedial actions for the excesses of the patent-based system (Bubela and Cook-Deegan 2015; de Beer and Gold 2020).
Challenges to open science
Despite the benefits of open science, its implementation further along the drug discovery continuum faces critical barriers to success. These barriers include: (1) strongly held norms, especially by government decision/policy-makers, academic institutions, and some prominent researchers, that early-stage patenting is necessary to support or finance drug discovery and delivery; (2) incentive structures for promotion and tenure, and public research grants that favour and often mandate patent holding; (3) a lack of knowledge about the ways in which open science reduces transaction costs and accelerates research across the research and drug discovery continuum; (4) the absence of funding models to support open academic and commercial research; (5) a peer-review and financing system that favours low-risk research; and (6) too few successful examples to build confidence among governments and industry in the benefits of open science. Most significantly, substantial and long-term funding by governments and philanthropies is required to even get these partnerships off the ground. Governments need to recognize the return on investment that derives from reductions in future health system costs, which are promised by open drug discovery initiatives.
Conclusion
In the context of emergent pandemic viruses, the status quo of proprietary drug discovery does not work. In implementing an open science drug discovery alternative, we are building on a deep history of State and philanthropy funded mission-oriented science. The political question remains, however: Are we more afraid that open science drug discovery will not work or that it will work? If we are afraid open science will work because it will disrupt entrenched interests, then we should at least test it in domains where those entrenched interests are clearly failing to deliver.
Specifically, we recommend:
1.
In addition to the Public Health Agency of Canada and financial support to individuals and firms, Canada ought to build a third pillar to its pandemic responsiveness: a flexible, open, and stable nonprofit, virtual drug discovery entity that coordinates and invests in a pipeline for the proactive development of anti-viral drugs (and possibly vaccines) for viruses with pandemic potential.
2.
The independent, nonprofit should be provided with long-term, stable funding to insulate it from day-to-day politics. The nonprofit will inevitably invest in anti-virals and other interventions that fail, as failure is part of innovation. Tolerance for failure requires an arms-length entity (Kenney and Patton 2009).
3.
The nonprofit and Canada’s pandemic innovation preparedness ought to be embedded in an international, open, effort to coordinate R&D of new products, such as the international environments in which the SGC and DNDi operate. The broader the collaborative network, the more efficient the discovery efforts will be. By participating in R&D international efforts, Canada increases its ability to access and to afford interventions developed elsewhere.
4.
To establish an equilibrium between open and proprietary R&D for drug discovery, funding councils and other funding bodies ought to establish specific open science calls, with significant funding, for those research projects that agree, upfront, to the following: (1) open and free availability to all data on an ongoing basis during the research endeavour; (2) publication in a journal complying with FAIR principles; and (3) no patenting on any research results, even if achieved through collaboration with outside partners. Such funding should include support for data standardization, entry, etc.
5.
Governments, granting councils, and philanthropies ought to establish funding to collect data on and analyse open science partnerships both in Canada and internationally to assess their impact, costs, and barriers to use. This will require data infrastructure, such as Canada’s New Digital Research Infrastructure Organization, which should provide data storage and collection for open data, develops, and maintains open data storage standards and requirements, establish rules and norms around data use (including privacy and security), and provide financial support for data entry.
6.
All government or quasi-government grants or contracts supporting pandemic-related research ought to impose requirement of no patenting of research results and rapid dissemination of data and results in accordance with FAIR principles.
7.
Philanthropies ought to prioritize pandemic-related research projects that are open and eschew patents.
8.
Canada ought to take a leadership role in advancing open science partnerships that comply with best practices recommended by international governmental bodies, such as the World Intellectual Property Office, the World Health Organization, and the Organization for Economic Co-operation and Development.
9.
Canada ought to lead the world in open science policymaking, for example, by supporting Health Canada (and (or) other regulators) to implement regulatory mechanisms that encourage open science drug development. Regulations might extend data protection periods for authorized products where the sponsor has made its preclinical and clinical trial data openly accessible to the research community, has not filed restrictive patents, and has agreed to make its product broadly accessible at affordable pricing.
References
Ali-Khan SE, Jean A, and Gold ER. 2018. Identifying the challenges in implementing open science. MNI Open Research, 2: 5.
Aoki R, and Schiff A. 2008. Promoting access to intellectual property: patent pools, copyright collectives, and clearinghouses. R&D Management, 38: 189–204.
Arshad Z, Smith J, Roberts M, Lee WH, Davies B, Bure K, et al. 2016. Open access could transform drug discovery: a case study of JQ1. Expert Opinion on Drug Discovery, 11: 321–332.
