Mercury in water
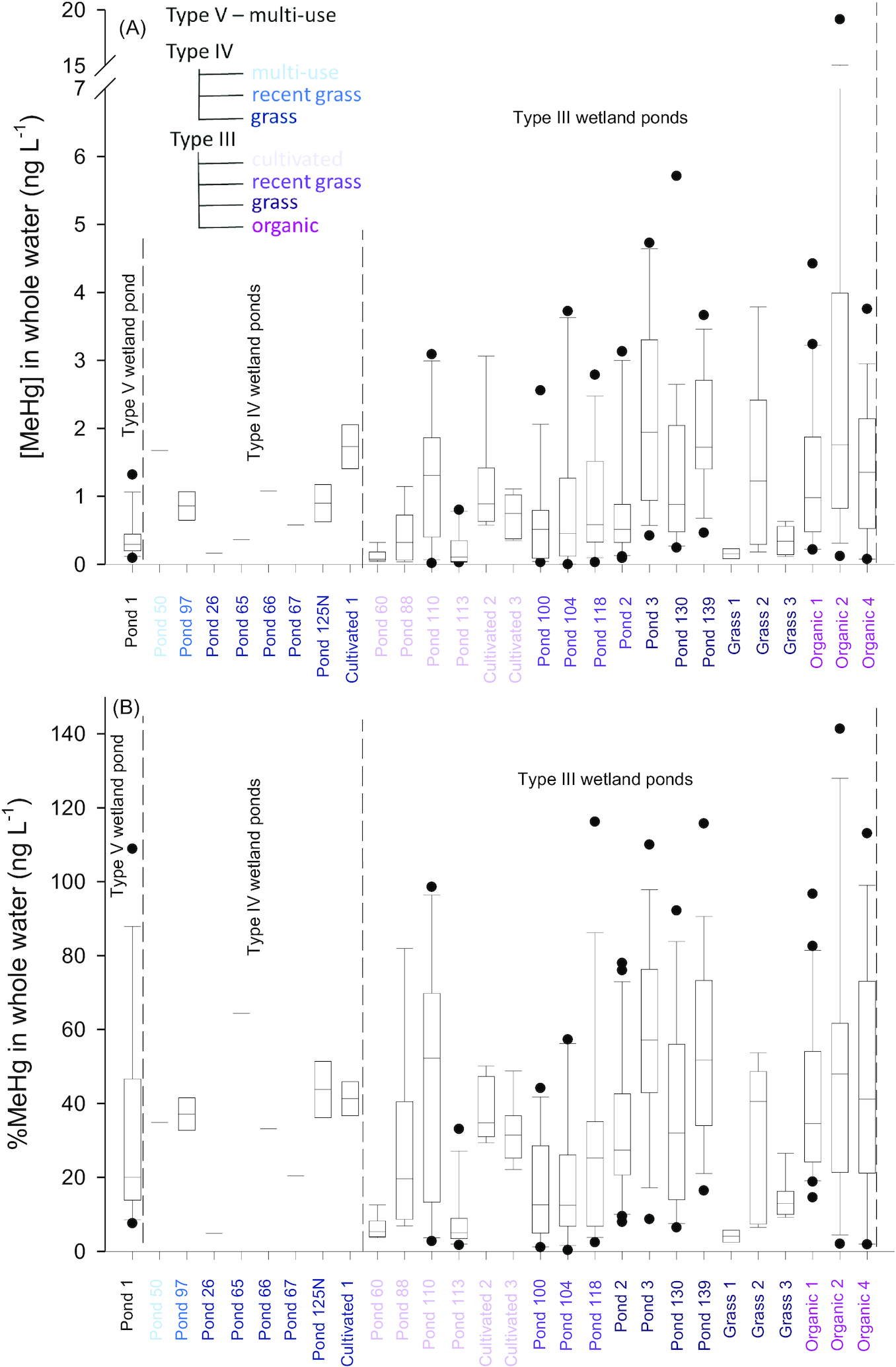
Average MeHg and total Hg concentrations (all forms of Hg, [THg]) in unfiltered water from individual ponds ranged from 0.123 to 9.947 ng·L
−1 and 0.57 to 7.32 ng·L
−1, respectively (
Figs. 2A and S2, Table S5). The %MeHg values ranged from 3.2% to 64.5% (
Fig. 2B, Table S5). All of these measurements were similar to values observed in other Saskatchewan ponds in 2006–2007 (
Hall et al. 2009) and in North Dakota in 2003–2004 (
Sando et al. 2007), with the exception of Organic 2, where average [MeHg] in water was significantly higher than all other ponds in this study (9.947 ± 5.054 ng·L
−1). Total Hg concentrations in Organic 2 (7.31 ± 2.15 ng·L
−1) were also elevated compared to other sites; however, the difference was not as striking as with [MeHg].
