Introduction
Global amphibian declines represent one of the hallmarks of the current biodiversity crisis. Amphibians are impacted by numerous stressors, including habitat loss and alteration, invasive species, disease, environmental pollution (
Hamer and McDonnell 2008;
Baldwin and DeMaynadier 2009;
Rollins-Smith 2017); however, one of the least understood threats is global climate change because its effects have yet to be fully realized (
Blaustein et al. 2010;
Li et al. 2013). Shifts in seasonal timings of the onset of freeze–thaw periods may cause uneven changes in the phenological cycles of interacting species (
Blaustein et al. 2001;
Ficetola and Maiorano 2016;
Green 2017), and environmental stress may increase the susceptibility of amphibians to disease (
Blaustein et al. 2010;
Rollins-Smith 2017). In addition, amphibians often have very specific breeding requirements that are tied to aquatic resources, so changes to climate that affect the availability of these resources could have detrimental effects on recruitment (
Corn 2005;
Brooks 2009). Our ability to predict and react to the direct effects of climate change and the negative synergistic effects with other stressors will rely on efforts to understand the diversity of amphibian habitats and how communities change across environmental gradients.
In glaciated northeastern North America, forested wetlands comprise a large proportion of amphibian breeding habitat. These wetlands vary in a number of qualities that dictate patterns in amphibian diversity across the landscape, including hydroperiod length (i.e., the amount of time a wetland is inundated;
Babbitt et al. 2003;
Baber et al. 2004), canopy cover (
Werner and Glennemeier 1999;
Werner et al. 2007), and surrounding land cover (
Hecnar and M’Closkey 1998;
Findlay et al. 2001;
Houlahan and Findlay 2003). Despite these differences leading to unique amphibian assemblages, conservation efforts are often unequal across wetland types. Certain protections are provided to large and conspicuous permanent wetlands, but temporary wetlands are frequently overlooked in legislation because of their relative obscurity in forested landscapes (
Mahaney and Klemens 2008). In northeastern North America, temporary wetlands that occur in the temperate forests are commonly referred to as vernal pools and are a primary breeding habitat for many amphibians that are intolerant of predatory fish populations, including salamanders in the genus