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. 2020. Remdesivir for the treatment of Covid-19—final report. The New England Journal of Medicine: NEJMoa2007764.
Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. 2020. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. The New England Journal of Medicine, 383: 517–525.
Bubela T, and Cook-Deegan R. 2015. Keeping score, strengthening policy and fighting bad actors over access to research tools. Nature Biotechnology, 33: 143–147.
Bubela T, Adams R, Chandrasekharan S, Mishra A, and Liu S. 2017. Governance of biomedical research commons to advance clinical translation: lessons from the mouse model community. In Governing medical knowledge commons. Edited by KJ Strandburg, BM Frischmann, and MJ Madison. Cambridge University Press, Cambridge, UK.
Bubela TM, and Caulfield T. 2010. Role and reality: technology transfer at Canadian universities. Trends in Biotechnology, 28: 447–451.
Centers for Disease Control and Prevention. 2020a. Influenza (flu) [online]: Available from cdc.gov/flu/vaccines-work/vaccineeffect.htm.
Centers for Disease Control and Prevention. 2020b. CDC takes action to prepare against “G4” swine flu viruses in China with pandemic potential.
Coronavirus Aid, Relief, and Economic Security (CARES) Act. 2020. Division B—emergency appropriations for coronavirus health response and agency operations. H.R. 748.
COVID-19 Vaccine Tracker. 2020. COVID-19 Vaccine Tracker [online]: Available from covid-19vaccinetracker.org/.
Darrow JJ, and Kesselheim AS. 2020. Incentivizing antibiotic development: why isn’t the generating antibiotic incentives now (GAIN) act working? Open Forum Infectious Diseases, 7: ofaa001.
David PA. 2008. The Historical origins of ‘open science’: an essay on patronage, reputation and common agency contracting in the scientific revolution. Capitalism and Society, 3: 1–103.
David PA. 2014. The republic of open science—the institution’s historical origins and prospects for continued vitality. SIEPR Discussion Paper No. 13-037. Stanford Institute for Economic Policy Research, Stanford University, Stanford, California.
de Beer J, and Gold ER. 2020. International trade, intellectual property, and innovation policy: lessons from a pandemic. In Vulnerable: the law, policy and ethics of COVID-19. Edited by CM Flood, V MacDonnell, J Philpott, S Thériault, and S Venkatapuram. University of Ottawa Press, Ottawa, Ontario.
DNDi. 2018. Intellectual Property Policy [online]: Available from dndi.org/wp-content/uploads/2018/03/DNDi_Intellectual_Property_Policy.pdf.
DNDi: Drugs for Neglected Diseases Initiative. 2020a. DNDi: Drugs for Neglected Diseases Initiative [online]: Available from dndi.org/.
DNDi: Drugs for Neglected Diseases Initiative. 2020b. First new chemical entity developed by DNDi and the first all-oral cure for all stages of sleeping sickness [online]: Available from dndi.org/research-development/portfolio/fexinidazole/.
DNDi: Drugs for Neglected Diseases Initiative. 2020c. Research & Development portfolio [online]: Available from dndi.org/research-development/portfolio/.
Edwards A, Morgan M, Al Chawaf A, Andrusiak K, Charney R, Cynader Z, et al. 2017. A trust approach for sharing research reagents. Science Translational Medicine, 9: eaai9055.
Edwards AM, Bountra C, Kerr DJ, and Willson TM. 2009. Open access chemical and clinical probes to support drug discovery. Nature Chemical Biology, 5: 436–440.
Edwards AM, Isserlin R, Bader GD, Frye SV, Willson TM, and Yu FH. 2011. Too many roads not taken. Nature, 470: 163–165.
Funck-Brentano C, and Salem JE. 2020. Chloroquine or hydroxychloroquine for COVID-19: why might they be hazardous? The Lancet: S0140-6736(20)31174-0.
Garde D. 23 July 2020. Ruling threatns to upend patens on Mederna’s Covid-19 vaccine. STAT.
Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. 2020. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. The New England Journal of Medicine, 382: 2411–2418.
Gold ER. 2016. Accelerating translational research through open science: the neuro experiment. PLoS Biology, 14: e2001259.
Gold ER, and Morgan M. 7 February 2019. For innovation, open science means open for business. The Globe and Mail.
Gold ER, Ali-Khan SE, Allen L, Ballell L, Barral-Netto M, Carr D, et al. 2019. An open toolkit for tracking open science partnership implementation and impact. Gates Open Research, 3: 1442.
Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. 2020. Remdesivir for 5 or 10 days in patients with severe Covid-19. The New England Journal of Medicine: NEJMoa2015301.
Guizzaro L. 2018. Be open about drug failures to speed up research. Nature, 563: 317–319.
Hay M, Thomas DW, Craighead JL, Economides C, and Rosenthal J. 2014. Clinical development success rates for investigational drugs. Nature Biotechnology, 32: 40–51.
Herder M, Graham JE, and Gold R. 2020. From discovery to delivery: public sector development of the rVSV-ZEBOV Ebola vaccine. Journal of Law and the Biosciences: lsz019.
Herper M. 2020. Gilead announces long-awaited price for Covid-19 drug remdesivir. STAT. Boston Globe Media, Boston, Massachusetts.
Janeway WH. 2012. Doing capitalism in the innovation economy: markets, speculation and the state. Cambridge University Press, Cambridge, UK.
Jasny BR, Wigginton N, McNutt M, Bubela T, Buck S, Cook-Deegan R, et al. 2017. Fostering reproducibility in industry-academia research. Science, 357: 759–761.
Jiang S, He Y, and Liu S. 2005. SARS vaccine development. Emerging Infectious Diseases, 11: 1016–1020.
Kay J. 2007. The failure of market failure. Prospect, Issue 137.
Kenney M, and Patton D. 2009. Reconsidering the Bayh-Dole Act and the current university invention ownership model. Research Policy, 38: 1407–1422.
Kmietowicz Z. 2020. Covid-19: selected NHS patients will be treated with remdesivir. BMJ, 369: m2097.
Lenzer J. 2020. Covid-19: US gives emergency approval to hydroxychloroquine despite lack of evidence. BMJ, 369: m1335.
Liu Y, Salwi S, and Drolet B. 2020. Multivalue ethical framework for fair global allocation of a COVID-19 vaccine. Journal of Medical Ethics, 46: 499–501.
Mahase E. 2020a. Covid-19: Remdesivir is helpful but not a wonder drug, say researchers. BMJ, 369: m1798.
Mahase E. 2020b. Covid-19: Remdesivir probably reduces recovery time, but evidence is uncertain, panel finds. BMJ, 370: m3049.
Mazzucato M. 2011. The entrepreneurial state. Soundings, 49: 131–142.
Medicines Patent Pool. 2020. About us [online]: Available from medicinespatentpool.org/.
Mehra MR, Desai SS, Kuy S, Henry TD, and Patel AN. 2020a. Cardiovascular disease, drug therapy, and mortality in Covid-19. The New England Journal of Medicine, 382: e102.
Mehra MR, Desai SS, Kuy S, Henry TD, and Patel AN. 2020b. Retraction: Cardiovascular disease, drug therapy, and mortality in Covid-19. The New England Journal of Medicine, 382: 2582.
Mehra MR, Desai SS, Ruschitzka F, and Patel AN. 2020c. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. The Lancet: S0140-6736(20)31180-6.
Milken Institute. 2020. COVID-19 treatment and vaccine tracker [online]: Available from covid-19tracker.milkeninstitute.org/#treatment_antibodies.
Morgan MR, Roberts OG, and Edwards AM. 2018. Ideation and implementation of an open science drug discovery business model—M4K Pharma. Wellcome Open Research, 3: 154.
Mullard A. 2020. COVID-19 vaccine development pipeline gears up. The Lancet, 395: 1751–1752.
OECD. 2020. Why open science is critical to combatting COVID-19.
Offord C. 4 June 2020. Lancet, NEJM retract surgisphere studies on COVID-19 patients. The Scientist.
OICR NEWS. 2019. New potential treatment for leukemia discovered by OICR scientists draws major industry investment [online]: Available from news.oicr.on.ca/2019/01/new-potential-treatment-for-leukemia-discovered-by-oicr-scientists-draws-major-industry-investment%EF%BB%BF/.
Olson M. 1965. The logic of collective action; public goods and the theory of groups. Harvard University Press, Cambridge, Massachusetts.
Open Access Scholarly Publishers Association (OASPA). 2020. COVID-19 Publishers Open Letter of Intent—rapid review [online]: Available from oaspa.org/covid-19-publishers-open-letter-of-intent-rapid-review/.
Open COVID Pledge. 2020. Let’s share intellectual property to fight COVID-19 [online]: Available from opencovidpledge.org/.
Ostrom E. 2005. Understanding institutional diversity. Princeton University Press, Princeton, New Jersey.
Ostrom E. 2015. Governing the commons: the evolution of institutions for collective action. Cambridge University Press, Cambridge, UK.