Shallow ponds in this area are often turbid due to shallow depths, loose sediments, and constant mixing due to wind (
de la Cruz 1979; cited in
Murkin 1989). Since we did not filter the majority of our samples, and although we made every effort to exclude particulates when sampling, the amount of particulate matter in some samples was substantial, potentially leading to %MeHg values that were greater than 100%. To assess the general contributions of particles to overall whole water concentrations, MeHg and THg in both the dissolved and particulate phases were examined from seven sites on two occasions in 2007 (Table S6). In six out of seven sites, the percentage of MeHg and THg in the dissolved phase ranged from 73.0% to 98.4% and 85.7% to 99.7%, respectively. These results are similar to ponds in North Dakota where the majority of MeHg was found in the dissolved phase (
Sando et al. 2007) or in the colloidal phase that may pass through 0.45 µm filters (
Hsu Kim et al. 2013). In Organic 2, 53% of MeHg and 62% of THg was in the particulate phase (Table S6). This site was shallow with extremely fine and flocculate sediments and it was impossible to avoid large particulates in the sample and, as such, increased particulate matter in Organic 2 may explain high whole water concentrations at that site. Variability within whole water samples in Organic 2 was large and %MeHg values ranged from 13.5% to 177.3%. Concentrations of MeHg and THg in insects were also high at this site compared to others (
Bates and Hall 2012), suggesting bioaccumulation in the invertebrate food chain was occurring in that pond. We reiterate a conclusion presented in
Hoggarth et al. (2015) that, from a management perspective, measuring MeHg concentrations, as well as the %MeHg, in surface water may be sufficient to predict and assess MeHg risks to biota.
Water chemistry controls on MeHg concentrations
Considering that our ponds were all within 3 km of each other, the variation of water chemistry parameters (Table S2) may be surprising if unfamiliar with the high temporal and spatial variability of these prairie wetland systems (
Euliss and Mushet 1996;
Nachson et al. 2014). Concentrations of DOC and SO
4−2 (range = 10.06–188.20 mg·L
−1 and 0.04–8168 mg·L
−1 for DOC and SO
4−2, respectively), conductivity (range = 0.168–11.220 mS·cm
−1), and pH (range = 6.1–9.4) varied widely among ponds over the course of our sampling period. Similarly water temperature varied (range = 7.64 °C–32.11 °C) likely as a function of the difference in depth and climatic conditions over the open water season. Despite the variability, forward multiple linear regression on data from Type III ponds identified SO
4−2 and DOC concentrations, SUVA values, conductivity, pH, and temperature to be significant variables predicting MeHg concentrations (
Table 2). Predictive relationships between [MeHg] in whole water and both SO
4−2 and DOC concentrations and SUVA is not surprising because previous studies have demonstrated that these parameters may control MeHg production by providing necessary substrates to methylating communities (
Benoit et al. 1999;
Aiken et al. 2003;
Gorski et al. 2008;
Hall et al. 2008;
Janssen et al. 2022) (see below). A positive relationship between conductivity and MeHg reflects the fact that most of our systems are SO
4−2 dominated. As methylation is by in large a microbially mediated process, we are also not surprised that temperature predicated [MeHg]. Finally, expectations on the processes in which pH may influence [MeHg] are less clear with early work showing inverse relationships between pH and Hg concentrations in fish (
Winfrey and Rudd 1990) and that increasing concentrations of H
+ facilitates the uptake of Hg by methylating organisms (
Kelly et al. 2003). We note, however, that these findings were made in low pH lakes. Studies on the impact of pH in less acidic lakes are rare.