Ambystoma and wood frogs (
Lithobates sylvaticus). To better conserve amphibian diversity on regional scales, underrepresented habitats like vernal pools need greater research and management attention.
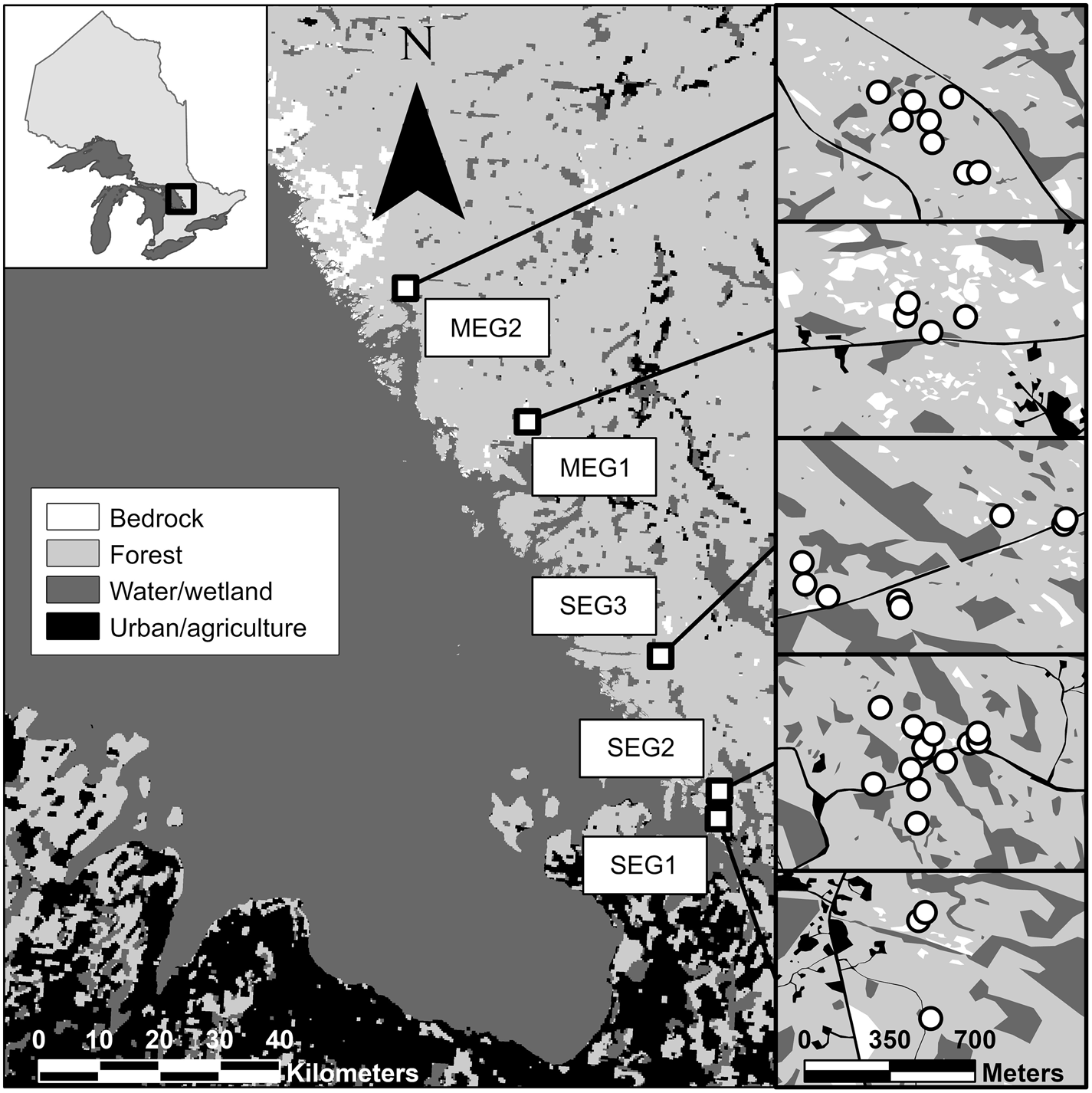
Our study focusses on vernal pool habitat in an intact forested region of eastern Georgian Bay, Ontario. Little is known about the aquatic resources in this region compared with more urban and agricultural regions in the southern part of the province. While breeding habitat associations for amphibians are well studied for southern Ontario (
Findlay and Houlahan 1997;
Hecnar and M’Closkey 1998;
Findlay et al. 2001), there are differences in climate, landscape characteristics, wetland types, and amphibian ranges that could affect the reliability of these relationships across the province. The primary objective of this study was to document baseline information on vernal pool amphibian assemblages in the Georgian Bay region through assessments of community structure and species–habitat relationships. In particular, we were interested in re-examining relationships with influential variables from previous studies, including hydroperiod length and canopy cover (
Babbitt et al. 2003;
Egan and Paton 2004;
Baldwin et al. 2006;
Veysey et al. 2011;
Semlitsch et al. 2015), to assess whether these variables were similarly important for community structure and abundance in vernal pools within a largely intact forest ecosystem. There is an urgent need to identify key habitat characteristics that structure amphibian communities in vernal pools of forests in the Ontario, especially in the face of anticipated threats associated with global climate change.
Results
Wetland characteristics
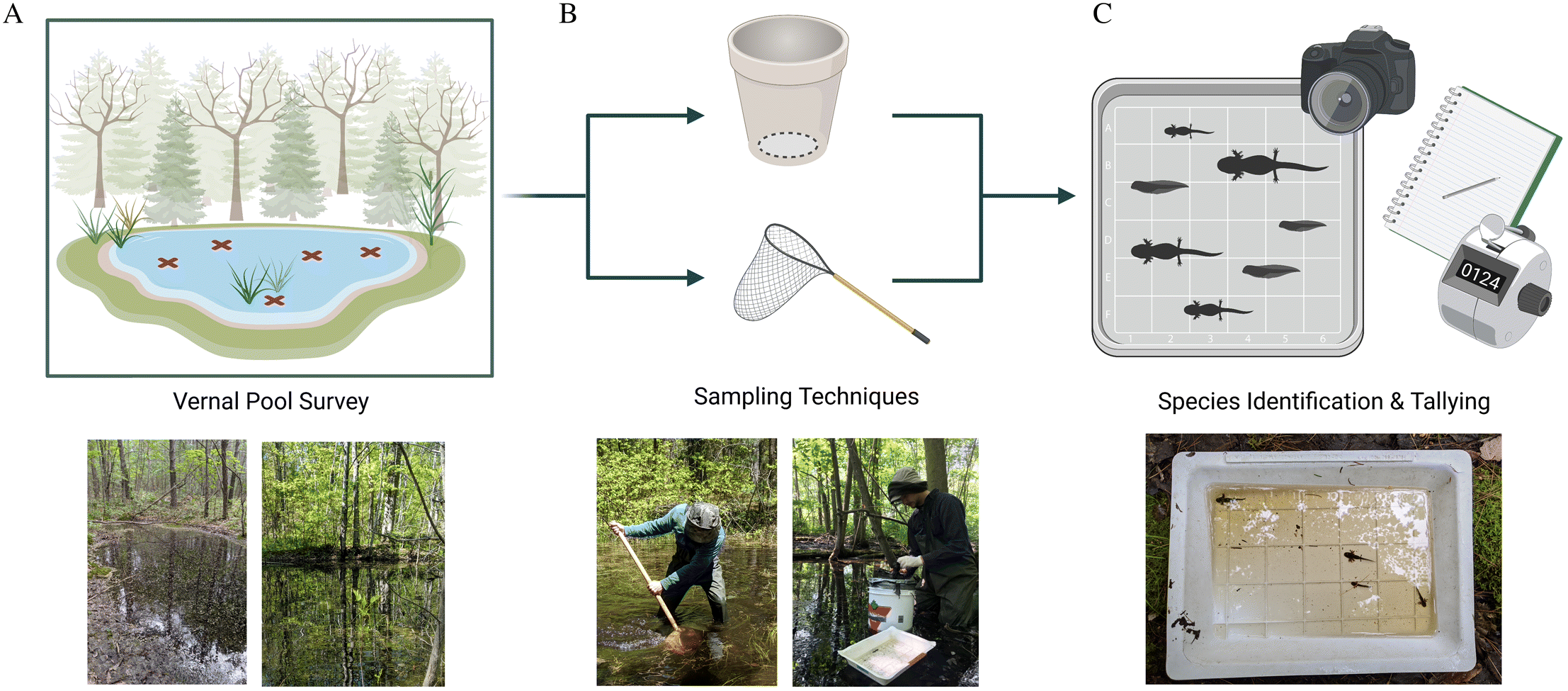
Pools varied in size and appearance (
Table 1), with substrate and vegetation conditions spanning hard packed leaf litter to loose muddy bottoms, and dense thicket cover to emergent grasses and sedges. All variables had VIF < 8 and Spearman’s rank correlation coefficients for most of the within-pool variables considered in this study fell between −0.2 and 0.2 with
P > 0.05. Pool vegetation cover and canopy openness were the only variables strongly correlated with one another (
ρ = 0.72.