Popp Berman E. 2012. Creating the market university: how academic science became an economic engine. Princeton University Press, Princeton, New Jersey.
Pronker ES, Weenen TC, Commandeur H, Claassen EHJHM, and Osterhaus ADME. 2013. Risk in vaccine research and development quantified. PLoS ONE, 8: e57755.
RECOVERY: Randomised Evaluation of COVID-19 Therapy. 2020a. No clinical benefit from use of lopinavir-ritonavir in hospitalised COVID-19 patients studied in RECOVERY [online]: Available from recoverytrial.net/news/no-clinical-benefit-from-use-of-lopinavir-ritonavir-in-hospitalised-covid-19-patients-studied-in-recovery.
RECOVERY: Randomised Evaluation of COVID-19 Therapy. 2020b. No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19 [online]: Available from recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19.
Roskams-Edris D, and Gold ER. 2019. OSFHOME: Open Science Agreements Toolkit (OSAT) [online]: Available from osf.io/4s8at/.
Sampat B. 2020. Whose drugs are these? Issues in Science and Technology, 36(Summer): 42–48.
Sattui SE, Liew JW, Graef ER, Coler-Reilly A, Berenbaum F, Duarte-García A, et al. 2020. Swinging the pendulum: lessons learned from public discourse concerning hydroxychloroquine and COVID-19. Expert Review of Clinical Immunology, 16: 659–666.
Schaffer Deroo S, Pudalov NJ, and Fu LY. 2020. Planning for a COVID-19 Vaccination Program. JAMA, 323: 2458–2459.
Silverman E. 28 May 2020. Pharma leaders shoot down WHO voluntary pool for patent rights on Covid-19 products. STAT.
Stiglitz JE. 1989. Markets, market failures, and development. The American Economic Review, 79: 197–203.
The Lancet Editors. 2020. Expression of concern: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. The Lancet, 395: e102.
The RECOVERY Collaborative Group. 2020. Dexamethasone in hospitalized patients with Covid-19—preliminary report. The New England Journal of Medicine: NEJMoa2021436.
Thomas K. 31 July 2020. Sanofi and GlaxoSmithKline snag biggest coronavirus vaccine deal yet. New York Times.
US Patent and Trademark Office Patent Trial and Appeal Board. 2020. IPR2019-00554—Moderna Therapeutics, Inc. et al. v. Arbutus Biopharma Corporation et al.
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. 2020. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet, 395: 1569–1578.
Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, et al. 2016. The FAIR Guiding Principles for scientific data management and stewardship. Scientific Data, 3: 160018.
World Health Organization. 2020a. SARS (severe acute respiratory syndrome) [online]: Available from who.int/ith/diseases/sars/en/.
World Health Organization. 2020b. WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19 [online]: Available from who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19.
Information & Authors
Information
Published In

FACETS
Volume 5 • Number 1 • January 2020
Pages: 1019 - 1036
Editor: Jules M. Blais
History
Received: 4 September 2020
Accepted: 20 October 2020
Version of record online: 17 December 2020
Notes
This paper is part of the Royal Society of Canada’s COVID-19 Task Force Collection.
Copyright
© 2020 Bubela et al. This work is licensed under a Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Data Availability Statement
All relevant data are within the paper.
Key Words
Sections
Subjects
Authors
Author Contributions
TB, ERG, MM, and AE conceived and designed the study.
All drafted or revised the manuscript.
Competing Interests
AE is the CEO, and AE, ERG, MM, and TB are Directors of the Viral Interruption Medicines Initiative (VIMI); AE is a Director of M4K Pharma and CEO of the Structural Genomics Consortium (SGC). MM is Legal Consultant & Corporate Secretary of M4K Pharma, CEO of Agora Open Science Trust, and Chief Policy Officer and Senior Counsel at the SGC. VIMI is a Canadian not-for-profit company; Agora Open Science Trust is a Canadian registered charity; SGC is a UK registered charity; and M4K Pharma Inc. is a Canadian corporation. DP, JN, KM and VG have no conflicts to declare.
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Tania Bubela, E. Richard Gold, Vivek Goel, Max Morgan, Karen Mossman, Jason Nickerson, David Patrick, and Aled Edwards. 2020. Open drug discovery of anti-virals critical for Canada’s pandemic strategy. FACETS.
5(1): 1019-1036. https://doi.org/10.1139/facets-2020-0079
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
Cited by
1. Canada has an opportunity to address antimicrobial resistance through COVID-19 recovery spending
2. The first five years of FACETS: Canada’s multidisciplinary open access academy journal