Controls of land use on MeHg concentrations
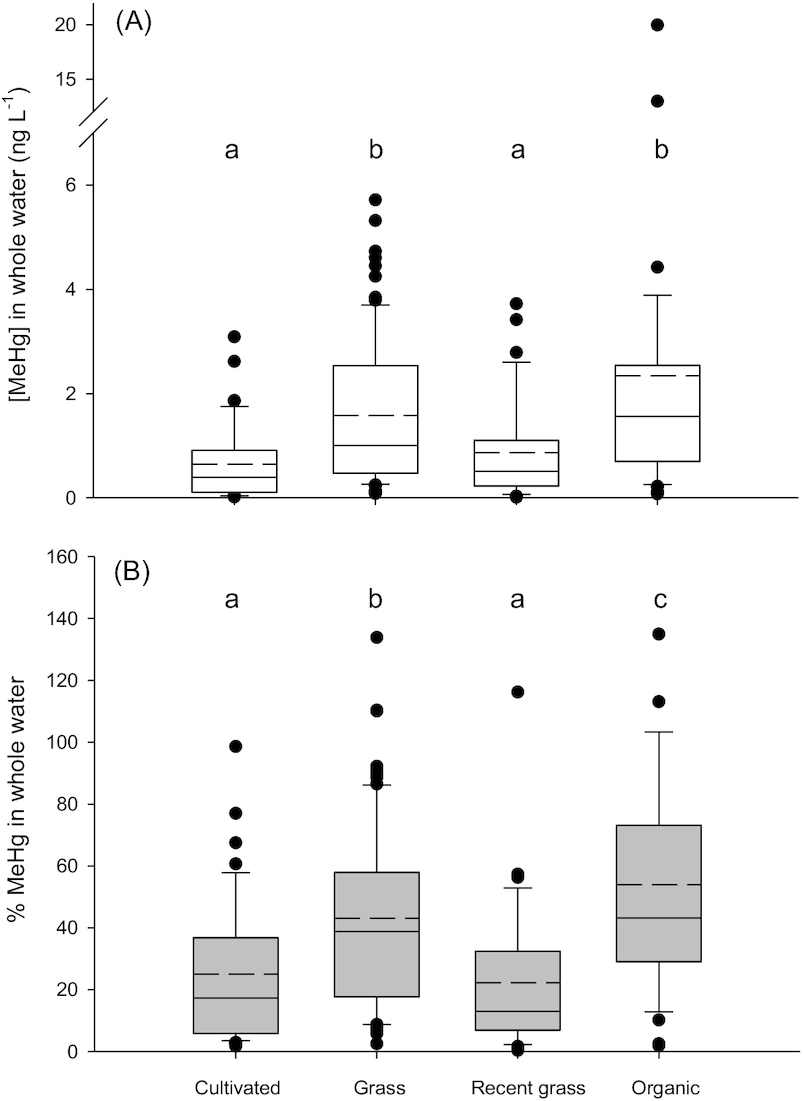
Average MeHg concentrations in water were higher in Type III wetland ponds surrounded by organic farming (
p < 0.001) and grasslands (
p < 0.001) compared to those surrounded by cultivated lands or lands that had been recently converted to grass (
Fig. 3). The significant difference in [MeHg] and %MeHg in the Organic land use group could have been driven by data from Organic 2, which had high concentrations due to high particle loads (see above). Concentrations of THg in ponds surrounded by cultivated land were significantly lower than in ponds with other land use (
p = 0.002; Fig. S3).