P < 0.00001), while hydroperiod length was moderately correlated with canopy openness (
ρ = 0.59,
P < 0.001) and maximum depth (
ρ = 0.49,
P < 0.01). Since the mechanism by which key factors affect the amphibian community may vary, all correlated variables were retained for further statistical analyses.
Larvae assemblages
During 2019 and 2020, we captured 2830 amphibian larvae from eight amphibian species (five anurans and three caudates). Of the 35 pools included in our study, 21 (60%) were surveyed four times, twice each year; eight (23%) were surveyed three times because the sites had dried before the second survey period in 2020; one (3%) was surveyed only twice in 2019 because it was dry during both 2020 survey periods; and five (14%) were surveyed only once because they only had standing water during the early part of the 2019 survey period. From these 35 pools, we excluded the 5 pools with extremely short hydroperiods from further analysis because they dried before any larvae could be captured (i.e., we found unhatched eggs of spotted salamander (Ambystoma maculatum)). The early surveys were generally characterized by early breeding species, including the spotted salamander, blue-spotted salamander (Ambystoma laterale), and wood frog (Lithobates sylvaticus), whereas later surveys were characterized by fewer encounters of wood frogs and more encounters of species with prolonged breeding periods, including the spring peeper (Pseudocris crucifer).
In pools with larvae, richness varied from 1 to 6, with a mean of 3.5. The most commonly encountered species, the spotted salamander, was captured in all but two pools (93% of the pools). Other common species included the blue-spotted salamander, wood frog, and spring peeper, which were found in more than 60% of the study pools (>17 pools). Together, spotted salamanders, blue-spotted salamanders, wood frogs, and spring peepers accounted for 95% of all larvae captured. Gray treefrogs (Hyla versicolor) and four-toed salamanders were less common but were still found in more than 20% of the study pools (>6 pools). American toads (Anaxyrus americanus) and green frogs (Lithobates clamitans) were the least common species in our study (one and two pools, respectively). Both of these rarely encountered species were excluded from the community analysis so as not to disproportionately influence overall trends.
Drivers of community structure
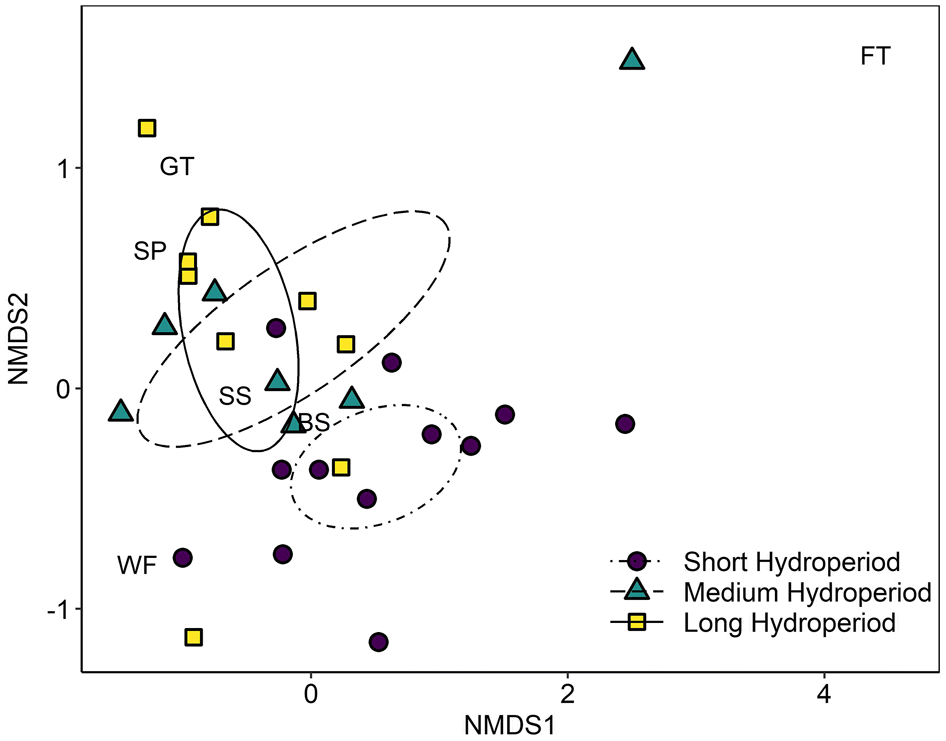
The stress of the two-dimensional NMDS model was 0.11, indicating a good representation of the dissimilarities between pools in reduced dimensions. Species with close phylogenetic relationships tended to be associated in ordination space. For instance, centroids for the two mole salamanders (spotted and blue-spotted salamanders) and for the two treefrogs (gray treefrogs and spring peepers) were found closer to each other than with centroids of other taxa (
Fig. 3). The centroid for the four-toed salamander (upper right corner of the
Fig. 3) was isolated from the other species in ordination space and this species tended to be captured in pools where other species were either absent or captured at low abundances. They were also the only species found in two sphagnum-dominated pools, which had environmental characteristics that were very different from those of the other pools. These two pools had a disproportionate influence on the appearance of initial NMDS ordinations and were excluded from further statistical analyses.
Results of the between-sites PERMANOVAs indicated that larvae community structure changed across hydroperiod classes (pseudo
F = 2.27,
P = 0.01;
Fig. 3) and across the gradient of canopy openness (pseudo
F = 2.25,
P = 0.04); however, neither variable had significant independent effects on community structure in the marginal-effects model (hydroperiod: pseudo
F = 1.59,
P = 0.09; canopy openness: pseudo
F = 1.00,
P = 0.4). Pairwise comparisons of hydroperiod classes revealed community structures in pools with short hydroperiods were dissimilar from those in pools with long hydroperiods (pseudo
F = 3.79,
P = 0.003) and somewhat dissimilar from those in pools with moderate hydroperiods (pseudo
F = 2.06,
P = 0.05). Community structures in pools with moderate hydroperiods were not significantly different from those in pools with long hydroperiods (pseudo
F = 0.51,
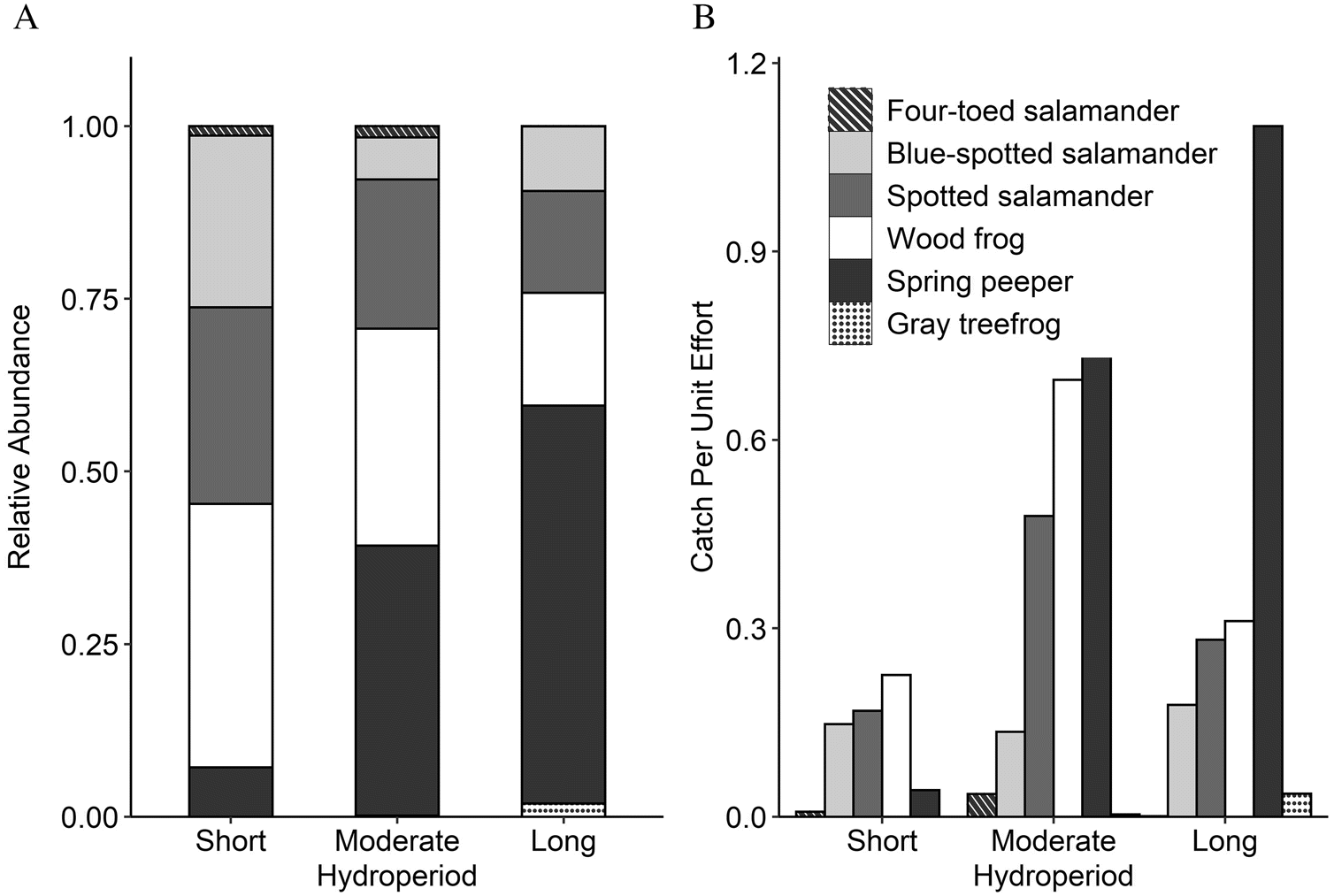
P = 0.8). Spring peepers contributed the most to average dissimilarity (SIMPER) between pools with short hydroperiods and pools with moderate and long hydroperiods (32% and 50%, respectively), followed by wood frogs (25% and 22%, respectively) and spotted salamanders (20% and 14%, respectively). The remaining species contributed <20% to average dissimilarity between hydroperiod classes. The proportion of spring peepers in total larvae captured increased with hydroperiod (
Fig. 4A); spring peepers had a low average CPUE in pools with short hydroperiods, whereas spring peepers in pools with moderate and long hydroperiods had the highest average CPUE (