We present two explanations for why land use might indirectly influence [MeHg] in these environments; both are based on the finding that DOC concentrations were significantly lower in Cultivated ponds (Fig. S4A). First, because organic matter may influence rates of Hg methylation, impacts of land use on DOC mobilization to ponds may increase net MeHg concentrations in waters. For example, ponds with grassland riparian areas may have increased organic matter inputs due to heavily vegetated shorelines compared to ponds where the riparian vegetation is removed in an annual harvest. Ponds surrounded by organic farms may have increased organic matter inputs due to the use of green manures as fertilizers (
Lundquist et al. 1999).
Second, ponds surrounded by grasslands tend to have increased hydraulic conductivity due to increased infiltration rates, macroporosity, and evapotranspiration in the riparian zone (
Conly and van der Kamp 2001;
Bodhinayake and Si 2004). As a result, infiltration of summer precipitation and snowmelt is likely to be higher and runoff smaller in undisturbed grasslands compared to cultivated lands. Increased upland infiltration may result in increased input of porewaters with high MeHg and DOC concentrations, or DOC that is more chemically complex. However, in very wet periods, water fluctuations tend to be smaller for grasslands wetlands compared to cultivated wetlands (
Euliss and Mushet 1996). In fact, the conversion of cultivated lands at the SDNWA to grass in the 1980s resulted in a decrease in water levels in grassland ponds compared to cultivated ponds in subsequent years (
van der Kamp et al. 1999,
2003).
Controls of period of inundation on MeHg concentrations
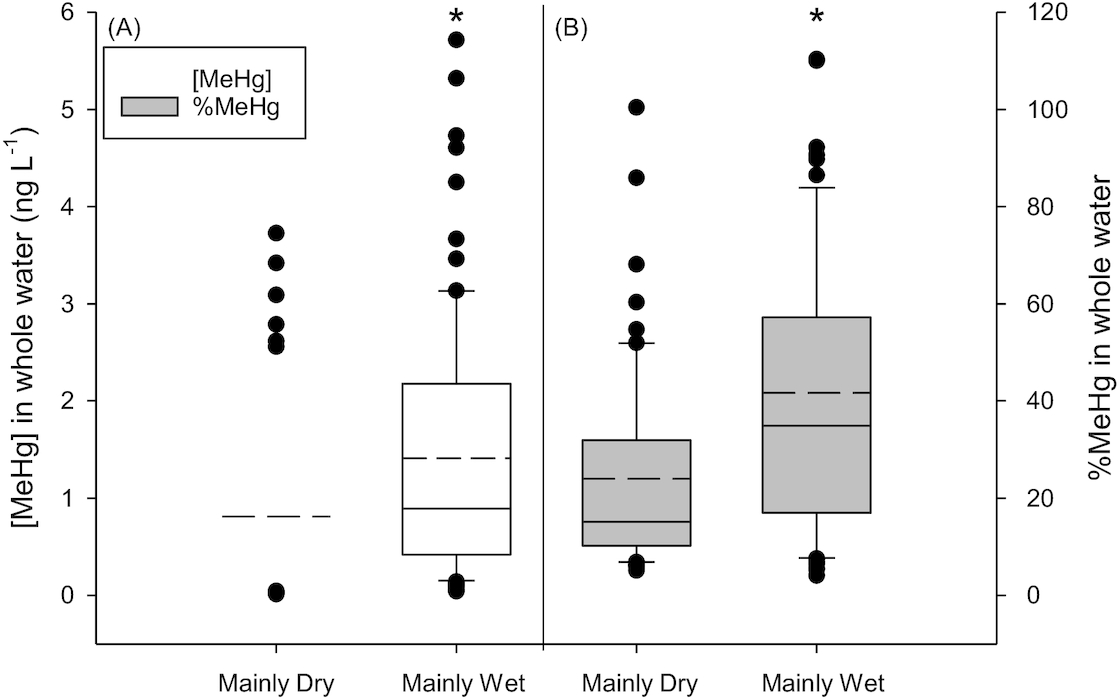
Ideally, we would have liked to examine differences in [MeHg] among Type III, IV, and V ponds to determine if length of hydroperiod controlled concentrations. However, samples numbers in the more permanent ponds (Type IV and V) were small and thus we restricted our analysis to Type III ponds. Average [MeHg] and %MeHg in ponds classified into the Mainly Wet category were significantly higher than ponds classified to the Mainly Dry category (
t test, [MeHg]
p < 0.001 and %MeHg
p < 0.001;
Fig. 4). There was no significant difference in average [THg] in ponds with different hydro-status (
p = 0.479; Fig. S5).
The high variability of hydrologic cycles that result in the alternation of wet–dry conditions of prairie wetlands may result in unusually high rates of MeHg production. We identify two strong possibilities to explain higher [MeHg] in ponds that had significant periods of flooding following years of drought conditions (Mainly Wet ponds). First, rewetting sediments increases the amount of labile DOC due to decomposition of flooded soils and vegetation growth in dried wetlands during periods of drought (
Lundquist et al. 1999;
Graham et al. 2013). We observed higher DOC in our Mainly Wet ponds (range = 15.0–181.8, mean = 63.9) than in the Mainly Dry ponds (range = 10.6–158.0 mg·L
−1, mean = 50.0 mg·L
−1;
p = 0.030) and this correlated with [MeHg] (
Figs. 4 and
5A). As well, infrequent, yet major, hydrological events (including snow melt and intense precipitation events) may result in temporary flooding of soils and terrestrial or riparian vegetation leading to increased DOC concentrations (cited in
Brothers et al. 2014;
Raymond et al. 2016;
Pang et al. 2021). As such, because flooding of organic matter stimulates MeHg methylation (
Hall et al. 2005;
Windham-Myers et al. 2014;
Eckley et al. 2015;
Herrero Ortega et al. 2018), this high-quality, labile DOC can support increased microbial activity and Hg bioavailability to cells (
Kelly et al. 1997;
Hall and St. Louis 2004;
St. Louis et al. 2004;
Bravo and Cosio 2020). In a study examining the impact of the length of hydroperiod on [MeHg] on similar types of prairie ponds at Lostwood Reserve in North Dakota,
Sando et al. (2007) found that whole water [MeHg] and %MeHg values were generally higher in seasonal and semipermanent wetlands compared to those from temporary and permanent wetlands. This was attributed to increased methylation due to the stimulation of the anaerobic microbial community following reflooding of dried ponds. Similar studies in the Everglades have also shown increases in [MeHg] in response elevated oxidized S due to wet–dry cycles (
Krabbenhoft and Fink 2000;
Janssen et al. 2022).
Second, water-level declines expose anoxic wetland sediments or inundated soils to oxygen, thereby changing redox conditions (
Coleman Wasik et al. 2015) resulting in the oxidation of sulfide to SO
4−2, a required substrate for SRB methylators. After such, rewetting and re-establishment of anoxic conditions, may stimulate MeHg production rates (
Eckley et al. 2015). At the SDNWA,
Hoggarth et al. (2015) found that ponds with elevated SO
4−2 concentrations had elevated MeHg concentrations compared to ponds with lower SO
4−2 concentrations, but found no significant difference in potential rates of MeHg production with the use of stable Hg isotope sediment incubations. The elevated SO
4−2 sites in
Hoggarth et al. (2015) correspond to our Mainly Wet ponds which had SO
4−2 concentrations ranges that were an order of magnitude higher than those in the Mainly Dry ponds (range = 0.19–2184 and 0.04–176 mg·L
−1 and mean = 703.5 and 25.9 mg·L
−1, respectively,
Fig. 5B;
p < 0.0001 for log [SO
4−2]). A number of studies (
Benoit et al. 1999;
Li and Cai 2012;
Poulin et al. 2017;
Jones et al. 2020;
Huang et al. 2022;
Janssen et al. 2022) have led to the development of a general paradigm that lakes or wetlands with lower concentrations of SO
4−2 have lower SRB activity, and therefore less MeHg production, whereas those with higher SO
4−2 concentrations result in inhibition of methylation due to formation of insoluble Hg–sulfide complexes not bioavailable to methylating organisms (
Gilmour et al. 1998). Although we suspect high sulfide concentrations may have been present based on occasional H
2S odours and visual observation of black sediments, our data suggest that our sites may not fit this paradigm because
Hoggarth et al. (2015) did not observe inhibition of potential methylation rates, and [MeHg] in sites with elevated SO
4−2 were high compared to those in sites with lower SO
4−2 concentrations. Prolonged flooding of the saline ring formed around the typical pond margin could also release SO
4−2 into the pond while water levels are high (
Mushet et al. 2018). Rates of SO
4−2 reduction can be rapid in prairie wetland sediment with turnover of the porewater SO
4−2 pool in hours to days (
Dalcin Martins et al. 2017).