Fig. 4B). Average percent canopy openness also increased across hydroperiod classes and was twice as large in pools with long hydroperiods compared with those with short hydroperiods. The other three common species (spotted salamanders, blue-spotted salamanders, and wood frogs) did not appear to differ as strongly among hydroperiod classes, though wood frogs and spotted salamanders appeared to have a somewhat higher average CPUE in pools with moderate hydroperiods.
Species-specific relationships with habitat variables
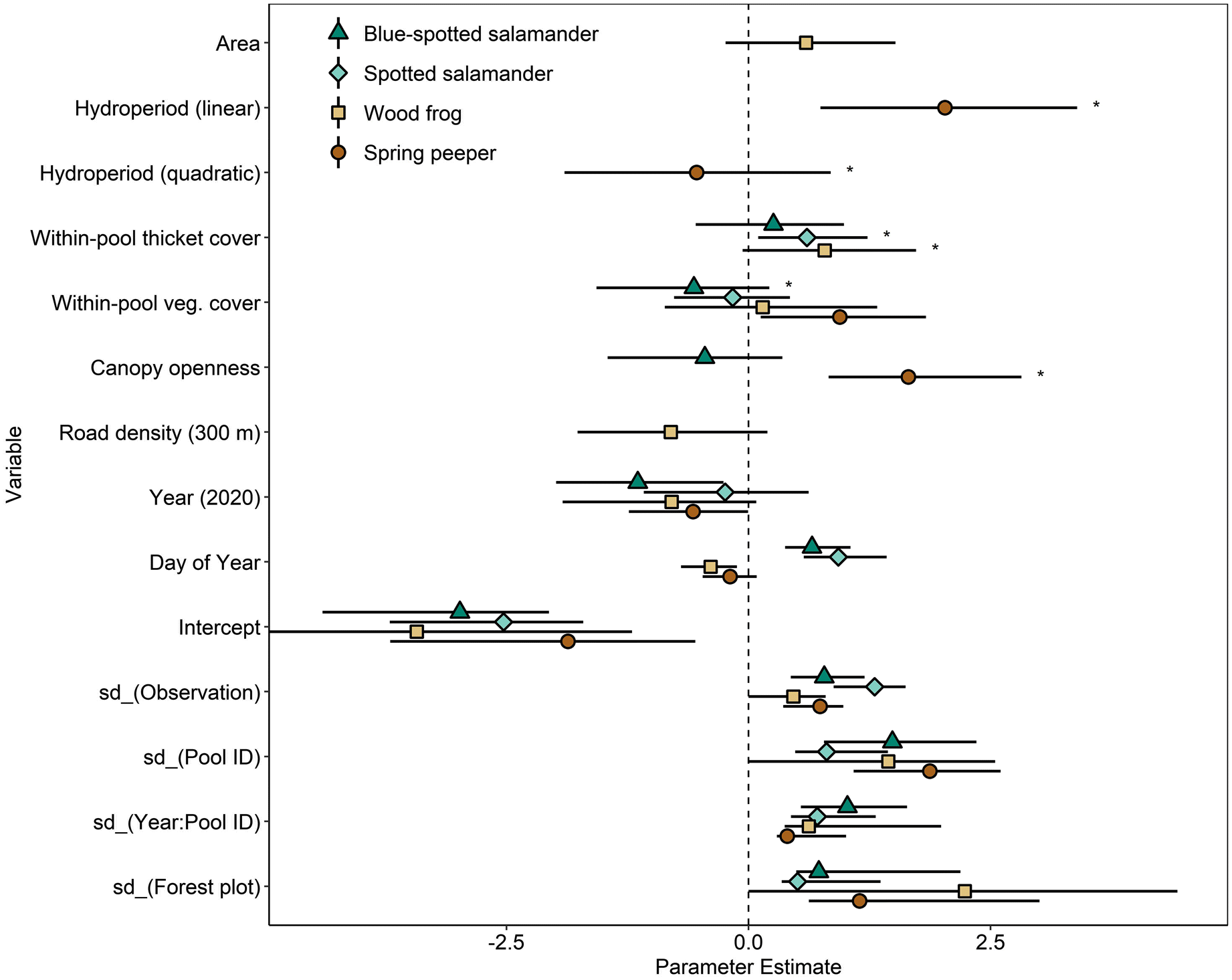
GLMMs were used to test the effect of habitat variables on the relative abundances of spotted salamanders, blue-spotted salamanders, wood frogs, and spring peepers. Gray treefrogs and four-toed salamanders were excluded because they were found in less than 30% of the study pools. Variables in models with strong support (ΔAICc < 2) were considered potential drivers of species abundances (
Table 2). Models without any habitat variables were strongly supported for two species (blue-spotted salamanders and wood frogs), but the best supported model for all species included some combination of habitat variables. The combinations of habitat variables in models with strong support did not overlap appreciably between species (
Fig. 5). Since parameter estimates for the most influential variables were mostly conserved across models, we did not perform model averaging. The exceptions to this were canopy openness and hydroperiod, where parameter estimates were slightly weaker in models including both variables. Parameter estimates for canopy openness and herbaceous vegetation cover were also substantially different in models including both variables, but these models were relatively weak for all four species and not included in the final model set.
Herbaceous vegetation cover, thicket cover, and canopy openness were the only habitat variables that were important for more than one species. Higher vegetation cover and canopy openness were strongly and positively correlated with the CPUE of spring peepers and weakly and negatively correlated with CPUE of blue-spotted salamanders. Herbaceous vegetation was also weakly correlated with CPUE of wood frogs and spotted salamanders; however, these relationships appear to be tied to the strong effects of other variables included in the same models. Accounting for the effect of hydroperiod length, CPUE of spring peepers in pools with the most open canopies was estimated to be 50 times greater than in pools that were almost completely shaded by canopy. Similarly, spring peeper CPUE was estimated to be 20 times greater in pools with near-complete cover of herbaceous vegetation compared to pools with no herbaceous vegetation. The presence of thicket cover in pools was strongly associated with CPUE of spotted salamanders and wood frogs and weakly associated with CPUE of blue-spotted salamanders. The CPUE of wood frogs and spotted salamanders was 6 and 4 times greater, respectively, in pools with thicket cover compared with pools with no thicket cover.
Models including hydroperiod were only strongly supported for spring peepers and had a positive effect on spring peeper CPUE. Accounting for the effect of canopy openness, the CPUE of spring peepers was estimated to be five times higher in pools with moderate hydroperiods compared with pools with short hydroperiods and twice as large in pools with long hydroperiods compared with pools with moderate hydroperiods.
Wood frogs were the only species with strongly supported models that included pool size and road density. Increases in pool size were associated with increases in the CPUE of wood frogs, though the effect did not appear to be very strong. Road density within a 300-m diameter buffer appeared to have a negative effect on CPUE of wood frogs in our study; CPUE of wood frogs was 16 times lower in pools that had high road density (2.7 km/km2) compared with pools without any roads in the 300-m buffer.
All species had lower CPUE in 2020 compared to 2019, though this effect was weak for spotted salamanders (
Fig. 5). We used Spearman’s rank correlation coefficients to investigate whether the rank order of CPUE in pools was conserved across years. CPUE exhibited a strong and positive correlation across years for spring peepers (
ρ = 0.82
P < 0.0001) but was only weakly correlated for wood frogs (
ρ = 0.35
P = 0.2), spotted salamanders (
ρ = 0.30
P = 0.2), and blue-spotted salamanders (
ρ = 0.26
P = 0.3).
Discussion
The four most common amphibian species identified in this study (
Ambystoma maculatum, A. laterale, Lithobates sylvaticus, Pseudocris crucifer) were either absent or detected less frequently compared with other amphibians in previous studies in Ontario (
Hecnar and M’Closkey 1998;
Gagné and Fahrig 2007;
Loder et al. 2019). For the most part, these studies investigated a wider range of wetland types, including permanent forested wetlands and wetlands in urban and agricultural settings. Species associated with permanent wetlands (e.g.,
Lithobates septentrionalis, L. catesbeianus, L. clamitans;
Semlitsch et al. 2015) and those associated with open-canopy and anthropogenically disturbed wetlands (e.g.,
Anaxyrus americanus;
Werner et al. 2007) were either absent or uncommon in our study pools. The species identified in our study generally aligned with species assemblages from previous studies that were found in temporary to semi-permanent forested wetlands with moderate to high canopy cover (
Werner and Glennemeier 1999;
Werner et al. 2007,
Semlitsch et al. 2015).