To further investigate the impact of S species on MeHg concentrations in our ponds, we used traditional geochemical speciation modelling. In the absence of details on DOC characterization, historic data from 18 ponds at SDNWA (
Bam et al. 2019) were input into the geochemical modeling software Visual MINTEQ ver. 3.1 (
Gustafsson 2014) to determine the dominant S species in anoxic bottom waters of our ponds. At pH = 8, models suggest that Hg species were dominated by small, ionic S containing species such as HgSH
2− and HgS
2−2 (Fig. S6). Under the assumption that bioavailable Hg enters methylating cells passively (which may or may not be the case;
Hsu-Kim et al. 2013), ionic Hg species tend to be less bioavailable, and if our sites fit the accepted paradigm of inhibition of methylation in high S systems, we would have expected to see lower concentrations of MeHg in high SO
4−2 sites. Higher MeHg concentrations in high SO
4−2 sites suggest that the formation of HgS complexes are not rate limiting for MeHg production. It is possible that DOC is outcompeting sulfide species for Hg binding and facilitating MeHg production in high DOC, high SO
4−2 sites.
The chemical composition of DOC also influences MeHg cycling in aquatic systems, in part because DOC and S species compete for Hg binding and thus controlling the ability of Hg to enter methylating cells (
Miller et al. 2007). Highly complex aromatic moieties in DOC measured using SUVA mobilizes both inorganic and organic Hg facilitating increased inorganic Hg methylation and increased MeHg mobilization from sediments into other components of aquatic systems (
Aiken et al. 2003,
2011). In our sites, SUVA ranged from 0.43 to 4.9 L mg·C
−1·cm
−1 and were not significantly different in Mainly Wet compared to Mainly Dry ponds (
t test,
p = 0.404 for SUVA;
Fig. 5A). The range in SUVA values in our sites was similar to those in dark water systems that had significant relationships between MeHg concentrations and SUVA values (Louisiana wetlands range = 2.6–4.0 L mg·C
−1·cm
−1 (
Hall et al. 2008), boreal lakes = 1.4–4.8 L mg·C
−1·cm
−1 (
Bravo et al. 2016), and European lotic systems = 2.4–5.2 L mg·C
−1·cm
−1). The highest SUVA values in our sites were higher than those in North Dakota (1.2–2.1 L mg·C
−1·cm
−1 (
Holloway et al. 2011)). Prairie wetland ponds typically experience intense irradiation due to long summer days, lower cloud cover, and reduced overhanging riparian or floating vegetation. As a result, due to intense photodegradation and despite high DOC concentrations, waters in many prairie ponds are not highly coloured and contain less aromatic and labile DOC (
Waiser and Robarts 2004); however, some of our ponds do not follow this trend. Finally, more complex DOC (more complex DOC could be more aromatic, have more complicated active side groups, and be less bio- or photodegraded) in our Mainly Wet ponds may inhibit photolytic demethylation to a greater extent compared to less complex DOC in our Mainly Dry ponds, facilitating increased net MeHg concentrations in the water.
Investigating possible explanations on why prairie wetland ponds may not fit the accepted model is on-going in our lab, including the identification of putative methylating and demethylating microbes in ponds with differing MeHg properties and on-going studies investigating how DOC with different in optical characteristics may clarify the role of DOC composition in Hg cycling in SDNWA ponds.