The influence of hydroperiod on amphibians in our study was relatively minor, contrasting the large body of literature documenting the strong influence of hydroperiod on numerous aspects of pond-breeding amphibian communities (
Snodgrass et al. 2000;
Babbitt et al. 2003 Werner et al. 2007). Though we identified a significant effect of hydroperiod length on community composition, the strong positive relationship between hydroperiod length and spring peeper abundance appeared to be the primary driver. We only found the rarely encountered gray treefrog (
Hyla versicolor) in pools with moderate or long hydroperiods, and this suggests that we may have found a similar positive relationship between hydroperiod length and abundance for this species if we had sampled more sites and for a longer duration. The apparent lack of an effect of hydroperiod on the other amphibian species may also be explained by our limited range in hydroperiod length. We focussed our study on forested vernal pools and did not sample any wetlands that were permanently inundated such as beaver ponds, thicket swamps, and marshes. At the other end of the spectrum, we lost seven of our pools with the shortest hydroperiods because pools dried before larvae hatched from their eggs. By expanding our sampling to include other wetland types and increasing sampling effort on pools that dry up quickly, future investigations may be able to obtain a more pronounced effect of hydroperiod on abundances of the vernal pool obligate amphibian species.
Canopy openness was positively correlated with herbaceous vegetation cover and hydroperiod length in our study and may have contributed to differences in amphibian community composition across hydroperiod classes. Spring peepers, in particular, had the highest larval abundance in open canopy pools with high herbaceous vegetation cover. By comparison, we found no evidence to suggest a strong effect of canopy openness or herbaceous cover on the abundance of wood frogs, spotted salamanders, or blue-spotted salamanders. The discrepancy between species responses to canopy openness is thought to be primarily linked to differences in resource requirements (
Earl et al. 2011). Caudate larvae are carnivores and are considered canopy generalists because prey species are available across a gradient of canopy cover (
Earl et al. 2011). Anurans in this region, however, are primarily herbivores/detritivores that feed on a range of food quality (
Schiesari 2006). For instance, filamentous algae and decaying nonwoody plants in open canopy pools are of higher nutritional quality than the decaying leaf litter that covers closed canopy pools (
Skelly et al. 2002;
Williams et al. 2008). Lower food quality can depress growth and development rates of larvae, which can reduce fitness and increase the risk of mortality from pond drying (
Werner and Glennemeier 1999;
Skelly et al. 2002). Compared to other anurans, wood frogs are more efficient at metabolizing lower quality detritus and regularly breed in heavily shaded wetlands (
Schiesari 2006).
The presence of vegetation may also influence breeding effort by increasing the number of available attachment sites for amphibian eggs. The anuran species and
Ambystoma salamanders are known to attach their eggs to a variety of sturdy structures within the pool basin, including floating logs and branches, submerged thicket, and the remnants of herbaceous vegetation from the previous year (
Egan and Paton 2004). Anecdotally, we have found the large egg masses of wood frogs and
Ambystoma salamanders to be more frequently attached to submerged branches of shrubs compared with herbaceous vegetation, which may explain the positive relationship we observed between the presence of thicket cover and the abundance of wood frogs and
Ambystoma salamanders. Unlike the other species encountered in our surveys, four-toed salamanders are known to lay their eggs in mossy clumps at the edges of pools (
Chalmers and Loftin 2006). Though they were not included in our species-specific analysis, four-toed salamanders appeared to be associated with sphagnum moss cover and were the only species encountered in the two pools that were completely covered by sphagnum moss.
Changes in canopy openness and vegetation communities can occur as a result of human intervention, as is the case with forest management practices and land-use changes, or from disturbance events such as forest fires. While clear-cut logging can be harmful to many amphibian species (
Quinn 2004), selective logging practices that maintain mature forests have been found to generally provide favourable conditions for amphibians in central Ontario (
Enright 1998). Natural fire disturbances can be important for maintaining breeding habitat for some species (
Skelly et al. 1999;
Gorman et al. 2013); however, increases in the frequency and severity of fires from climate change may lead to more severe consequences, especially for species that depend on upland forest habitat for most of the year (
Blaustein et al. 2010).
Prolonged periods of drought and more frequent disease outbreaks are also expected to affect forest health (
Sturrock et al. 2011), which may further reduce the prevalence of closed canopy habitats. More research is needed to determine the effect of widespread canopy changes on the performance of amphibians in this region. Although changes in canopy openness in our study were modest, we saw obvious shifts in vegetation communities and dominant amphibian species. More pronounced canopy gradients have been associated with larger shifts in amphibian communities and can lead to drastically different ecosystems (
Werner et al. 2007). Regardless of the resiliency of pond-breeding amphibians to widespread canopy changes, closed canopy wetlands represent unique ecosystems and efforts should be made to ensure sufficient land is protected to allow patches of these wetlands to persist in the event of forest loss.
Forests in our study region have had relatively minor changes in surrounding land use. Roads accounted for the highest percentage of urban land use and the only buildings within a kilometer radius of each forest plot were cottages or marinas. Despite low levels of anthropogenic stress, we found evidence to suggest that road density had negative effects on the abundance of wood frogs. These results are consistent with those in more impacted regions (
Findlay et al. 2001;
Veysey et al. 2011) and suggest wood frogs may be particularly sensitive to land-use changes that disrupt seasonal migration and dispersal. Impacts of land-use changes have also been found to extend to other species of pond-breeding amphibians in more anthropogenically impacted regions (
Houlahan and Findlay 2003;
Veysey et al. 2011). Many pond-breeding amphibians are hypothesized to be governed by metapopulation dynamics on large spatial scales and may rely on landscape connectivity between wetlands for gene flow and population rescue (
Marsh and Trenham 2001;
Semlitsch 2008). Future development plans for the Georgian Bay region may necessitate the inclusion of the protection of landscape connectivity to surrounding wetlands in management plans.
Despite the narrow range of hydroperiod lengths encountered in this study, our results suggested that wetland hydroperiod had an effect on the structure of vernal pool amphibian communities. The relationship between hydroperiod and amphibian community composition is especially important to consider in the face of global climate change. In northeastern North America, climate change is projected to lead to increased temperatures along with more frequent and longer periods of drought (
Bush and Lemmen 2019). Although there is evidence that shifts in the timing of spring melt may promote earlier migration to breeding pools (
Blaustein et al. 2001;
Todd et al. 2011;
Green 2017), the average length of hydroperiods is predicted to decrease and low recruitment years for amphibians are expected to occur more frequently (
Brooks 2009;
Blaustein et al. 2010).
How amphibian populations will respond to these changes remains unclear. The pools with the shortest hydroperiods will likely become unreliable for most amphibians, whereas wetlands that previously held fish populations may dry more frequently and become suitable for a greater range of amphibians. On the landscape level, reductions in wetland density could limit dispersal across the landscape, leading to reduced genetic diversity and slower rates of recolonization following local extinctions (
Willson and Hopkins 2013;
Coster et al. 2015). Within breeding pools, differences in species-specific phenological responses to earlier warming could alter predator–prey dynamics and lead to competitive advantages for species that have high adaptive capacity (
Todd et al. 2009;
Blaustein et al. 2010;
Walls et al. 2013). The adaptive capacity of individual amphibian species may depend on their ability to colonize wetlands that become suitable due to climate-induced changes in hydroperiod or canopy openness. Conservation should prioritize the preservation of diverse assemblages of wetlands at the landscape scale.
While we did find associations between amphibians and habitat characteristics, it is clear that vernal pools and their communities are dynamic, and short-term studies will undoubtedly miss information. Hydroperiod length in this study is only useful as a relative measure, as annual weather conditions will ultimately affect the duration that pools are available in any given year (
Brooks 2009). Longer-term studies could examine the effect of year-to-year fluctuations in hydroperiods on changes in the composition of species assemblages. Given the short nature of this study, we acknowledge the possibility that we did not obtain a complete list of species that use these vernal pools on a long-term basis. In addition, the relative performance of species in one year can have trickle-down effects in subsequent years; population booms and busts for short-lived species like wood frogs or spring peepers have the potential to affect breeding effort in subsequent years (
Berven 1990;
Werner et al. 2009).
Vernal pools in south-central Ontario are diverse in appearance and vary with respect to their suitability as breeding habitat for amphibian species. Vernal pools and forested wetlands in general are responsible for a considerable amount of energy and biomass that is then transferred to terrestrial habitats (
Leibowitz 2003). Conservation of forested wetlands is necessary for the maintenance of the important ecological services provided by forests (
Nyman 2011) and healthy forests, in turn, are important for the amphibians that spend a majority of their lives in forested environments (
Skidds et al. 2007;
Todd et al. 2009). Large remote forests like those in central Ontario support numerous forested wetlands and are thought to provide indispensable environmental values (
Watson et al. 2018). Across Ontario, there is increased recognition of the importance of intact forest and wetland ecosystems, especially for building adaptive capacity to climate change and improving the ecological functions of watersheds (
Environmental Commissioner of Ontario 2018). Sustainable management of these forests appears to be compatible with healthy amphibian communities and is attractive from an economic perspective due to the large number of cottagers, tourists, and campers that visit these forests every year (
Davidson 2015).
This study represents one of the first to explore the diversity of pond-breeding amphibian communities in south-central Ontario. There is an urgent need to understand the hydrology and physical characteristics of amphibian breeding habitats, especially in the face of global climate change. A shift in timing of rain events or onset of hot weather could severely reduce the reproductive success of species with constrained breeding periods, such as wood frogs or
Ambystoma salamanders (
Brooks 2009;
Blaustein et al. 2010). In areas where land-use changes already alter hydrological conditions, including changes in the rate and direction of overland flow, the effect of climate change on the flashiness of precipitation events is of even more concern (
Semlitsch and Skelly 2008). In addition, climate change may also affect forest health in this region, as well as increase the frequency of forest fires and disease (
Gillett et al. 2004;
Bush and Lemmen 2019). Since the abundance of vernal pool obligate amphibians (wood frogs, spotted salamanders, blue-spotted salamanders) appeared to be influenced by changes in the composition of vegetation communities in this study, any change in forest health/canopy may also affect the assemblages of these species. Unfortunately, most climate investigations have focused on regions where the effect of climate change is difficult to separate from other amphibian stressors (
Li et al. 2013). Small-scale ecosystems in large remote forests, such as forested wetlands, have not been explored as extensively as those in more urban settings, but may provide opportunities to study the effects of climate change in the absence of other major stressors. There is clearly a need for more research focused on forested ecosystems in these regions that support high diversity of wetland types and wetland-dependent biota.